Bioengineering Human Upper Respiratory Mucosa: A Systematic Review of the State of the Art of Cell Culture Techniques
Abstract
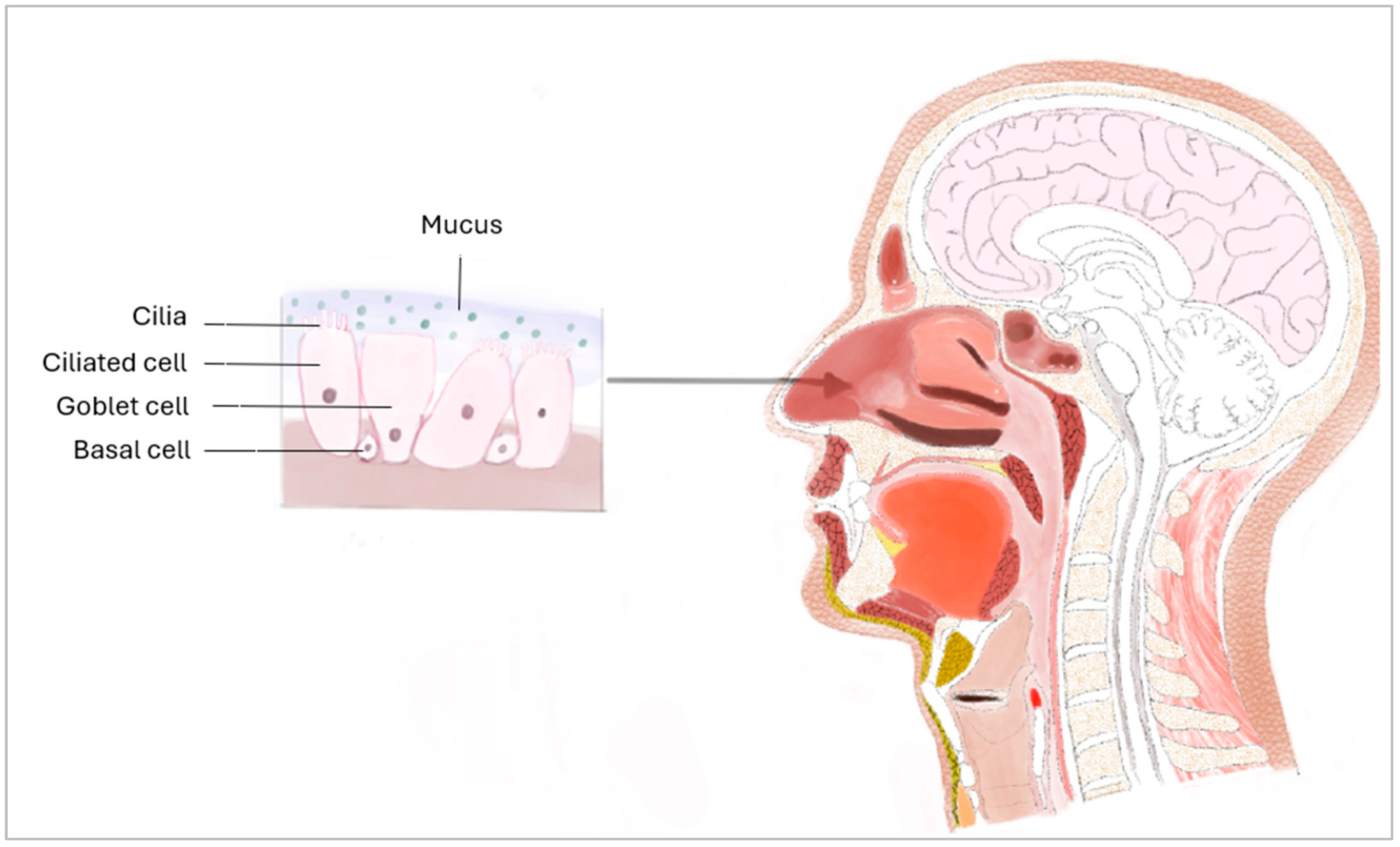
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Management and Risk of Bias Assessment
3. Results
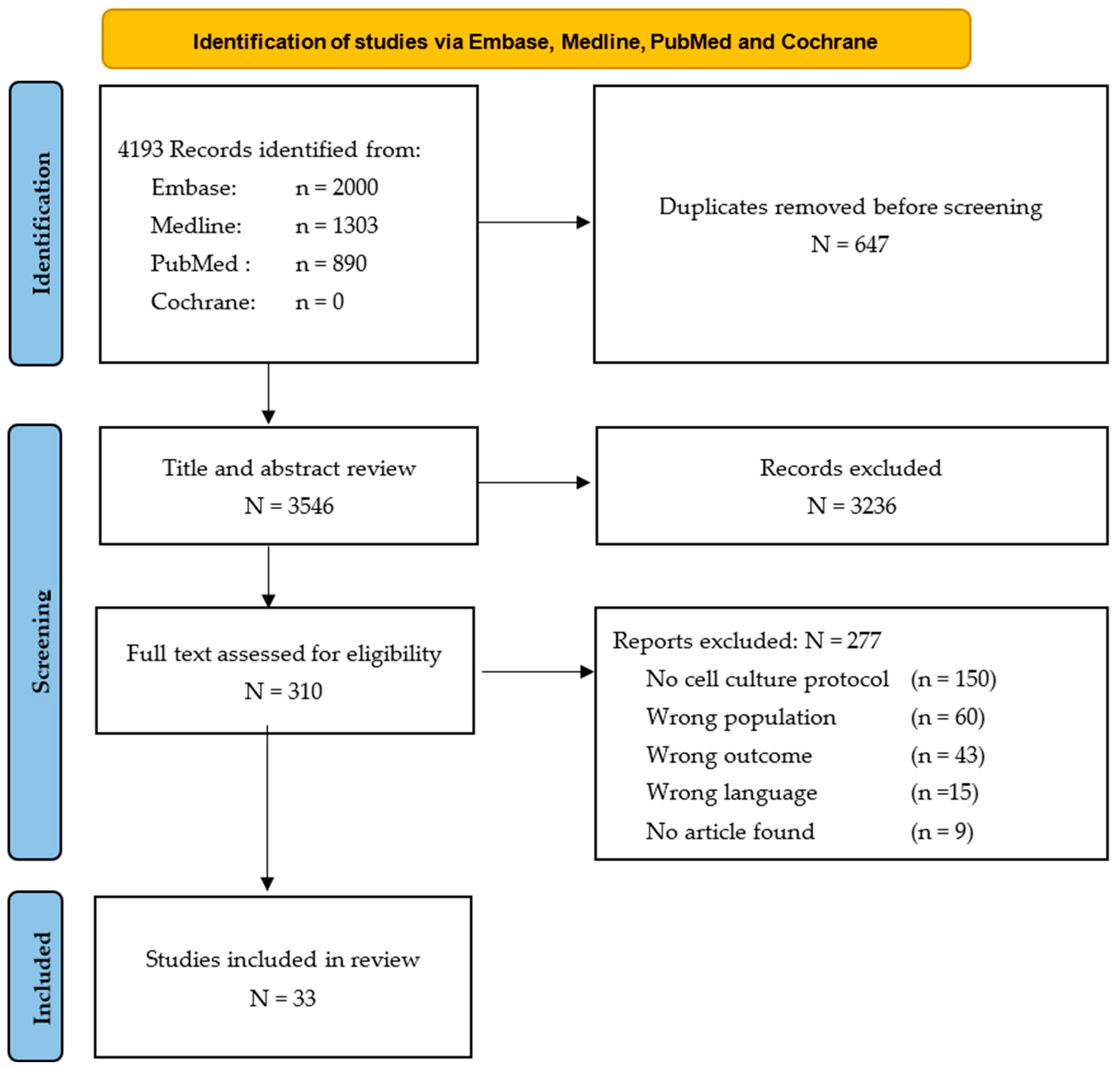
3.1. Study Selection
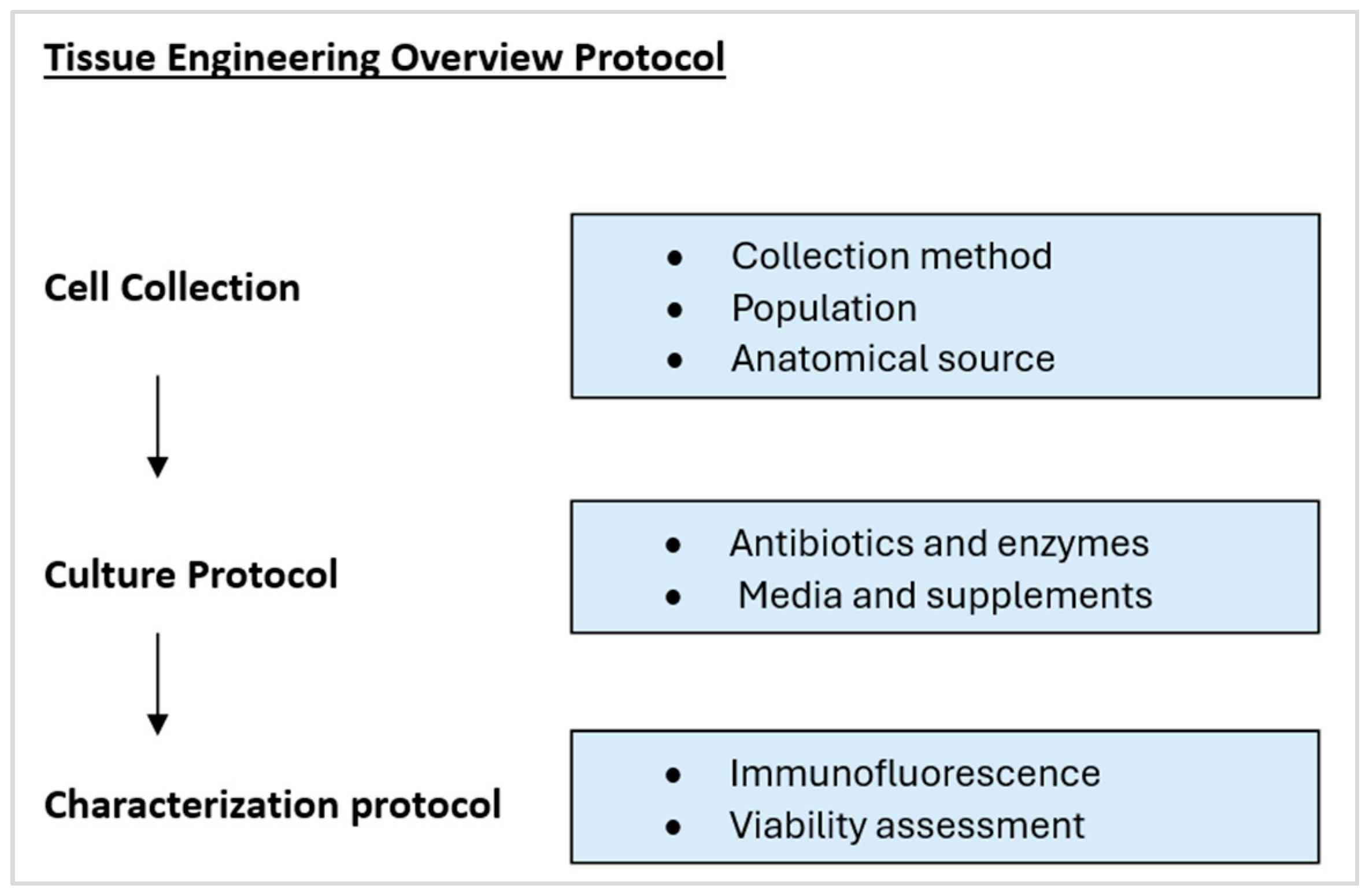
3.2. Tissue Engineering Protocol Overview
3.3. Cell Collection and Intended Use
3.4. Cell Culture Protocol
3.5. Cell Characterisation Protocol
3.6. Risk of Bias Assessment
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
Appendix A. Medline Ovid Search Results
| # | Search Strategy | Results |
| 1 | exp culture techniques/or exp cell culture techniques/or exp cell engineering/or exp tissue engineering/ | 239,186 |
| 2 | Cell culture.ti,ab,kw. | 90,366 |
| 3 | Cell tissue culture.ti,ab,kw | 128 |
| 4 | exp respiratory mucosa/or exp nasal mucosa/or exp olfactory mucosa/ | 38,824 |
| 5 | Respiratory mucosa.ti,ab,kw. | 1204 |
| 6 | Nasal mucosa.ti,ab,kw. | 9593 |
| 7 | Respiratory epithelium.ti,ab,kw. | 3405 |
| 8 | 1 or 2 or 3 | 314,016 |
| 9 | 4 or 5 or 6 or 7 | 45,556 |
| 10 | 8 and 9 | 1855 |
| 11 | exp Animals/not Humans/ | 5,185,986 |
| 12 | 10, not 11 | 1303 |
Appendix B. Embase Search Results
| # | Search Strategy | Results |
| 1 | ‘Cell, tissue or organ culture’/exp | 891,835 |
| 2 | ‘Cell culture’:ti,ab,kw | 125,869 |
| 3 | ‘Cell tissue culture’:ti,ab,kw | 148 |
| 4 | ‘Bioengineering’/exp | 235,955 |
| 5 | ‘Respiratory mucosa’/exp | 36,311 |
| 6 | ‘Respiratory mucosa’:ti,ab,kw | 1524 |
| 7 | ‘Nasal mucosa’:ti,ab,kw | 11,985 |
| 8 | ‘Respiratory epithelium’:ti,ab,kw | 4461 |
| 9 | ‘Respiratory epithelium’/exp | 40,974 |
| 10 | ‘Nasal epithelium’:ti,ab,kw | 2296 |
| 11 | ‘Nose epithelium’:ti,ab,kw | 11 |
| 12 | #1 OR #2 OR #3 OR #4 | 1,148,854 |
| 13 | #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 | 80,462 |
| 14 | ‘animal’/exp NOT ‘human’/exp | 6,069,638 |
| 15 | #12 AND #13 | 8595 |
| 16 | #15 NOT #14 | 6774 |
| 17 | #16 AND [Embase]/lim NOT ([Embase]/lim AND [medline]/lim) | 2000 |
Appendix C. PubMed Search Results
| # | Search Strategy | Results |
| 1 | (“Cell Culture Techniques”[Mesh]) OR “Bioengineering”[Mesh] | 125,240 |
| 2 | ((Cell culture[Title/Abstract]) OR (Cell tissue culture[Title/Abstract])) OR (Bioengineering[Title/Abstract]) | 100,835 |
| 3 | “Respiratory Mucosa”[Mesh] | 38,809 |
| 4 | ((Respiratory Mucosa[Title/Abstract]) OR (Nasal mucosa[Title/Abstract])) OR (respiratory epithelium[Title/Abstract]) | 13,987 |
