Advances in siRNA Drug Delivery Strategies for Targeted TNBC Therapy
Abstract
1. Introduction
2. Significant Roles of Biomarkers, TILs, and Genes in TNBC
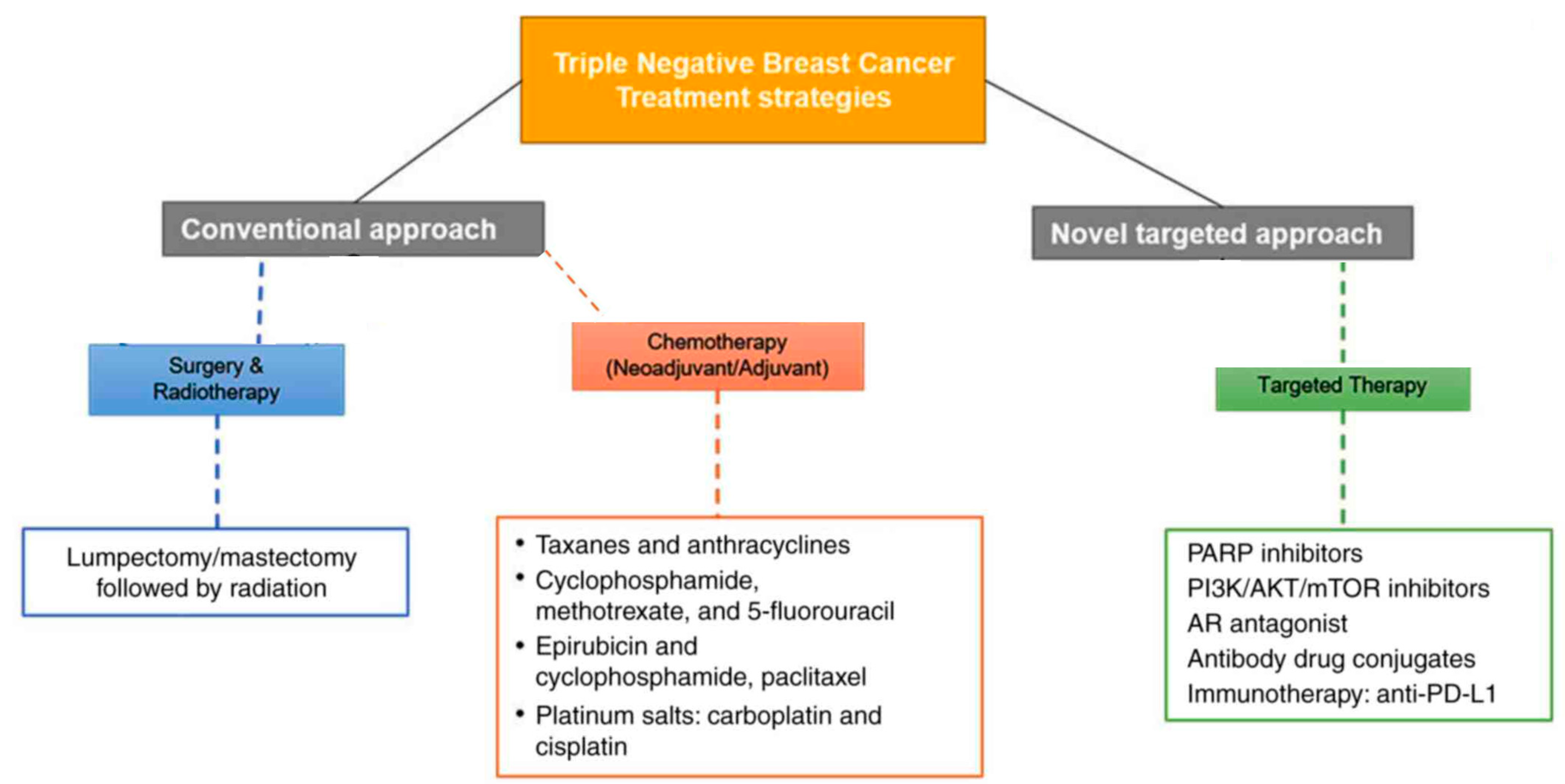
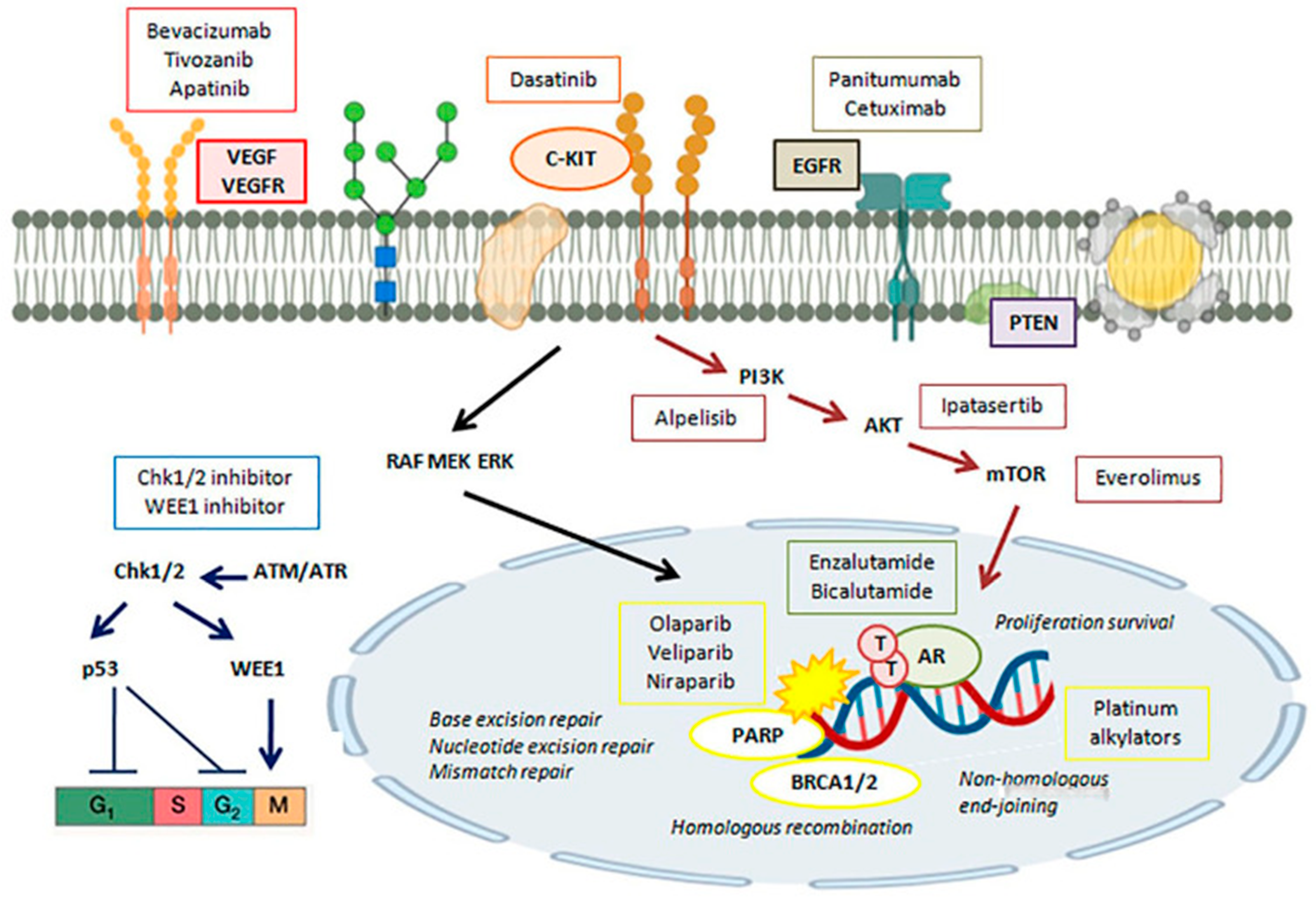
3. Currently Utilized Therapeutic Targets in TNBC
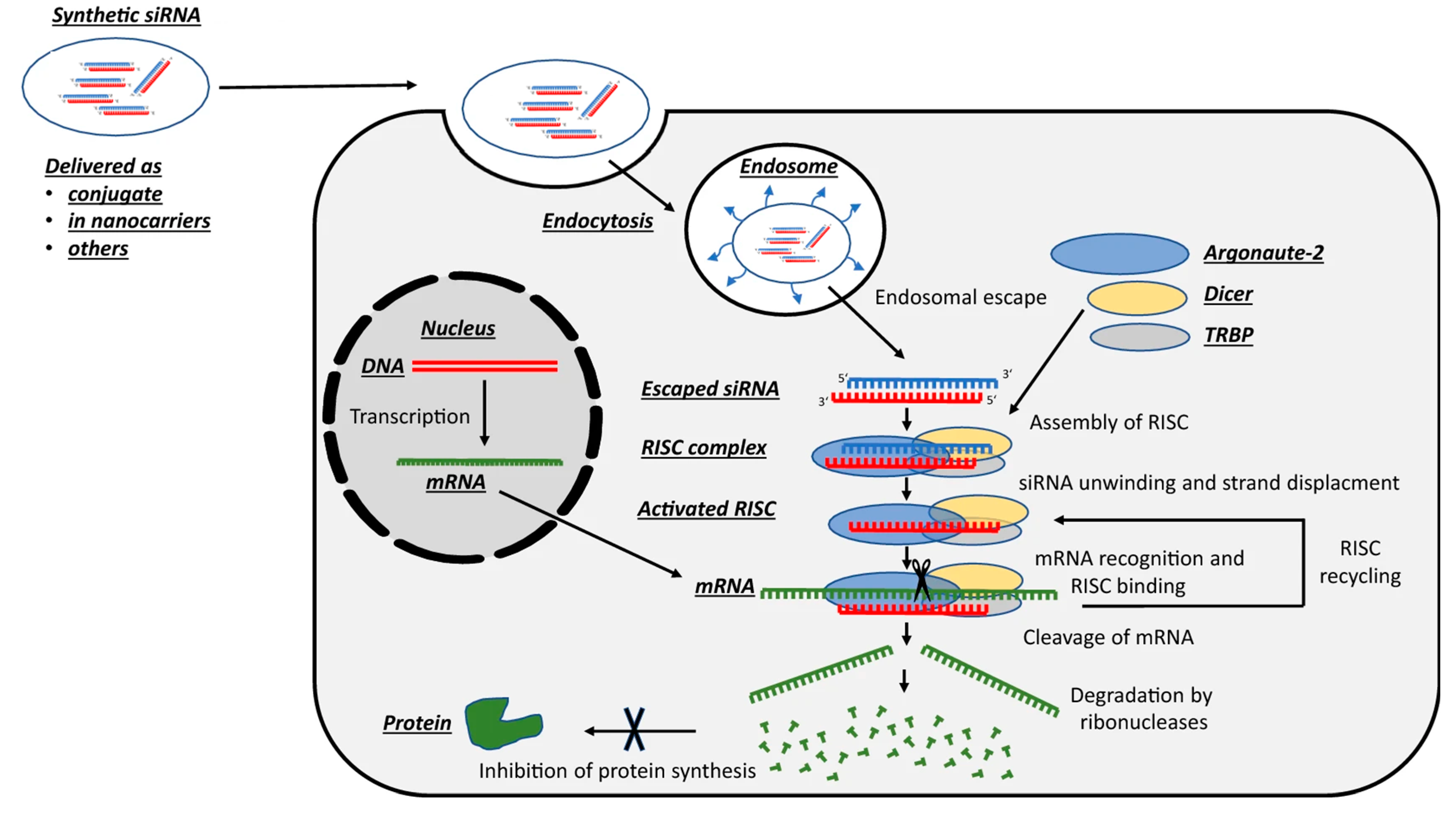
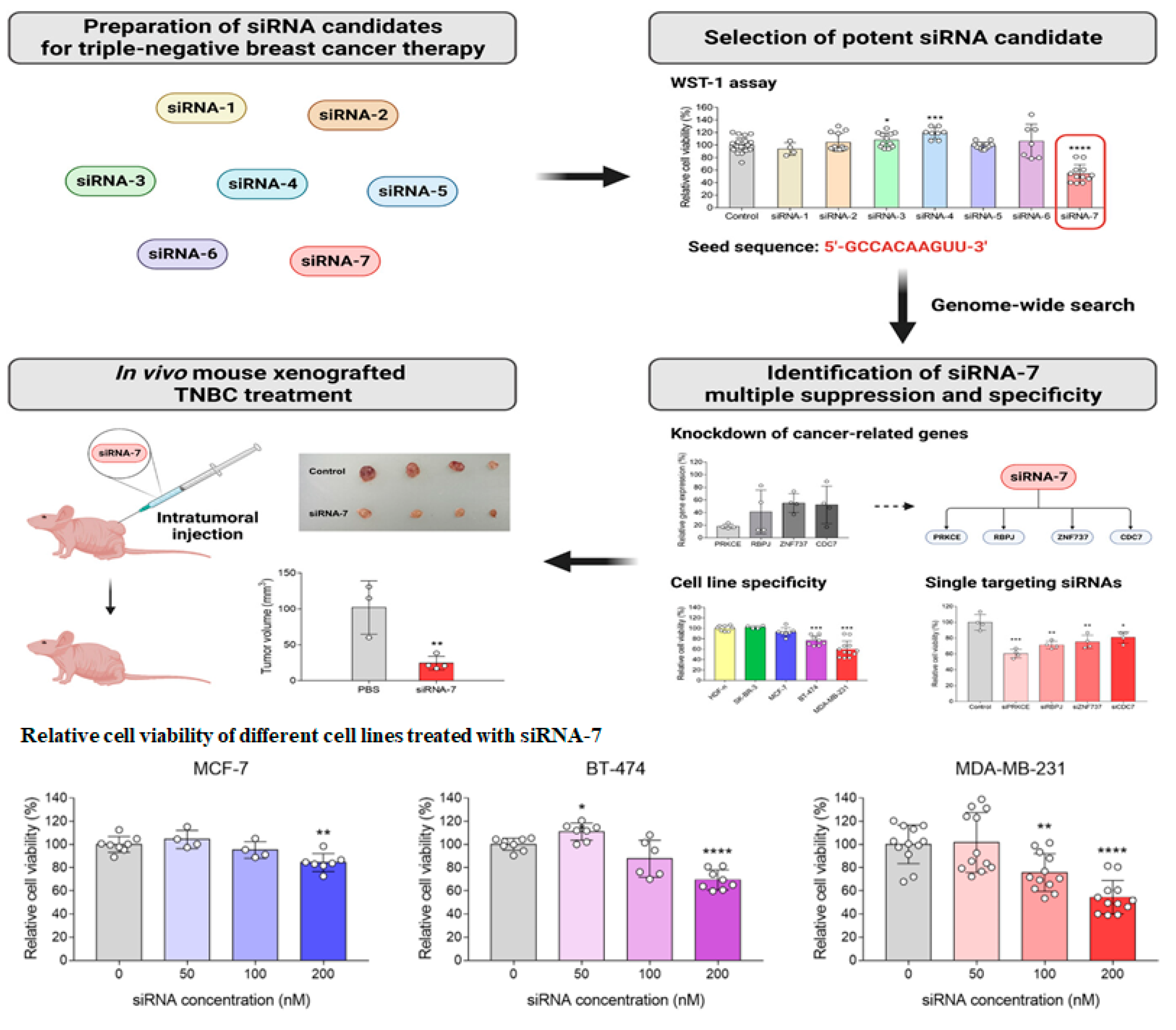
4. siRNA-Based Therapies Facilitating an Antitumor Effect in Cancer Therapy
5. Targeted siRNA Therapy in TNBC
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Data and Statistics. Available online: https://www.cdc.gov/cancer/data/?CDC_AAref_Val=https://www.cdc.gov/cancer/dcpc/data/index.htm (accessed on 9 April 2024).
- American Cancer Society. Global Cancer Facts & Figures, 5th ed.; American Cancer Society: Atlanta, GA, USA, 2024; p. 2. Available online: https://www.cancer.org/research/cancer-facts-statistics/global.html (accessed on 9 April 2024).
- Abad, M.N.; Calabuing-Farinas, S.; Lobo de Mena, M.; Sanz de Bremond, M.J.G.; González, C.G.; Martínez, S.T.; García-García, J.A.; González-Cruz, V.I.; Herrero, C.C. Update on systemic treatment in early triple-negative breast cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835920986749. [Google Scholar]
- Manjunath, M.; Choudhary, B. Triple-negative breast cancer: A run-through of features, classification and current therapies. Oncol. Lett. 2021, 22, 512. [Google Scholar] [CrossRef] [PubMed]
- Triple-Negative Breast Cancer: Symptoms, Treatment, Research. BCRF. 20 February 2024. Available online: https://www.bcrf.org/blog/triple-negative-breast-cancer-treatment-symptoms-research (accessed on 9 April 2024).
- Ensenyat-Mendez, M.; Llinàs-Arias, P.; Orozco, J.I.J.; Iñiguez-Muñoz, S.; Salomon, M.P.; Sesé, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front. Oncol. 2021, 11, 681476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhan, Z.; Yin, X.; Fu, S.; Deng, X. Targeted Therapeutic Strategies for Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 731535. [Google Scholar] [CrossRef] [PubMed]
- Parvani, J.G.; Jackson, M.W. Silencing the roadblocks to effective triple-negative breast cancer treatments by siRNA nanoparticles. Endocr.-Relat. Cancer 2017, 24, R81–R97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Song, B.; Yi, M.; Yan, Y.; Mei, Q.; Wu, K. Recent advances in targeted strategies for triple-negative breast cancer. J. Hematol. Oncol. 2023, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Ning, Z.; Chen, H.; Wu, Y. Nanomaterial Technology and Triple Negative Breast Cancer. Front. Oncol. 2022, 11, 828810. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, C.; Akakuru, O.U.; Su, Z.; Wu, A.; Wei, G. The design and biomedical Applications of self-assembled two-dimensional organic biomaterials. Chem. Soc. Rev. 2019, 48, 5564–5595. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Alu, A.; Liu, H.; Shi, Y.; Wei, X.; Cai, L.; Wei, Y. Biomaterial-assisted biotherapy: A brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact. Mater. 2022, 17, 29–48. [Google Scholar] [CrossRef]
- Wei, P.S.; Chen, Y.J.; Lin, S.Y.; Chuang, K.H.; Sheu, M.T.; Ho, H.O. In situ subcutaneously injectable thermosensitive PEG-PLGA diblock and PLGA-PEG-PLGA triblock copolymer composite as sustained delivery of bispecific anti-CD3 scFv T-cell/anti-EGFR Fab Engager (BiTEE). Biomaterials 2021, 278, 121166. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.H.; Wu, Y.; Varadhan, A.K.; Curvino, E.J.; Chong, A.S.; Collier, J.H. Enabling sublingual peptide immunization with molecular self-assemblies. Biomaterials 2020, 241, 119903. [Google Scholar] [CrossRef]
- Zhang, N.; Mei, K.; Guan, P.; Hu, X.; Zhao, Y. Protein-based artificial nanosystems in cancer therapy. Small 2020, 16, e1907256. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Butkovich, N.; Li, E.; Ramirez, A.; Burkhardt, A.M.; Wang, S.W. Advancements in protein nanoparticle vaccine platforms to combat infectious disease. Wiley Interdiscipl. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1681. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Smanski, M.J.; Zhou, H.; Claesen, J.; Shen, B.; Fischbach, M.A.; Voigt, C.A. Synthetic biology to access and expand nature’s chemical diversity. Nat. Rev. Microbiol. 2016, 14, 135–149. [Google Scholar] [CrossRef]
- Cai, A.-Y.; Zhu, Y.-J.; Qi, C. Biodegradable Inorganic Nanostructured Biomaterials for Drug Delivery. Adv. Mater. Interfaces 2020, 819, 2000819. [Google Scholar] [CrossRef]
- Lee, J.; Bang, J.H.; Ryu, Y.C.; Hwang, B.H. Multiple suppressing small interfering RNA for cancer treatment—Application to triple-negative breast cancer. Biotechnol. J. 2023, 18, 2300060. [Google Scholar] [CrossRef]
- Abdulmalek, S.A.; Saleh, A.M.; Shahin, Y.R.; El Azab, E.F. Functionalized siRNA-chitosan nanoformulations promote triple-negative breast cancer cell death via blocking the miRNA-21/AKT/ERK signaling axis: In-silico and in vitro studies. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D. Cancer and the Immune System: Basic Concepts and Targets for Intervention. Semin. Oncol. 2015, 42, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. Immune blockade inhibition in breast cancer. Anticancer. Res. 2016, 36, 5607–5622. [Google Scholar] [CrossRef] [PubMed]
- Muenst, V.; Schaerli, A.R.; Gao, F.; Däster, S.; Trella, E.; Droeser, R.A.; Muraro, M.G.; Zajac, P.; Zanetti, R.; Gillanders, W.E.; et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2014, 146, 15–24. [Google Scholar] [CrossRef]
- Wen, W.X.; Leong, C. Association of BRCA1- and BRCA2-deficiency with mutation burden, expression of PD-L1/PD-1, immune infiltrates, and T cell-inflamed signature in breast cancer. PLoS ONE 2019, 14, e0215381. [Google Scholar] [CrossRef]
- Cyprian, F.S.; Akhtar, S.; Gatalica, Z.; Vranic, S. Targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy: A new clinical paradigm in the treatment of triple-negative breast cancer. Bosn. J. Basic Med. Sci. 2019, 19, 227–233. [Google Scholar] [CrossRef]
- Friese, C.; Harbst, K.; Borch, T.H.; Westergaard, M.C.W.; Pedersen, M.; Kverneland, A.; Jönsson, G.; Donia, M.; Svane, I.M.; Met, O. CTLA-4 blockade boosts the expansion of tumor-reactive CD8+ tumor-infiltrating lymphocytes in ovarian cancer. Sci. Rep. 2020, 10, 3914. [Google Scholar] [CrossRef]
- Kassardjian, A.; Shintaku, P.I.; Moatamed, N.A. Expression of immune checkpoint regulators, cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1), in female breast carcinomas. PLoS ONE 2018, 13, e0195958. [Google Scholar] [CrossRef]
- Marra, A.; Viale, G.; Curigliano, G. Recent advances in triple-negative breast cancer: The immunotherapy era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef]
- Wesolowski, J.; Tankiewicz-Kwedlo, A.; Pawlak, D. Modern Immunotherapy in the Treatment of Triple-Negative Breast Cancer. Cancers 2022, 14, 3860. [Google Scholar] [CrossRef] [PubMed]
- Afghahi, A.; Telli, M.L.; Kurian, A.W. Genetics of triple-negative breast cancer: Implications for patient care. Curr. Probl. Cancer 2016, 40, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, J.; Zhang, Z.; Tang, Y.; Li, X.; Liu, S.; Cao, S.; Li, X. Association between BRCA status and triple-negative breast cancer: A meta-analysis. Front. Pharmacol. 2018, 9, 909. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, J.; Loibl, S. Optimal Systemic Treatment for Early Triple-Negative Breast Cancer. Breast Care 2020, 15, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Weber, K.E.; Timms, K.M.; Elkin, E.P.; Hahnen, E.; Fasching, P.A.; Lederer, B.; Denkert, C.; Schneeweiss, A.; Braun, S.; et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann. Oncol. 2018, 29, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Pohl-Rescigno, E.; Hauke, J.; Rhiem, K.; Möbus, V.; Furlanetto, J.; Denkert, C.; Fasching, P.A.; Hanusch, C.; Tesch, H.; Weber-Lassalle, N.; et al. Germline mutation status and therapy response in high-risk early breast cancer: Results of the GeparOcto study (NCT02125344). J. Clin. Oncol. 2019, 37 (Suppl. S15), 573. [Google Scholar] [CrossRef]
- Walsh, C.S. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol. Oncol. 2015, 137, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zgura, A.; Galesa, L.; Bratila, E.; Anghel, R. Relationship between tumor-infiltrating lymphocytes and progression in breast cancer. Maedica 2018, 13, 317–320. [Google Scholar]
- Denkert, C.; von Minckwitz, G.; Brase, J.C.; Sinn, B.V.; Gade, S.; Kronenwett, R.; Pfitzner, B.M.; Salat, C.; Loi, S.; Schmitt, W.D.; et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 2015, 33, 983–991. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, S.; Li, M.; Li, F.; Wei, F.; Liu, J.; Ren, X. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front. Immunol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Sukumar, J.; Gast, K.; Quiroga, D.; Lustberg, M.; Williams, N. Triple-negative breast cancer: Promising prognostic biomarkers currently in development. Expert. Rev. Anticancer Ther. 2021, 21, 135–148. [Google Scholar] [CrossRef]