| 5 | #1 or #2 | 210,922 |
| 6 | #3 or #4 | 45,700 |
| 7 | #5 and #6 | 1158 |
| 8 | (“Animals”[Mesh]) NOT “Humans”[Mesh] | 5,185,583 |
| 9 | #7 NOT #8 | 890 |
Appendix D. Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Qualitative Research
| Question | Yes | No | Unclear | Not Applicable | |
| 1 | Is there congruity between the stated philosophical perspective and the research methodology? | ||||
| 2 | Is there congruity between the research methodology and the research question or objectives? | ||||
| 3 | Is there congruity between the research methodology and the methods used to collect data? | ||||
| 4 | Is there congruity between the research methodology and the representation and analysis of data? | ||||
| 5 | Is there congruity between the research methodology and the interpretation of results? | ||||
| 6 | Is there a statement locating the researcher culturally or theoretically? | ||||
| 7 | Is the influence of the researcher on the research, and vice-versa, addressed? | ||||
| 8 | Are participants, and their voices, adequately represented? | ||||
| 9 | Is the research ethical according to current criteria or, for recent studies, and is there evidence of ethical approval by an appropriate body? | ||||
| 10 | Do the conclusions drawn in the research report flow from the analysis, or interpretation, of the data? |
Appendix E. Risk of Bias Assessment Using the Joanna Briggs Institute (JBI) Critical Appraisal Tools for Qualitative Research
| Study | Q1 a | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | % No | Risk of Bias b |
| Golec et al. [22] | Yes | Yes | Yes | Yes | N/A | Yes | N/A | N/A | Yes | N/A | 0 | Low |
| Michi et al. [34] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Lokanathan et al. [42] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Manna et al. [43] | Yes | Yes | Yes | Yes | N/A | Yes | Yes | N/A | N/A | Yes | 0 | Low |
| Leung et al. [61] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Lee et al. [45] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Kreft et al. [31] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Bergougnan et al. [44] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Everman et al. [68] | Yes | Yes | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | Low |
| Jiang et al. [62] | Yes | Yes | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | Low |
| Gianotti et al. [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Schagen et al. [46] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Schogler et al. [47] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Broadbent et al. [33] | Yes | Yes | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | Low |
| Butler et al. [48] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Raredon et al. [63] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Hussain et al. [49] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Müller et al. [50] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Fulcher et al. [64] | Yes | Yes | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | Low |
| Zhao et al. [51] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Prytherch et al. [65] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Randell et al. [54] | Yes | Yes | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | Low |
| Even TZur [52] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Norruddin et al. [53] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Choe et al. [58] | Yes | Yes | Yes | N/A | N/A | Yes | Yes | N/A | N/A | N/A | 0 | Low |
| Widicombe et al. [67] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | No | Yes | 10 | Low |
| Bals et al. [66] | Yes | Yes | Yes | N/A | N/A | Yes | Yes | Yes | Yes | N/A | 0 | Low |
| Paquette et al. [59] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Yoo et al. [32] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Ross et al. [55] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Werner et al. [57] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Steinsvag et al. [56] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Lechner et al. [60] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Acronym: N/A: Not applicable. Answer options: Yes, no, unclear, not applicable. a Q1–Q10 represents questions 1 to 10 according to the JBI risk assessment (Appendix D). b The risk of bias was measured as low when the study reached up to 30% of “no” scores, moderate if the study attained between 30 to 60% of “no” scores, and high if the study obtained more than 60% “no” scores. | ||||||||||||
References
- Sahin-Yilmaz, A.; Naclerio, R.M. Anatomy and physiology of the upper airway. Proc. Am. Thorac. Soc. 2011, 8, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, L.; Li, P.; Pang, K.; Liu, H.; Tian, L. Epithelial Barrier in the Nasal Mucosa, Related Risk Factors and Diseases. Int. Arch. Allergy Immunol. 2023, 184, 481–501. [Google Scholar] [CrossRef]
- Nakisa, K.i.; Tushar, B. Histology, Respiratory Epithelium. [Updated 2023 May 1]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541061/ (accessed on 17 October 2023).