- El Hejjioui, B.; Lamrabet, S.; Amrani Joutei, S.; Senhaji, N.; Bouhafa, T.; Malhouf, M.A.; Bennis, S.; Bouguenouch, L. New Biomarkers and Treatment Advances in Triple-Negative Breast Cancer. Diagnostics 2023, 13, 1949. [Google Scholar] [CrossRef]
- Sporikova, Z.; Koudelakova, V.; Trojanec, R.; Hajduch, M. Genetic Markers in Triple-Negative Breast Cancer. Clin. Breast Cancer 2018, 18, e841–e850. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Harris, C.C. p53 Biological Network: At the Crossroads of the Cellular-Stress Response Pathway and Molecular Carcinogenesis. J. Nippon. Med. Sch. 2006, 73, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Zilfou, J.T.; Lowe, S.W. Tumor Suppressive Functions of p53. Cold Spring Harb. Perspect. Biol. 2009, 1, a001883. [Google Scholar]
- Coates, A.S.; Millar, E.K.; O’Toole, S.A.; Molloy, T.J.; Viale, G.; Goldhirsch, A.; Regan, M.M.; Gelber, R.D.; Sun, Z.; Castiglione-Gertsch, M.; et al. Prognostic interaction between expression of p53 and estrogen receptor in patients with node-negative breast cancer: Results from IBCSG Trials VIII and IX. Breast Cancer Res. 2012, 14, R143. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, F.; Jalal, A.; Jalal, A.; Gul, Z.; Mubeen, H.; Rizvi, S.Z.; Un-Nisa, E.A.; Asghar, A.; Butool, F. Multi-omic analysis of dysregulated pathways in triple-negative breast cancer. Asia-Pac. J. Clin. Oncol. 2024, 20, 450–462. [Google Scholar] [CrossRef]
- Speiser, J.J.; Er¸sahin, Ç.; Osipo, C. The Functional Role of Notch Signaling in Triple-Negative Breast Cancer. Vitam. Horm. 2013, 93, 277–306. [Google Scholar]
- Broner, E.C.; Alpert, G.; Gluschnaider, U.; Mondshine, A.; Solomon, O.; Sloma, I.; Rauch, R.; Izumchenko, E.; Aster, J.C.; Davis, M. AL101 mediated tumor inhibition in notch-altered TNBC PDX models. J. Clin. Oncol. 2019, 37, 1064. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wang, L.; Cao, M.; Zhang, S.; Zhang, H.; Zhou, Y.; Wang, J. 270P Apatinib added to taxanes and platinum neoadjuvant chemotherapy for patients with triple-negative and HER2-positive breast cancer: A multicenter, randomized, phase II, open-label trial. Ann. Oncol. 2020, 31, S346. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.B.; Lei, K.-J.; Jiang, M.Q.; Jia, Y.M. Significant response of low-dose apatinib monotherapy in brain metastases of triple-negative breast cancer. Medicine 2019, 98, e14182. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, C.; Shen, Y.; Chen, Q.; Huang, Z.; Liu, J.; Lin, X.; Wang, L.; Wu, F.; Chen, X.; et al. A real-world study of the effectiveness and safety of apatinib-based regimens in metastatic triple-negative breast cancer. BMC Cancer 2024, 24, 39. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Conte, P. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.H.; Fehrenbacher, L.; Blum, J.L. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients with Operable Breast Cancer with a Germline BRCA Pathogenic Variant. J. Clin. Oncol. 2020, 38, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Abraham, J.; Chan, S.; Wheatley, D.; Brunt, A.M.; Nemsadze, G.; Baird, R.D.; Park, Y.H.; Hall, P.S.; Perren, T.; et al. Capivasertib Plus Paclitaxel Versus Placebo Plus Paclitaxel As First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial. J. Clin. Oncol. 2020, 38, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Dent, R.; Im, S.A.; Espié, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. LOTUS investigators. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef]
- Sofianidi, A.; Dumbrava, E.E.; Syrigos, K.N.; Nasrazadani, A. Triple-Negative Breast Cancer and Emerging Therapeutic Strategies: ATR and CHK1/2 as Promising Targets. Cancers 2024, 16, 1139. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, X.; Wang, H.; Zhong, L.; Zou, D. Advances in ATM, ATR, WEE1, and CHK1/2 inhibitors in the treatment of PARP inhibitor-resistant ovarian cancer. Cancer Biol. Med. 2023, 20, 915–921. [Google Scholar] [CrossRef]
- Matsuda, N.; Wang, X.; Lim, B.; Krishnamurthy, S.; Alvarez, R.H.; Willey, J.S.; Parker, C.A.; Song, J.; Shen, Y.; Hu, J.; et al. Safety and efficacy of panitumumab plus neoadjuvant chemotherapy in patients with primary her2-negative inflammatory breast cancer. JAMA Oncol. 2018, 4, 1207–1213. [Google Scholar] [CrossRef]
- Damodaran, S.; Litton, J.K.; Hess, K.R. Abstract OT2-06-01: A phase-2 trial of neoadjuvant alpelisib and nab-paclitaxel in anthracycline refractory triple negative breast cancers with PIK3CA or PTEN alterations. Cancer Res. 2020, 80 (Suppl. S4), OT2-06-01. [Google Scholar] [CrossRef]
- Basho, R.K.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Herbrich, S.M.; Valero, V.; Albarracin, C.; et al. Targeting the PI3K/AKT/mTOR Pathway for the Treatment of Mesenchymal Triple-Negative Breast Cancer Evidence From a Phase 1 Trial of mTOR Inhibition in Combination with Liposomal Doxorubicin and Bevacizumab. JAMA Oncol. 2017, 3, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Sahota, S.; Vahdat, L.T. Sacituzumabgovitecan: An antibody-drug conjugate. Expert. Opin. Biol. Ther. 2017, 17, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Rugo, H.S.; Brufsky, A.; Kalinsky, K.; Cortés, J.; O’Shaughnessy, J.; et al. LBA17 ASCENT: A randomized phase III study of sacituzumabgovitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with previously treated metastatic triple-negative breast cancer (mTNBC). Ann. Oncol. 2020, 31, S1142–S1215. [Google Scholar] [CrossRef]
- Subhan, M.A.; Torchilin, V.P. Advances with antibody-drug conjugates in breast cancer treatment. Eur. J. Pharm. Biopharm. 2021, 169, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Untch, M.; Jackisch, C.; Schneeweiss, A.; Conrad, B.; Aktas, B.; Denkert, C.; Eidtmann, H.; Wiebringhaus, H.; Kümmel, S.; Hilfrich, J.; et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): A randomised, phase 3 trial. Lancet Oncol. 2016, 17, 345–356. [Google Scholar] [PubMed]
- Untch, M.; Jackisch, C.; Schneeweiss, A.; Schmatloch, S.; Aktas, B.; Denkert, C.; Schem, C.; Wiebringhaus, H.; Kümmel, S.; Warm, M.; et al. NAB-Paclitaxel Improves Disease-Free Survival in Early Breast Cancer: GBG 69-GeparSepto. J. Clin. Oncol. 2019, 37, 2226–2234. [Google Scholar] [CrossRef]
- Furlanetto, J.; Jackisch, C.; Untch, M.; Schneeweiss, A.; Schmatloch, S.; Aktas, B.; Denkert, C.; Wiebringhaus, H.; Kümmel, S.; Warm, M.; et al. Efficacy and safety of nab-paclitaxel 125 mg/m2 and nab-paclitaxel 150 mg/m2 compared to paclitaxel in early high-risk breast cancer. Results from the neoadjuvant randomized GeparSepto study (GBG 69). Breast Cancer Res. Treat. 2017, 163, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Mansutti, M.; Anton, A.; Martínez, L.C.; Bisagni, G.; Bermejo, B.; Semiglazov, C.; Thill, M.; Chacon, J.I.; Chan, A.; et al. Event-free survival analysis of the prospectively randomized phase III ETNA study with neoadjuvant nab-paclitaxel (nab-P) versus paclitaxel (P) followed by anthracycline regimens in women with HER2-negative high-risk breast cancer. J. Clin. Oncol. 2019, 37, 515. [Google Scholar] [CrossRef]
- AGO Breast Committee. Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer: Recommendations 2019. Available online: www.ago-online.de (accessed on 9 April 2024).
- National Comprehensive Cancer Network. Breast Cancer (Version 1.2020). 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 9 April 2024).