- Kia’i, N.; Bajaj, T. Histology, Respiratory Epithelium. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Geurkink, N. Nasal anatomy, physiology, and function. J. Allergy Clin. Immunol. 1983, 72, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.P. Nasal anatomy and physiology. Facial Plast. Surg. Clin. N. Am. 2004, 12, 387–395. [Google Scholar] [CrossRef]
- Larsson, K.; Tornling, G.; Gavhed, D.; Müller-Suur, C.; Palmberg, L. Inhalation of cold air increases the number of inflammatory cells in the lungs in healthy subjects. Eur. Respir. J. 1998, 12, 825–830. [Google Scholar] [CrossRef]
- Shephard, R.J. Does cold air damage the lungs of winter athletes? Curr. Sports Med. Rep. 2004, 3, 289–291. [Google Scholar] [CrossRef]
- Sobiesk, J.L.; Munakomi, S. Anatomy, Head and Neck, Nasal Cavity. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Altunbulakli, C.; Reiger, M.; Neumann, A.U.; Garzorz-Stark, N.; Fleming, M.; Huelpuesch, C.; Castro-Giner, F.; Eyerich, K.; Akdis, C.A.; Traidl-Hoffmann, C. Relations between epidermal barrier dysregulation and Staphylococcus species-dominated microbiome dysbiosis in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2018, 142, 1643–1647. [Google Scholar] [CrossRef]
- Xian, M.; Ma, S.; Wang, K.; Lou, H.; Wang, Y.; Zhang, L.; Wang, C.; Akdis, C.A. Particulate Matter 2.5 Causes Deficiency in Barrier Integrity in Human Nasal Epithelial Cells. Allergy Asthma Immunol. Res. 2020, 12, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.K.; Grammer, L.C. An overview of allergens. Allergy Asthma Proc. 2019, 40, 362–365. [Google Scholar] [CrossRef]
- Schamberger, A.C.; Mise, N.; Jia, J.; Genoyer, E.; Yildirim, A.; Meiners, S.; Eickelberg, O. Cigarette smoke-induced disruption of bronchial epithelial tight junctions is prevented by transforming growth factor-β. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1040–1052. [Google Scholar] [CrossRef]
- Hong, Z.; Guo, Z.; Zhang, R.; Xu, J.; Dong, W.; Zhuang, G.; Deng, C. Airborne Fine Particulate Matter Induces Oxidative Stress and Inflammation in Human Nasal Epithelial Cells. Tohoku J. Exp. Med. 2016, 239, 117–125. [Google Scholar] [CrossRef]
- Levine, C.G.; Casiano, R.R. Revision Functional Endoscopic Sinus Surgery. Otolaryngol. Clin. N. Am. 2017, 50, 143–164. [Google Scholar] [CrossRef]
- Ahmad, R.; Norie, A. Endonasal endoscopic resection of intranasal haemangioma. Med. J. Malaysia 2006, 61, 644–646. [Google Scholar]
- Nadeau, S.; Côté, M.; Champagne, P.O. Endoscopic Endonasal Resection of a Pontine Brainstem Cavernoma. World Neurosurg. 2021, 150, 19. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vilchis, R.A.; Piedra-Ramirez, A.; Patiño-Morales, C.C.; Sanchez-Gomez, C.; Beltran-Vargas, N.E. Sources, Characteristics, and Therapeutic Applications of Mesenchymal Cells in Tissue Engineering. Tissue Eng. Regen. Med. 2022, 19, 325–361. [Google Scholar] [CrossRef]
- Blais, M.; Parenteau-Bareil, R.; Cadau, S.; Berthod, F. Concise review: Tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl. Med. 2013, 2, 545–551. [Google Scholar] [CrossRef]
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Duranceau, L.; Bortoluzzi, P.; Beaudoin Cloutier, C.; Genest, H.; Caouette-Laberge, L.; Dumas, A.; et al. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts: A case series of 14 severely burned patients indicating clinical effectiveness. Eur. Cell Mater. 2018, 36, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, A.; Delpiano, L.; Caci, E. In vitro methods for the development and analysis of human primary airway epithelia. Front. Pharmacol. 2018, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Golec, A.; Pranke, I.; Scudieri, P.; Hayes, K.; Dreano, E.; Dunlevy, F.; Hatton, A.; Downey, D.G.; Galietta, L.; Sermet, I. Isolation, cultivation, and application of primary respiratory epithelial cells obtained by nasal brushing, polyp samples, or lung explants. STAR Protoc. 2022, 3, 101419. [Google Scholar] [CrossRef]
- Paré, B.; Lehmann, M.; Beaudin, M.; Nordström, U.; Saikali, S.; Julien, J.P.; Gilthorpe, J.D.; Marklund, S.L.; Cashman, N.R.; Andersen, P.M.; et al. Misfolded SOD1 pathology in sporadic Amyotrophic Lateral Sclerosis. Sci. Rep. 2018, 8, 14223. [Google Scholar] [CrossRef]
- Brodeur, A.; Roy, V.; Touzel-Deschênes, L.; Bianco, S.; Droit, A.; Fradette, J.; Ruel, J.; Gros-Louis, F. Transcriptomic Analysis of Mineralized Adipose-Derived Stem Cell Tissues for Calcific Valve Disease Modelling. Int. J. Mol. Sci. 2024, 25, 2291. [Google Scholar] [CrossRef] [PubMed]
- Louit, A.; Beaudet, M.J.; Gros-Louis, F.; Berthod, F. Tissue-engineered in vitro modeling of the impact of Schwann cells in amyotrophic lateral sclerosis. Biotechnol. Bioeng. 2022, 119, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Brodeur, A.; Touzel Deschênes, L.; Dupré, N.; Gros-Louis, F. RNF213 Loss-of-Function Promotes Angiogenesis of Cerebral Microvascular Endothelial Cells in a Cellular State Dependent Manner. Cells 2022, 12, 78. [Google Scholar] [CrossRef]
- Roy, V.; Lamontagne, R.; Talagas, M.; Touzel-Deschênes, L.; Khuong, H.T.; Saikali, S.; Dupré, N.; Gros-Louis, F. Biofabrication of a three dimensional human-based personalized neurofibroma model. Biotechnol. J. 2021, 16, e2000250. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Magne, B.; Vaillancourt-Audet, M.; Blais, M.; Chabaud, S.; Grammond, E.; Piquet, L.; Fradette, J.; Laverdière, I.; Moulin, V.J.; et al. Human Organ-Specific 3D Cancer Models Produced by the Stromal Self-Assembly Method of Tissue Engineering for the Study of Solid Tumors. BioMed Res. Int. 2020, 2020, 6051210. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Paquet, A.; Touzel-Deschênes, L.; Khuong, H.T.; Dupré, N.; Gros-Louis, F. Heterozygous NF1 dermal fibroblasts modulate exosomal content to promote angiogenesis in a tissue-engineered skin model of neurofibromatosis type-1. J. Neurochem. 2023, 167, 556–570. [Google Scholar] [CrossRef]