- Han, J.; Lim, W.; You, D.; Jeong, Y.; Kim, S.; Lee, J.E.; Shin, T.H.; Lee, G.; Park, S. Chemoresistance in the Human Triple-Negative Breast Cancer Cell Line MDA-MB-231 Induced by Doxorubicin Gradient Is Associated with Epigenetic Alterations in Histone Deacetylase. J. Oncol. 2019, 2019, 1345026. [Google Scholar] [CrossRef] [PubMed]
- Jazaeri, A.A.; Awtrey, C.S.; Chandramouli, G.V.R.; Chuang, Y.E.; Khan, J.; Sotiriou, C.; Aprelikova, O.; Yee, C.J.; Zorn, K.K.; Birrer, M.J.; et al. Gene expression profiles associated with response to chemotherapy in epithelial ovarian cancers. Clin. Cancer Res. 2005, 11, 6300–6310. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. ESMO Guidelines Committee. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Michiels, S.; Adams, S.; Loibl, S.; Budczies, J.; Denkert, C.; Salgado, R. The Journey of Tumor-Infiltrating Lymphocytes as a Biomarker in Breast Cancer: Clinical Utility in an Era of Checkpoint Inhibition. Ann. Oncol. 2021, 32, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Dudley, M.E. Adoptive Cell Therapy for the Treatment of Patients with Metastatic Melanoma. Curr. Opin. Immunol. 2009, 21, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Wu, Y.-H.; Song, Y.; Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Ward, E.M.; Sung, H. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef]
- Ward, D.M.; Shodeinde, A.B.; Peppas, N.A. Innovations in Biomaterial Design toward Successful RNA Interference Therapy for Cancer Treatment. Adv. Healthc. Mater. 2021, 10, 2100350. [Google Scholar] [CrossRef]
- Maik Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef]
- Hattab, D.; Bakhtiar, A. Bioengineered siRNA-Based Nanoplatforms Targeting Molecular Signaling Pathways for the Treatment of Triple Negative Breast Cancer: Preclinical and Clinical Advancements. Pharmaceutics 2020, 12, 929. [Google Scholar] [CrossRef]
- Navarro, G.; Sawant, R.R.; Biswas, S.; Essex, S.; Tros de Ilarduya, C.; Torchilin, V.P. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine 2012, 7, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Zhang, W.; Sun, C.; Wu, J.; Tang, J. Reversing of multidrug resistance breast cancer by co-delivery of P-gp siRNA and doxorubicin via folic acid-modified core-shell nanomicelles. Colloids Surf. B Biointerfaces 2016, 138, 60–69. [Google Scholar] [CrossRef]
- Yu, M.; Han, S.; Kou, Z.; Dai, J.; Liu, J.; Wei, C.; Li, Y.; Jiang, L.; Sun, Y. Lipid nanoparticle-based co-delivery of epirubicin and BCL-2 siRNA for enhanced intracellular drug release and reversing multidrug resistance. Artif. Cells Nanomed. Biotechnol. 2018, 46, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yu, A.-M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Shen, M.; Teng, Y.; Sun, Y.; Li, F.; Zhang, X.; Xu, Y.; Duan, Y.; Du, L. Enhanced therapeutic effect of Adriamycin on multidrug-resistant breast cancer by the ABCG2-siRNA loaded polymeric nanoparticles assisted with ultrasound. Oncotarget 2015, 6, 43779–43790. [Google Scholar] [CrossRef]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopsowitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sun, H.; Xu, P.; Yin, Q.; Zhang, Z.; Wang, S.; Yu, H.; Li, Y. Simultaneous inhibition of metastasis and growth of breast cancer by co-delivery of twist shRNA and paclitaxel using pluronic P85-PEI/TPGS complex nanoparticles. Biomaterials 2013, 34, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Riehle, R.; Navarro, G.; Perche, F.; De Rosa, G.; Torchilin, V.P. Polymeric micelles containing reversibly phospholipid-modified anti-survivin siRNA: A promising strategy to overcome drug resistance in cancer. Cancer Lett. 2014, 343, 224–231. [Google Scholar] [CrossRef]
- Kara, G. siRNA targeting ABCB1 potentiates the efficacy of chemotherapy in human triple-negative breast cancer cells. Hacet. J. Biol. Chem. 2022, 50, 349–358. [Google Scholar] [CrossRef]
- Kesharwani, P.; Sheikh, A.; Abourehab, M.A.S.; Salve, R.; Gajbhiye, V. A combinatorial delivery of survivin targeted siRNA using cancer-selective nanoparticles for triple-negative breast cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 80, 104164. [Google Scholar] [CrossRef]
- Hamurcu, Z.; Ashour, A.; Kahraman, N.; Ozpolat, B. FOXM1 regulates expression of eukaryotic elongation factor 2 kinase and promotes proliferation, invasion and tumorigenesis of human triple negative breast cancer cells. Oncotarget 2016, 7, 16619–16635. [Google Scholar] [CrossRef]
- Wang, M.; Gartel, A.L. The suppression of FOXM1 and its targets in breast cancer xenograft tumors by siRNA. Oncotarget 2011, 2, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Asik, E.; Kahraman, N.; Turk, M.; Ozpolat, B.; Ulubayram, K. Modified gold-based siRNA nanotherapeutics for targeted therapy of triple-negative breast cancer. Nanomedicine 2017, 12, 1961–1973. [Google Scholar] [CrossRef]
- Jan, A.; Jansonius, B.; Delaidelli, A.; Bhanshali, F.; An, Y.A.; Ferreira, N.; Smits, L.M.; Negri, G.L.; Schwamborn, J.C.; Jensen, P.H.; et al. Activity of translation regulator eukaryotic elongation factor-2 kinase is increased in Parkinson disease brain and its inhibition reduces alpha synuclein toxicity. Acta Neuropathol. Commun. 2018, 6, 54. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Han, C.; Wan, G.; Huang, X.; Ivan, C.; Jiang, D.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Rao, P.H.; et al. TP53 loss creates therapeutic vulnerability in colorectal cancer. Nature 2015, 520, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Pileczki, V.; Pop, L.; Petric, R.C.; Chira, S.; Pointiere, E.; Achimas-Cadariu, P.; Ioana Berindan-Neagoe, D. Dual Targeted Therapy with p53 siRNA and Epigallocatechingallate in a Triple Negative Breast Cancer Cell Model. PLoS ONE 2015, 10, e0120936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Liu, H.; Jain, A.; Patel, P.V.; Cheng, K. Co-delivery of IKBKE siRNA and cabazitaxel by hybrid nanocomplex inhibits invasiveness and growth of triple-negative breast cancer. Sci. Adv. 2020, 6, eabb0616. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, Y.; Wang, H.; Stewart, S.; Van der Jeught, K.; Agarwal, P.; Zhang, Y.; Liu, S.; Zhao, G.; et al. Precise targeting of POLR2A as a therapeutic strategy for human triple-negative breast cancer. Nat. Nanotechnol. 2019, 14, 388–397. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Lee, K.-M.; Jennifer, M.; Giltnane, M.J.; Balko, J.M.; Guerrero-Zotano, A.L.; Hutchinson, K.E.; Nixon, M.J.; Estrada, M.V.; Sánchez, V.; Sanders, M.E.; et al. MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metab. 2017, 26, 633–647. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Patel, S.S.; Cavnar, A.B.; Lo, J.H.; Babb, L.M.; Francini, N.; Patil, P.; Colazo, J.M.; Michell, D.L.; Sanchez, V.M.; et al. Structural optimization of siRNA conjugates for albumin binding achieves effect tive MCL1-directed cancer therapy. Nat. Commun. 2024, 15, 1581. [Google Scholar] [CrossRef] [PubMed]
- Maire, V.; Némati, F.; Richardson, M.; Vincent-Salomon, A.; Tesson, B.; Rigaill, G.; Gravier, E.; Marty-Prouvost, B.; Coning, L.D.; Lang, G.; et al. Polo-like kinase 1: A potential therapeutic Option in combination with conventional chemotherapy for the management of patients with triple-negative breast cancer. Cancer Res. 2013, 73, 813–823. [Google Scholar] [CrossRef]
- Hu, K.; Law, J.H.; Fotovati, A.; Dunn, S.E. Small interfering RNA library screen identified polo-like kinase-1 (PLK1) as a potential therapeutic target for breast cancer that uniquely eliminates tumor-initiating cells. Breast Cancer Res. 2012, 14, R22. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Gu, S.; Reda, M.; Castro, D.J.; Sangvanich, T.; Gray, G.W.; Yantasee, W. Targeted treatment of metastatic breast cancer by PLK1 siRNA delivered by an antioxidant nanoparticle platform. Mol. Cancer Ther. 2017, 16, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Asai, T.; Hirai, Y.; Shimizu, K.; Koide, H.; Minamino, T.; Oku, N. Systemic Administration of siRNA with Anti-HB-EGF Antibody-Modified Lipid Nanoparticles for the Treatment of Triple-Negative Breast Cancer. Mol. Pharm. 2018, 15, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Glackin, C.A. Nanoparticle Delivery of TWIST Small Interfering RNA and Anticancer Drugs: A Therapeutic Approach for Combating Cancer. Enzymes 2018, 44, 83–101. [Google Scholar] [PubMed]
- Finlay, J.; Roberts, C.M.; Lowe, G.; Loeza, J.; Rossi, J.J.; Glackin, C.A. RNA-Based TWIST1 Inhibition via Dendrimer Complex to Reduce Breast Cancer Cell Metastasis. BioMed Res. Int. 2015, 2015, 382745. [Google Scholar] [CrossRef] [PubMed]
- Pillé, J.-Y.; Denoyelle, C.; Varet, J.; Bertrand, J.-R.; Soria, J.; Opolon, P.; Lu, H.; Pritchard, L.-L.; Vannier, J.-P.; Malvy, C.; et al. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol. Ther. 2005, 11, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yang, J.; Di Jia, M.A.M.; Auguste, D.T. ICAM-1-targeted, Lcn2 siRNA-encapsulating liposomes are potent anti-angiogenic agents for triple-negative breast cancer. Theranostics 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Ye, Z.; Abdelmoaty, M.M.; Curran, S.M.; Dyavar, S.R.; Kumar, D.; Alnouti, Y.; Coulter, D.W.; Podany, A.T.; Singh, R.K.; Vetro, J.A. Direct Comparison of Chol-siRNA Polyplexes and Chol-DsiRNA Polyplexes Targeting STAT3 in a Syngeneic Murine Model of TNBC. Non-Coding RNA 2022, 8, 8. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, M.; Weng, B.; Mao, H.; Zhao, J. Exosome-based delivery nanoplatforms: Next-generation theranostic platforms for breast cancer. Biomater. Sci. 2022, 10, 1607–1625. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.N.; Chaudhuri, A.; Aqil, F.; Dehari, D.; Munagala, R.; Singh, S.; Gupta, R.C.; Agrawal, A.K. Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application. Cancers 2022, 14, 1435. [Google Scholar] [CrossRef] [PubMed]
- Ochiya, T.; Takenaga, K.; Endo, H. Silencing of S100A4, a metastasis-associated protein, in endothelial cells inhibits tumor angiogenesis and growth. Angiogenesis 2014, 17, 17–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Logue, S.E.; Samali, A.; Cleary, P.; Greene, S.; Mnich, K.; Almanza, A.; Chevet, E.; Dwyer, R.M.; Oommen, A.; Legembre, P.; et al. Inhibition of IRE RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun. 2018, 9, 3267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mu, C.; Zhang, T.; Wang, Y.; Wang, Y.; Fan, L.; Liu, C.; Chen, H.; Shen, J.; Wei, K.; et al. Systemic Delivery of Aptamer-Conjugated XBP1 siRNA Nanoparticles for Efficient Suppression of HER2+ Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 32360–32371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mu, C.; Zhang, T.; Yang, D.; Wang, C.; Chen, Q.; Tang, L.; Fan, L.; Liu, C.; Shen, J.; et al. Development of targeted therapy therapeutics to sensitize triple-negative breast cancer chemosensitivity utilizing bacteriophage phi29 derived packaging RNA. J. Nanobiotechnol. 2021, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Mao, T.; Yang, H.; Wang, H.; Wang, Y. Silencing of TGIF sensitizes MDA-MB-231 human breast cancer cells to cisplatin-induced apoptosis. Exp. Ther. Med. 2018, 15, 2978–2984. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.N.; Suri, S.; Li, K.; Casas, C.G.; Stigliano, G.; Riley, R.S.; Scully, M.A.; Hoover, E.C.; Aboeleneen, S.B.; Kramarenko, G.C.; et al. Antibody and siRNA Nanocarriers to Suppress Wnt Signaling, Tumor Growth, and Lung Metastasis in Triple-Negative Breast Cancer. Adv. Ther. 2024, 7, 2300426. [Google Scholar] [CrossRef]
- Jardin, I.; Diez-Bello, R.; Lopez, J.; Redondo, P.C.; Salido, G.M.; Samni, T.; Rosado, J.A. TRPC6 channels are required for proliferation, migration and invasion of breast cancer cell lines by modulation of Orai1 and Orai3 surface exposure. Cancers 2018, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.V.; Pippin, J.W.; Kaiser, C.; Krofft, R.D.; Brinkkoetter, P.T.; Hudkins, K.L. Novel siRNA Delivery System to Target Podocytes In Vivo. PLoS ONE 2010, 5, e9463. [Google Scholar] [CrossRef]
- Jing, H.; Cheng, W.; Li, S.; Wu, B.; Leng, X.; Xu, S.; Tian, J. Novel cell-penetrating peptide-loaded nanobubbles synergized with ultrasound irradiation enhance EGFR siRNA delivery for triple negative Breast cancer therapy. Colloids Surf. B Biointerfaces 2016, 146, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.V.; Hervé-Aubert, K.; David, S.; Lautram, N.; Passirani, C.; Igor Chourpa, I.; Aubrey, N.; Allard-Vannier, E. Targeted nanomedicine with anti-1 EGFR scFv for siRNA delivery into triple-negative breast cancer cells. Eur. J. Pharm. Biopharm. 2020, 157, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Lee, J.S.; et al. Anti-EGF Receptor Aptamer-Guided Co-Delivery of Anti-Cancer siRNAs and Quantum Dots for Theranostics of Triple-Negative Breast Cancer. Theranostics 2019, 9, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Granata, I.; Collina, F.; Leonetti, F.; Cantile, M.; Botti, G.; Fedele, M.; Guarracino, M.R.; Cerchia, L. Novel Aptamers Selected on Living Cells for Specific Recognition of Triple-Negative Breast Cancer. iScience 2020, 23, 100979. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, R.; Pei, X.; Chai, M.; Sun, L.; Huang, Y.; Wang, J.; Barth, S.; Yu, F.; He, H. Antibody–siRNA conjugates (ARC): Emerging siRNA drug formulation. Med. Drug Discov. 2022, 15, 100128. [Google Scholar] [CrossRef]
- Fang, H.; Xie, J.; Zhang, M.; Zhao, Z.; Wan, Y.; Yao, Y. miRNA-21 promotes proliferation and invasion of triple-negative breast cancer cells through targeting PTEN. Am. J. Transl. Res. 2017, 9, 953–961. [Google Scholar] [PubMed]
- Zhang, Z.; Richmond, A.; Yan, C. Immunomodulatory Properties of PI3K/AKT/mTOR and MAPK/MEK/ERK Inhibition Aug-ment Response to Immune Checkpoint Blockade in Melanoma and Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 7353. [Google Scholar] [CrossRef]
- Alshaer, W.; Hillaireau, H.; Vergnaud, J.; Mura, S.; Deloménie, C.; Sauvage, F.; Ismail, S.; Fattal, E. Aptamer-guided siRNA-loaded nanomedicines for systemic gene silencing in CD-44 expressing murine triple-negative breast cancer model. J. Control. Release 2018, 271, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Dey, A.; Taliyan, R.; Puri, A.; Kharavtekar, S.; Dubey, S.K. Recent Advances in Targeted Nanocarriers for the Management of Triple Negative Breast Cancer. Pharmaceutics 2023, 15, 246. [Google Scholar] [CrossRef]