- Dakiw Piaceski, A.; Larouche, D.; Ghani, K.; Bisson, F.; Cortez Ghio, S.; Larochelle, S.; Moulin, V.J.; Caruso, M.; Germain, L. Translating the combination of gene therapy and tissue engineering for treating recessive dystrophic epidermolysis bullosa. Eur. Cell Mater. 2018, 35, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M.E.; Tratnjek, L.; Lasic, E.; Hevir, N.; Rizner, T.L.; Kristan, K. Different Culture Conditions Affect Drug Transporter Gene Expression, Ultrastructure, and Permeability of Primary Human Nasal Epithelial Cells. Pharm. Res. 2020, 37, 170. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Kim, Y.-S.; Lee, S.-H.; Lee, M.-K.; Roh, H.-J.; Jhun, B.-H.; Lee, C.-H.; Kim, D.-D. Serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Pharm. Res. 2003, 20, 1690–1696. [Google Scholar] [CrossRef]
- Broadbent, L.; Villenave, R.; Guo-Parke, H.; Douglas, I.; Shields, M.D.; Power, U.F. In Vitro Modeling of RSV Infection and Cytopathogenesis in Well-Differentiated Human Primary Airway Epithelial Cells (WD-PAECs). Methods Mol. Biol. 2016, 1442, 119–139. [Google Scholar] [CrossRef]
- Michi, A.N.; Proud, D. A toolbox for studying respiratory viral infections using air-liquid interface cultures of human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L263–L280. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Research. PROSPERO International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 30 October 2022).
- Covidence Systematic Review Software, V.H.I., Melbourne, Australia. Covidence. Available online: https://www.covidence.org/ (accessed on 4 January 2023).
- Lockwood, C.; Munn, Z.; Porritt, K. Qualitative research synthesis: Methodological guidance for systematic reviewers utilizing meta-aggregation. Int. J. Evid. Based Healthc. 2015, 13, 179–187. Available online: https://jbi.global/critical-appraisal-tools (accessed on 4 January 2023). [CrossRef]
- Melo, G.; Dutra, K.L.; Rodrigues Filho, R.; Ortega, A.O.L.; Porporatti, A.L.; Dick, B.; Flores-Mir, C.; De Luca Canto, G. Association between psychotropic medications and presence of sleep bruxism: A systematic review. J. Oral. Rehabil. 2018, 45, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Goplen, C.M.; Verbeek, W.; Kang, S.H.; Jones, C.A.; Voaklander, D.C.; Churchill, T.A.; Beaupre, L.A. Preoperative opioid use is associated with worse patient outcomes after Total joint arthroplasty: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 234. [Google Scholar] [CrossRef] [PubMed]
- Segeritz, C.-P.; Vallier, L. Chapter 9—Cell Culture: Growing Cells as Model Systems In Vitro. In Basic Science Methods for Clinical Researchers; Jalali, M., Saldanha, F.Y.L., Jalali, M., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 151–172. [Google Scholar] [CrossRef]
- Lokanathan, Y.; Fauzi, M.B.; Che Man, R.; Rashidbenam, Z.; Bin Saim, A.; Binti Hj Idrus, R.; Mohd Yunus, M.H. Preliminary Study on the Development of In Vitro Human Respiratory Epithelium Using Collagen Type I Scaffold as a Potential Model for Future Tracheal Tissue Engineering. Appl. Sci. 2021, 11, 1787. [Google Scholar] [CrossRef]
- Manna, V.; Caradonna, S. Isolation, expansion, differentiation, and histological processing of human nasal epithelial cells. STAR Protoc. 2021, 2, 100782. [Google Scholar] [CrossRef]
- Bergougnan, C.; Dittlein, D.C.; Hümmer, E.; Riepl, R.; Eisenbart, S.; Böck, D.; Griesbaum, L.; Weigl, A.; Damialis, A.; Hartwig, A.; et al. Physical and immunological barrier of human primary nasal epithelial cells from non-allergic and allergic donors. World Allergy Organ. J. 2020, 13, 100109. [Google Scholar] [CrossRef]
- Lee, D.D.H.; Petris, A.; Hynds, R.E.; O’Callaghan, C. Ciliated Epithelial Cell Differentiation at Air-Liquid Interface Using Commercially Available Culture Media. Methods Mol. Biol. 2020, 2109, 275–291. [Google Scholar] [CrossRef]
- Schagen, J.; Sly, P.D.; Fantino, E. Characterizing well-differentiated culture of primary human nasal epithelial cells for use in wound healing assays. Lab. Investig. 2018, 98, 1478–1486. [Google Scholar] [CrossRef]
- Schogler, A.; Blank, F.; Brugger, M.; Beyeler, S.; Tschanz, S.A.; Regamey, N.; Casaulta, C.; Geiser, T.; Alves, M.P. Characterization of pediatric cystic fibrosis airway epithelial cell cultures at the air-liquid interface obtained by non-invasive nasal cytology brush sampling. Respir. Res. 2017, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.R.; Hynds, R.E.; Gowers, K.H.C.; Lee, D.D.H.; Brown, J.M.; Crowley, C.; Teixeira, V.H.; Smith, C.M.; Urbani, L.; Hamilton, N.J.; et al. Rapid Expansion of Human Epithelial Stem Cells Suitable for Airway Tissue Engineering. Am. J. Respir. Crit. Care Med. 2016, 194, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Hugosson, S.; Roomans, G.M. Isolation and culture of primary human nasal epithelial cells from anesthetized nasal epithelia. Acta Oto-Laryngol. 2014, 134, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Brighton, L.E.; Carson, J.L.; Fischer, W.A., 2nd; Jaspers, I. Culturing of human nasal epithelial cells at the air liquid interface. J. Vis. Exp. JoVE 2013, 80, e50646. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, F.; Li, C.; Li, Y.; Chao, S.-S.; Loh, W.-S.; Pan, X.; Shi, L.; Wang, D.-Y. The use of nasal epithelial stem/progenitor cells to produce functioning ciliated cells in vitro. Am. J. Rhinol. Allergy 2012, 26, 345–350. [Google Scholar] [CrossRef]
- Even-Tzur, N.; Jaffa, A.; Gordon, Z.; Gottlieb, R.; Kloog, Y.; Einav, S.; Wolf, M.; Elad, D. Air-liquid interface culture of nasal epithelial cells on denuded amniotic membranes. Cell. Mol. Bioeng. 2010, 3, 307–318. [Google Scholar] [CrossRef]