- Patel, S.S.; Hoogenboezem, E.N.; Yu, F.; Sorets, A.G.; Cherry, F.K.; Lo, J.H.; Francini, N.; d’Arcy, R.; Cook, R.S.; Duvall, C.L. Abstract 2706: Therapeutic silencing of Rictor using siRNA nanoparticles to selectively block mTORC2 signaling in triple negative breast cancer. Cancer Res. 2023, 83 (Suppl. S7), 2706. [Google Scholar] [CrossRef]
- Malik, A.M.; Biswas, A.; Mishra, S.; Jadhav, S.; Chakraborty, K.; Tripathi, A.; Mukherjee, A.; Roy, R.S. Engineered vitamin E-tethered non-immunogenic facial lipopeptide for developing improved siRNA-based combination therapy against metastatic breast cancer. Chem. Sci. 2023, 14, 7842–7866. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, H.; Liu, H.; Xu, R. Liposomal ATM siRNA delivery for enhancing triple-negative breast cancer immune checkpoint blockade therapy. J. Biomater. Appl. 2023, 37, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.M.; Sun, Z.; Ayat, N.; Schilb, A.; Liu, X.; Jiang, H.; Sun, D.; Scheidt, J.; Qian, V.; He, S.; et al. Systemic Delivery of Tumor-Targeting siRNA Nanoparticles against an Oncogenic LncRNA Facilitates Effective Triple-Negative Breast Cancer Therapy. Bioconjugate Chem. 2019, 30, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, C.; Schilb, A.; Kim, J.; Sun, D.; Hall, R.; Gao, S.; Gilmore, H.; Schiemann, W.P.; Lu, Z.R. Evaluating Dual-Targeted ECO/siRNA Nanoparticles against an Oncogenic lncRNA for Triple Negative Breast Cancer Therapy with Magnetic Resonance Molecular Imaging. Chem. Biomed. Imaging. 2023, 1, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, X.; Li, Z.; Shi, Q.; Zheng, W.; Liu, Y.; Wang, P. Functionalization of single-walled carbon nanotubes enables efficient intracellular delivery of siRNA targeting MDM2 to inhibit breast cancer cells growth. Biomed. Pharmacother. 2012, 66, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kren, B.T.; Unger, G.M.; Abedin, M.J.; Vogel, R.I.; Henzler, C.M.; Ahmed, K.; Trembley, J.H. Preclinical evaluation of cyclin-dependent kinase 11 and casein kinase 2 survival kinases as RNA interference targets for triple-negative breast cancer therapy. Breast Cancer Res. 2015, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.H.; Mao, C.Q.; Dou, S.; Shen, S.; Tan, Z.B.; Wang, J. Triple-negative breast cancer therapy with CDK1 siRNA delivered by cationic lipid-assisted PEG-PLA nanoparticles. J. Control. Release 2014, 192, 114–121. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Landry, B.; Mahdipoor, P.; Uludag, H. Induction of apoptosis by survivin silencing through siRNA delivery in a human breast cancer cell line. Mol. Pharm. 2011, 8, 1821–1830. [Google Scholar] [CrossRef]
- Li, F.; Aljahdali, I.; Ling, X. Cancer therapeutics using survivin BIRC5 as a target: What can we do after over two decades of study? J. Exp. Clin. Cancer Res. 2019, 38, 368. [Google Scholar] [CrossRef]
- Werfel, T.A.; Wang, S.; Jackson, M.A.; Kavanaugh, T.E.; Joly, M.M.; Lee, L.H.; Hicks, D.J.; Sanchez, V.; Ericson, P.G.; Kilchrist, K.V.; et al. Selective mTORC2 inhibitor therapeutically blocks breast cancer cell growth and survival. Cancer Res. 2018, 78, 1845–1858. [Google Scholar] [CrossRef]
- Xu, R.; Huang, Y.; Mai, J.; Zhang, G.; Guo, X.; Xia, X.; Koay, E.J.; Qin, G.; Li, Q.; Liu, X.; et al. Multistage Vectored siRNA Targeting Ataxia-Telangiectasia Mutated for Breast Cancer Therapy. Small 2013, 9, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.B.; Ballesteros, B.E.A.; Fu, T.; Bahadur, K.C.R.; Alibadi, H.M.; Hugh, J.C.; Löbenberg, R.; Uludağ, H. Multiple siRNA delivery against cell cycle and anti-apoptosis proteins using lipid-substituted polyethylenimine in triple-negative breast cancer and non-malignant cells. J. Biomed. Mater. Res. Part A 2016, 104, 3031–3044. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, W.; Xia, Y.; Li, W.; Sun, J.; Chen, H.; Li, B.; Zhang, D.; Qian, W.; Meng, Y.; et al. The promotion of siRNA delivery to breast cancer overexpressing epidermal growth factor receptor through anti-EGFR antibody conjugation by immunoliposomes. Biomaterials 2011, 32, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Patil, R.; Portilla-Arias, J.; Ding, H.; Konda, B.; Espinoza, A.; Mongayt, D.; Markman, J.L.; Elramsisy, A.; Phillips, H.W.; et al. Nanobiopolymer for direct targeting and inhibition of EGFR expression in triple-negative breast cancer. PLoS ONE 2012, 7, e31070. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.C.; Luker, K.E.; Durmaz, H.; Luker, G.D.; Lahann, J. CXCR4-targeted nanocarriers for triple-negative breast cancers. Biomacromolecules 2015, 16, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.A.; Osooly, M.; Strutt, D.; Masin, D.; Yang, Y.; Yan, H.; Bally, M. Characterization of long-circulating cationic nanoparticle formulations consisting of a two-stage PEGylation step for the delivery of siRNA in a breast cancer tumor model. J. Pharm. Sci. 2013, 102, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Chadar, R.; Kesharwani, P. Nanotechnology-based siRNA delivery strategies for treatment of triple-negative breast cancer. Int. J. Pharm. 2021, 605, 120835. [Google Scholar] [CrossRef]
- Kanugo, A.; Gautam, R.K.; Kamal, M.A. Recent Advances of Nanotechnology in Diagnosis and Therapy of Triple-Negative Breast cancer (TNBC). Curr. Pharm. Biotechnol. 2022, 23, 1581. [Google Scholar] [CrossRef] [PubMed]
- Guha, L.; Bhat, I.A.; Rahman, J.U.; Pottoo, F.H. Nanotechnological Approaches for the Treatment of Triple-Negative Breast Cancer: A Comprehensive Review. Curr. Drug Metab. 2022, 23, 781–799. [Google Scholar] [CrossRef]
- Subhan, M.A.; Filipczak, N.; Torchilin, V.P. Advances with Lipid-Based Nanosystems for siRNA Delivery to Breast Cancers. Pharmaceuticals 2023, 16, 970. [Google Scholar] [CrossRef]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Rhym, L.H.; Anderson, D.G. Nanoscale delivery platforms for RNA therapeutics: Challenges and the current state of the art. Med 2022, 3, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Hattab, D.; Gazzali, A.M.; Bakhtiar, A. Clinical Advances of siRNA-Based Nanotherapeutics for Cancer Treatment. Pharmaceutics 2021, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Gomes-da-Silva, L.C.; Santos, A.O.; Bimbo, L.M.; Moura, V.; Ramalho, J.S.; De Lima, M.C.P.; Simões, S.; Moreira, J.N. Toward a siRNA-containing nanoparticle targeted to breast cancer cells and the tumor microenvironment. Int. J. Pharm. 2012, 434, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, S.; Gore, K.R. Advances in siRNA therapeutics and synergistic effect on siRNA activity using emerging dual ribose modifications. RNA Biol. 2022, 19, 452–468. [Google Scholar] [CrossRef]

| Gene | Associated with TNBC | Breast Cancer Risk Factor | Clinical Guideline |
|---|---|---|---|
| Ataxia-telangiectasia mutated (ATM) | Likely yes | Two- to three-fold | National Comprehensive Cancer Network (NCCN): screening breast MRI |
| BARD1 | Likely yes | - | - |
| BRCA1 | Yes; high prevalence of 185delAG and 5382insC founder mutations in TNBC | 10-fold | American Cancer Society (ACS) and NCCN: Screening breast MRI, recommending risk-reducing bilateral Salpingo-Oophorectomy (BSO), discussing risk-reducing mastectomy |
| BRCA2 | Yes | 10-fold | ACS and NCN: Screening breast MRI, recommending (BSO), discussing risk-reducing mastectomy |
| MR | Likely yes | - | - |
| NBN | Likely yes | Two- to three-fold | - |
| PALB2 | Yes | Three- to five-fold | NCCN: Screening breast MRI, discussing risk-reducing mastectomy |
| PTEN | Likely yes | Five-fold or more | NCCN: Screening breast MRI, discussing risk-reducing mastectomy |
| RAD50 | Yes | - | - |
| RAD51C | Yes | - | NCCN: Considering BSO |
| RAD51D | Yes | _ | NCCN: Considering BSO |
| XRCC2 | Likely yes | - | - |
| Biomarker | Prevalence in TNBC | Mechanism | References |
|---|---|---|---|
| BRCA 1/2 germline mutations | 10–20% | Homologous recombination and DNA double-strand break repair | [35,36,37] |
| Elevated HRD score | 40–70% | Homologous recombination and DNA double-strand break repair | [41] |
| PD-L1 | Variability (immune vs. tumor), disease stage, antibody: 40% on immune cells (SP142 antibody) in metastatic disease, 80% by CPS ≥ 1 (22C3) in primary disease | Evasion of tumor immune surveillance | [27,28] |
| TILs | Variability (intra-tumoral vs. stromal, primary vs. metastatic) | Stromal lymphocytic infiltration of tumor microenvironment | [33,42,43] |
| High tumor mutational burden | 3–11% | Somatic mutations per megabase of DNA | [33] |
| AR (androgen receptor) | 30–35% | Steroid nuclear transcription factor | [45] |
| EGFR | 13–76% EGFR1 overexpression: 18% EGFR1 gene amplification 33% EGFR2 gene amplification: <5% | Receptor tyrosine kinase involved in cell proliferation/survival | [46,47] |
| VEGF | 30–60% | Bind to receptor tyrosine kinase and promote angiogenesis | [45] |
| TP53 mutations | 80% | Encodes transcription factor protein that promotes cell cycle arrest | [48,49,50] |
| PI3K/AKT/mTOR | PI3K 7–9%, PTEN 30–50% | PI3K: intracellular lipid kinases in a signaling cascade that promote cell proliferation/activate survival, PTEN: tumor suppressor gene that downregulates signaling cascade | [45,51] |
| NTRK gene fusion | <1% | Gene fusion results in constitutively active TRK proteins which promotes tumor growth | [45] |
| Notch signaling | 10% | Oncogenes involved in cell proliferation, cell death, cell differentiation, and stem cell maintenance | [52,53] |
| Target Gene or Protein | siRNA-Conjugate with NPs | TNBC Cell Lines | Anticancer Effect | References |
|---|---|---|---|---|
| MDM2 | PEG-functionalized SWNTs | Breast cancer B-cap 37 | MDM2 silencing, reduced proliferation, and enhanced apoptotic cell death in breast tumors | [100] |
| FOXM1 | Liposomal NPs | MDA-MB-231 | Downregulated FOXM1 expression and inhibited cell-cycle regulation, migration/invasion, and survival, decreased growth of the TNBC cell line, MDA-MB-231 in mice. Also downregulated eEF2K expression in TNBC tumors. | [96,98,99] |
| FOXM1 | PEI–cationic polymer | MDA-MB-231 | Reduced FOXM1 protein expression level in TNBC tumor | [97] |
| PLK1 | Mesoporous silica NPs | MDA-MB-231, BT549 | Suppressed PLK1 proteins in TNBC xenografted mice and reduced tumor growth. Inhibited ROS, induced apoptotic cell death in TNBC, and reduced metastasis. | [107,108,109,110] |
| RhOA | Chitosan-coated PIHCA NPs | MDA-MB-231 | Silenced RhoA in TNBC, and reduced TNBC tumor without toxicity | [113] |
| CDK1 and c-Myc | PEG–PLA NPs | SUM149 and BT549 | Inhibition of CDK1 expression in cMyc overexpressed TNBC reduced cell viability through apoptotic cell death demonstrating synthetic lethality between cMyc with CDK1 in TNBC cells | [142] |
| Survivin | Lipid-substituted polymer NPs | MDA-MB-231 | Decreased cancer cell viability, down-regulated survivin protein, inhibited tumor cell growth reduced chemoresistance | [93] |
| Survivin | PEG2K–PE–PM | MDA-MB-231 | Reduced survivin expression in resistant cancer cells, triggered microtubule destabilization and significantly inhibited TNBC tumor growth | [93] |
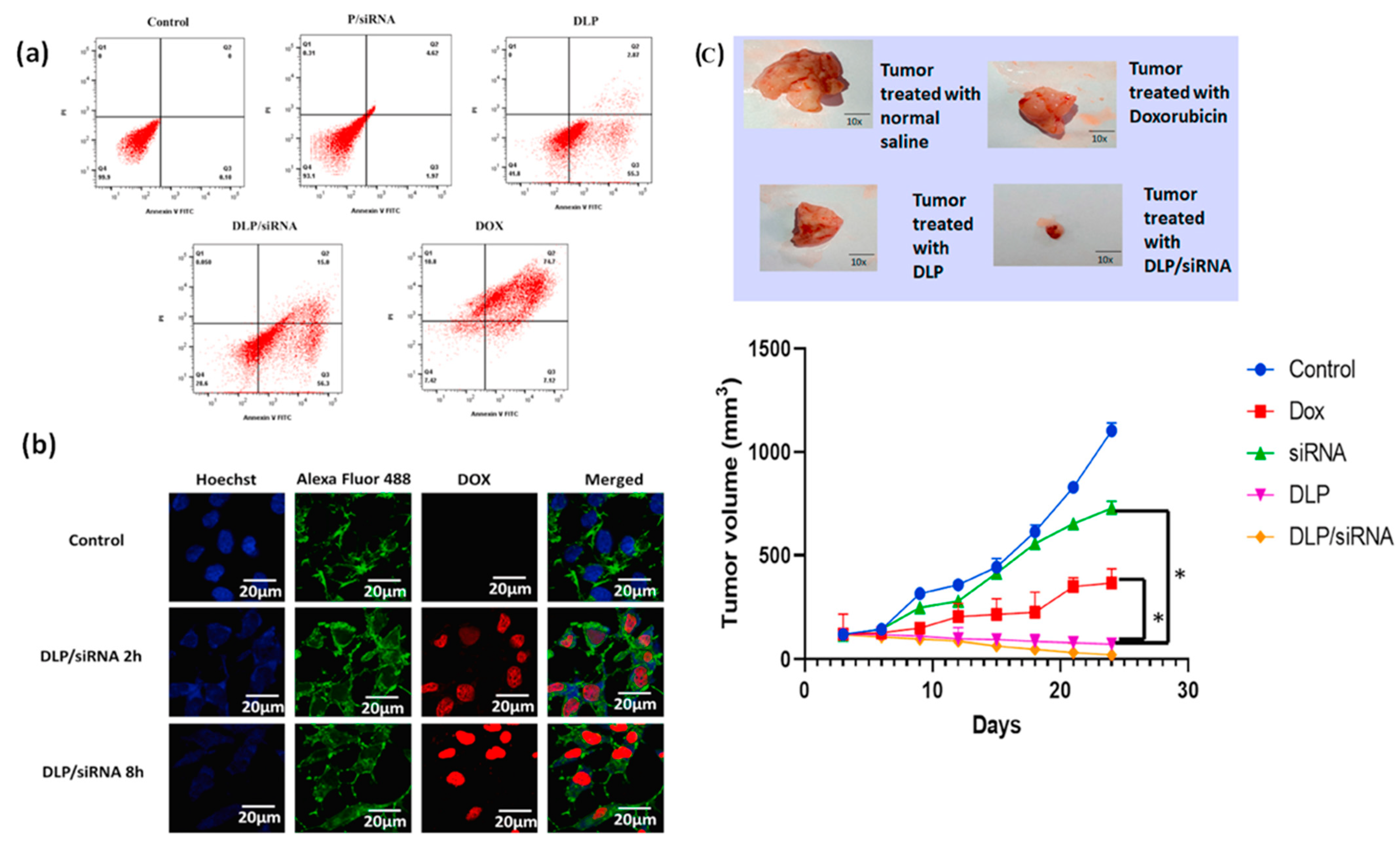
| Survivin | DLP/siRNA | MDA-MB-231 | Induced apoptotic cell death of TNBC cell lines effectively, suppressed cancer cell stemness, and inhibited tumor development | [95] |
| TWIST | PAMAM–dendrimer NPs | SUM1315 | Decreased TWIST expression along with phenotypic variations, and reduced cancer cell migration, inhibited TWIST-inspired amplification of mesenchymal marker, preventing TNBC tumor cells | [111,112] |
| Lipocalin 2 | ICAM-1 conjugated Liposomes NPs | MDA-MB-231 | Demonstrated noteworthy inhibition of VEGF from MDA-MB-231 cells, resulting in diminished angiogenesis both in vitro and in vivo | [114] |
| EGFR | CPP-loaded Nanobubbles | MDA-MB-231 | Exhibited significant downregulation of EGFR mRNA and protein in a xenografted tumor model of TNBC cells; suppressed miRNA-21, proliferation of TNBC cells via controlling EGFR and subsequently inhibiting the PI3K/AKT and ERK1/2 signaling axis | [10,126,127,128,129] |
| DANCR | RGD–PEG–ECO NPs | MDA-MB-231 | Reduced EDB-FN expression, demonstrated effectiveness against both TNBC and MCF-7 cell lines | [138,139] |
| MDR1 | Layer by layer NPs (depositing alternately siRNA and poly-L-arginine on NPs) | MDA-MB-468 | Inhibited MDR1 protein, increased doxorubicin sensitivity 4-fold and significantly decreased tumor volume | [91] |
| MDR1 | siRNA–NPs with PLL and hyaluronic acid | MDA-MB-231 | Downregulated ABCB1 and ABCG2 and increased the sensitivity of TNBC cells to doxorubicin and paclitaxel | [90,91] |
| mTORC2 | Silicon NPs | BT474, MDA-MB-361, MDA-MB-231, SKBR3 | Demonstrated selective mTOR2 inhibition in TNBC, decreased Akt phosphorylation, and tumor growth in TNBC | [145] |
| Rictor (mTORC2) | 50B8–DP100 siNP | MDA-MB-231 | Significantly inhibited mTOR2 activity in TNBC, potentially reduced Rictor expression in mTOR/PI3K active TNBC tumors | [134,135] |
| ATM protein | Nanoliposome | MDA-MB-231, SKBR3 | Reduced ATM protein expression in TNBC, stimulated cytotoxic T lymphocytes and controlled the immune-responsive tumor microenvironment triggering the cGAS-STING pathway | [137] |
| STAT3 | Cholesterol–siRNA and cationic PLL [30]-PEG5K | 4T1 | Downregulated STAT3, suppressed mRNA in 4T1 cells | [115] |
| TRPC6 | Shamporter coupled with siTRPC6 | MDA-MB-231 | Substantially silenced TRPC6 protein level, inhibited TNBC growth | [125] |
| MCL1 | siRNA–lipid–albumin conjugates | MDA-MB-231 | Silenced MCL1 expression and cMyc expression, reduced cancer cell stemness, and inhibited TNBC cell growth | [105,106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhan, M.A.; Torchilin, V.P. Advances in siRNA Drug Delivery Strategies for Targeted TNBC Therapy. Bioengineering 2024, 11, 830. https://doi.org/10.3390/bioengineering11080830
Subhan MA, Torchilin VP. Advances in siRNA Drug Delivery Strategies for Targeted TNBC Therapy. Bioengineering. 2024; 11(8):830. https://doi.org/10.3390/bioengineering11080830
Chicago/Turabian StyleSubhan, Md Abdus, and Vladimir P. Torchilin. 2024. "Advances in siRNA Drug Delivery Strategies for Targeted TNBC Therapy" Bioengineering 11, no. 8: 830. https://doi.org/10.3390/bioengineering11080830
APA StyleSubhan, M. A., & Torchilin, V. P. (2024). Advances in siRNA Drug Delivery Strategies for Targeted TNBC Therapy. Bioengineering, 11(8), 830. https://doi.org/10.3390/bioengineering11080830