- Noruddin, N.A.A.; Saim, A.B.; Chua, K.H.; Idrus, R. Human nasal turbinates as a viable source of respiratory epithelial cells using co-culture system versus dispase-dissociation technique. Laryngoscope 2007, 117, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Randell, S.H.; Fulcher, M.L.; O’Neal, W.; Olsen, J.C. Primary epithelial cell models for cystic fibrosis research. Methods Mol. Biol. 2011, 742, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Ross, U.H.; Wittmann, G. Living epithelial-mesenchymal compounds formed in vitro suitable for autografting. Eur. Arch. Oto-Rhino-Laryngol. 1997, 254 (Suppl. S1), S12–S17. [Google Scholar] [CrossRef]
- Steinsvag, S.K.; Strand, M.; Berg, O.; Miaguchi, M.; Olofsson, J. Human respiratory mucosa in a nonadhesive stationary organ culture system. Laryngoscope 1991, 101, 1323–1331. [Google Scholar] [CrossRef]
- Werner, U.; Kissel, T. Development of a human nasal epithelial cell culture model and its suitability for transport and metabolism studies under in vitro conditions. Pharm. Res. 1995, 12, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.M.; Tomei, A.A.; Swartz, M.A. Physiological 3D tissue model of the airway wall and mucosa. Nat. Protoc. 2006, 1, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Paquette, J.S.; Tremblay, P.; Bernier, V.; Auger, F.A.; Laviolette, M.; Germain, L.; Boutet, M.; Boulet, L.P.; Goulet, F. Production of tissue-engineered three-dimensional human bronchial models. Vitr. Cell. Dev. Biol.-Anim. 2003, 39, 213–220. [Google Scholar]
- Lechner, J.F.; LaVeck, M.A. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J. Tissue Cult. Methods 1985, 9, 43–48. [Google Scholar] [CrossRef]
- Leung, C.; Wadsworth, S.J.; Yang, S.J.; Dorscheid, D.R. Structural and functional variations in human bronchial epithelial cells cultured in air-liquid interface using different growth media. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1063–L1073. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Schaefer, N.; Chu, H.W. Air-Liquid Interface Culture of Human and Mouse Airway Epithelial Cells. Methods Mol. Biol. 2018, 1809, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Raredon, M.S.B.; Ghaedi, M.; Calle, E.A.; Niklason, L.E. A Rotating Bioreactor for Scalable Culture and Differentiation of Respiratory Epithelium. Cell Med. 2015, 7, 109–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fulcher, M.L.; Randell, S.H. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol. Biol. 2013, 945, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Prytherch, Z.; Job, C.; Marshall, H.; Oreffo, V.; Foster, M.; BeruBe, K. Tissue-Specific stem cell differentiation in an in vitro airway model. Macromol. Biosci. 2011, 11, 1467–1477. [Google Scholar] [CrossRef]
- Bals, R.; Beisswenger, C.; Blouquit, S.; Chinet, T. Isolation and air-liquid interface culture of human large airway and bronchiolar epithelial cells. J. Cyst. Fibros. 2004, 3 (Suppl. 2), 49–51. [Google Scholar] [CrossRef]
- Widdicombe, J.H.; Sachs, L.A.; Morrow, J.L.; Finkbeiner, W.E. Expansion of cultures of human tracheal epithelium with maintenance of differentiated structure and function. BioTechniques 2005, 39, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Everman, J.L.; Rios, C.; Seibold, M.A. Utilization of Air-Liquid Interface Cultures as an In Vitro Model to Assess Primary Airway Epithelial Cell Responses to the Type 2 Cytokine Interleukin-13. Methods Mol. Biol. 2018, 1799, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.B.; Kieninger, E.; Schögler, A.; Kopf, B.S.; Casaulta, C.; Geiser, T.; Regamey, N.; Alves, M.P. Comparison of three different brushing techniques to isolate and culture primary nasal epithelial cells from human subjects. Exp. Lung Res. 2014, 40, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Chabaud, S.; Fradette, J.; Bolduc, S. Evaluation of a Serum-Free Medium for Human Epithelial and Stromal Cell Culture. Int. J. Mol. Sci. 2022, 23, 10035. [Google Scholar] [CrossRef] [PubMed]
- Llames, S.; García-Pérez, E.; Meana, Á.; Larcher, F.; del Río, M. Feeder Layer Cell Actions and Applications. Tissue Eng. Part B Rev. 2015, 21, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Hynds, R.E.; Bonfanti, P.; Janes, S.M. Regenerating human epithelia with cultured stem cells: Feeder cells, organoids and beyond. EMBO Mol. Med. 2018, 10, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kang, W.; Du, L.; Ge, S. Rho-kinase inhibitor Y-27632 facilitates the proliferation, migration and pluripotency of human periodontal ligament stem cells. J. Cell Mol. Med. 2017, 21, 3100–3112. [Google Scholar] [CrossRef]
- Van-Seuningen, I.; Davril, M. A rapid periodic acid-Schiff staining procedure for the detection of glycoproteins using the PhastSystem. Electrophoresis 1992, 13, 97–99. [Google Scholar] [CrossRef]
- Hauber, H.P.; Zabel, P. PAS staining of bronchoalveolar lavage cells for differential diagnosis of interstitial lung disease. Diagn. Pathol. 2009, 4, 13. [Google Scholar] [CrossRef]
- Tabatabaei Shafiei, M.; Carvajal Gonczi, C.M.; Rahman, M.S.; East, A.; François, J.; Darlington, P.J. Detecting glycogen in peripheral blood mononuclear cells with periodic acid schiff staining. J. Vis. Exp. 2014, 94, e52199. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring Cell Death by Propidium Iodide Uptake and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087163. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095497. [Google Scholar] [CrossRef] [PubMed]
- Gray, T.E.; Guzman, K.; Davis, C.W.; Abdullah, L.H.; Nettesheim, P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1996, 14, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Ahmad, S.; Jian, A.; Li, B.; Smith, R.W.; Helm, K.M.; Seibold, M.A.; Groshong, S.D.; White, C.W.; Reynolds, S.D. Human tracheobronchial basal cells. Normal versus remodeling/repairing phenotypes in vivo and in vitro. Am. J. Respir. Cell Mol. Biol. 2013, 49, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Damian, S.; Gunther, N.; Smithhisler, M.R.; Klarmann, G.J. An air-liquid interface culture system for small airway epithelial cells. Am. J. Respir. Crit. Care Med. 2011, 183. Available online: https://lonza.picturepark.com/Website/?Action=downloadAsset&AssetId=30596 (accessed on 4 January 2023).
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, C.; Chen, P.; Hu, J.; Hu, R.; Huang, M.; Bi, H. Development, validation, and application of a novel 7-day Caco-2 cell culture system. J. Pharmacol. Toxicol. Methods 2014, 70, 175–181. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- von der Mark, K.; Gauss, V.; von der Mark, H.; Müller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Bissell, M.J. Of extracellular matrix, scaffolds, and signaling: Tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2006, 22, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O.W.; Rønnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef]
- Li, C.; Kato, M.; Shiue, L.; Shively, J.E.; Ares, M., Jr.; Lin, R.J. Cell type and culture condition-dependent alternative splicing in human breast cancer cells revealed by splicing-sensitive microarrays. Cancer Res. 2006, 66, 1990–1999. [Google Scholar] [CrossRef]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef]
- Yamada, K.M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef]
- Clément, V.; Roy, V.; Paré, B.; Goulet, C.R.; Deschênes, L.T.; Berthod, F.; Bolduc, S.; Gros-Louis, F. Tridimensional cell culture of dermal fibroblasts promotes exosome-mediated secretion of extracellular matrix proteins. Sci. Rep. 2022, 12, 19786. [Google Scholar] [CrossRef]
- Paré, B.; Touzel-Deschênes, L.; Lamontagne, R.; Lamarre, M.S.; Scott, F.D.; Khuong, H.T.; Dion, P.A.; Bouchard, J.P.; Gould, P.; Rouleau, G.A.; et al. Early detection of structural abnormalities and cytoplasmic accumulation of TDP-43 in tissue-engineered skins derived from ALS patients. Acta Neuropathol. Commun. 2015, 3, 5. [Google Scholar] [CrossRef]
- Frieboes, H.B.; Zheng, X.; Sun, C.H.; Tromberg, B.; Gatenby, R.; Cristini, V. An integrated computational/experimental model of tumor invasion. Cancer Res. 2006, 66, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Bennet, T.J.; Randhawa, A.; Hua, J.; Cheung, K.C. Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease. Cells 2021, 10, 1602. [Google Scholar] [CrossRef]
- Park, J.Y.; Ryu, H.; Lee, B.; Ha, D.H.; Ahn, M.; Kim, S.; Kim, J.Y.; Jeon, N.L.; Cho, D.W. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 2018, 11, 015002. [Google Scholar] [CrossRef]
- Chiu, M.C.; Li, C.; Liu, X.; Song, W.; Wan, Z.; Yu, Y.; Huang, J.; Xiao, D.; Chu, H.; Cai, J.P.; et al. Human Nasal Organoids Model SARS-CoV-2 Upper Respiratory Infection and Recapitulate the Differential Infectivity of Emerging Variants. mBio 2022, 13, e0194422. [Google Scholar] [CrossRef]
- Maughan, E.F.; Hynds, R.E.; Proctor, T.J.; Janes, S.M.; Elliott, M.; Birchall, M.A.; Lowdell, M.W.; De Coppi, P. Autologous Cell Seeding in Tracheal Tissue Engineering. Curr. Stem Cell Rep. 2017, 3, 279–289. [Google Scholar] [CrossRef]
- Gras, D.; Petit, A.; Charriot, J.; Knabe, L.; Alagha, K.; Gamez, A.S.; Garulli, C.; Bourdin, A.; Chanez, P.; Molinari, N.; et al. Epithelial ciliated beating cells essential for ex vivo ALI culture growth. BMC Pulm. Med. 2017, 17, 80. [Google Scholar] [CrossRef]
- Niermeyer, W.L.; Rodman, C.; Li, M.M.; Chiang, T. Tissue engineering applications in otolaryngology-The state of translation. Laryngoscope Investig. Otolaryngol. 2020, 5, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Brugger, S.D.; Bomar, L.; Lemon, K.P. Commensal-Pathogen Interactions along the Human Nasal Passages. PLoS Pathog. 2016, 12, e1005633. [Google Scholar] [CrossRef]
- Chaves, B.J.; Tadi, P. Gentamicin. [Updated 2023 Apr 10]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557550/ (accessed on 3 March 2023).
- Ryu, A.H.; Eckalbar, W.L.; Kreimer, A.; Yosef, N.; Ahituv, N. Use antibiotics in cell culture with caution: Genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci. Rep. 2017, 7, 7533. [Google Scholar] [CrossRef]
- Llobet, L.; Montoya, J.; López-Gallardo, E.; Ruiz-Pesini, E. Side Effects of Culture Media Antibiotics on Cell Differentiation. Tissue Eng. Part C Methods 2015, 21, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Erickson, V.R.; Antunes, M.; Chen, B.; Cohen, N.A.; Hwang, P.H. The effects of retinoic acid on ciliary function of regenerated sinus mucosa. Am. J. Rhinol. 2008, 22, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Sachs, L.A.; Finkbeiner, W.E.; Widdicombe, J.H. Effects of media on differentiation of cultured human tracheal epithelium. In Vitro Cell. Dev. Biol. Anim. 2003, 39, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Gentzsch, M.; Boyles, S.E.; Cheluvaraju, C.; Chaudhry, I.G.; Quinney, N.L.; Cho, C.; Dang, H.; Liu, X.; Schlegel, R.; Randell, S.H. Pharmacological Rescue of Conditionally Reprogrammed Cystic Fibrosis Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2017, 56, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, R.; Parenteau-Bareil, R.; Larouche, D.; Marcoux, H.; Bisson, F.; Bonnet, A.; Auger, F.A.; Bolduc, S.; Germain, L. Dynamic mechanical stimulations induce anisotropy and improve the tensile properties of engineered tissues produced without exogenous scaffolding. Acta Biomater. 2011, 7, 3294–3301. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Tremblay, A.; Morin, S.; Martin, C.; Julien, P.; Fradette, J.; Flamand, N.; Pouliot, R. α-Linolenic acid and linoleic acid modulate the lipidome and the skin barrier of a tissue-engineered skin model. Acta Biomater. 2022, 140, 261–274. [Google Scholar] [CrossRef]
- Attiogbe, E.; Larochelle, S.; Chaib, Y.; Mainzer, C.; Mauroux, A.; Bordes, S.; Closs, B.; Gilbert, C.; Moulin, V.J. An in vitro autologous, vascularized, and immunocompetent Tissue Engineered Skin model obtained by the self-assembled approach. Acta Biomater. 2023, 168, 361–371. [Google Scholar] [CrossRef]
| REFERENCE | YEAR | CELL SOURCE | POPULATION TYPE a | CELL COLLECTION | MUCOSA PURPOSE b |
|---|---|---|---|---|---|
| GOLEC ET AL. [22] | 2022 | Bronchial and Nasal cells | N/A | Nasal brushing, Lung explants | Cystic fibrosis research |
| MICHI ET AL. [34] | 2021 | Bronchial and Nasal cells | Cadaver | Bronchial biopsy | Viral research |
| LOKANATHAN ET AL. [42] | 2021 | Nasal cells | N/A | Nasal biopsy | Reconstructive surgical research |
| MANNA ET AL. [43] | 2021 | Nasal cells | Adults | Nasal brushing | Protocol description |
| LEUNG ET AL. [61] | 2020 | Bronchial and Nasal cells | Cadaver | Bronchial biopsy | Physiology research |
| LEE ET AL. [45] | 2020 | Nasal cells | N/A | Nasal brushing | Protocol description |
| KREFT ET AL. [31] | 2020 | Nasal cells | N/A | N/A | Pharmacology research |
| BERGOUGNAN ET AL. [44] | 2020 | Nasal cells | N/A | Nasal biopsy, Bronchial brushing | Physiology research |
| EVERMAN ET AL. [68] | 2018 | Primary basal epithelial cells | N/A | N/A | Physiology research |
| JIANG ET AL. [62] | 2018 | Bronchial and Nasal cells | N/A | Bronchial biopsy | Protocol description |
| GIANOTTI ET AL. [21] | 2018 | Nasal cells | N/A | Nasal brushing, Lung explants | Cystic fibrosis research |
| SCHAGEN ET AL. [46] | 2018 | Nasal cells | Adults | Nasal brushing | Physiology research |
| SCHOGLER ET AL. [47] | 2017 | Nasal cells | Pediatrics | Nasal brushing | Cystic fibrosis research |
| BROADBENT ET AL. [33] | 2016 | Bronchial and nasal cells | Pediatrics | Bronchial brushing | Viral research |
| BUTLER ET AL. [48] | 2016 | Nasal cells | N/A | Bronchial biopsy | Reconstructive surgical research |
| RAREDON ET AL. [63] | 2015 | Bronchial and Nasal cells | Adults | N/A | Protocol description |
| HUSSAIN ET AL. [49] | 2014 | Nasal cells | Adults | Nasal brushing | Pharmacology research |
| MULLER ET AL. [50] | 2013 | Nasal cells | N/A | Nasal biopsy | Protocol description |
| FULCHER ET AL. [64] | 2013 | Bronchial and Nasal cells | Cadaver | Bronchial biopsy | Protocol description |
| ZHAO ET AL. [51] | 2012 | Nasal cells | Adults | Nasal biopsy | Physiology research |
| PRYTHERCH ET AL. [65] | 2011 | Bronchial and Nasal cells | Cadaver | N/A | Protocol description |
| RANDELL ET AL. [54] | 2011 | Nasal cells | N/A | N/A | Cystic fibrosis research |
| EVEN-TZUR ET AL. [52] | 2010 | Nasal cells | N/A | Nasal biopsy | Protocol description |
| NORRUDDIN ET AL. [53] | 2007 | Nasal cells | N/A | Nasal biopsy | Reconstructive surgical research |
| CHOE ET AL. [58] | 2006 | Bronchial cells | N/A | N/A | Protocol description |
| WIDDICOMBE ET AL. [67] | 2005 | Bronchial and Nasal cells | Cadaver | Bronchial biopsy | Protocol description |
| BALS ET AL. [66] | 2004 | Bronchial and Nasal cells | N/A | Bronchial biopsy | Cystic fibrosis research |
| PAQUETTE ET AL. [59] | 2003 | Bronchial cells | N/A | Bronchial brushing | Protocol description |
| YOO ET AL. [32] | 2003 | Nasal cells | N/A | Nasal biopsy | Pharmacology research |
| ROSS ET AL. [55] | 1997 | Nasal cells | N/A | Nasal brushing | Reconstructive surgical research |
| WERNER ET AL. [57] | 1995 | Nasal cells | N/A | Nasal biopsy | Pharmacology research |
| STEINSVAG ET AL. [56] | 1991 | Nasal cells | Pediatrics | Nasal biopsy | Protocol description |
| LECHNER ET AL. [60] | 1985 | Bronchial cells | Cadaver | Bronchial biopsy | Protocol description |
| REFERENCE | 2D VS 3D CULTURE a | PROLIFERATIVE MEDIUM | SERUM | DIFFERENTIATION ALI MEDIUM | ANTIBIOTICS PROTOCOL | ENZYME |
|---|---|---|---|---|---|---|
| GOLEC ET AL. [22] | 3D | DMEM/F12 | FBS | DMEM/F12 | Penicillin, streptomycin, Tazocilin, Colomycin, ciprofloxacin, | Pronase |
| MICHI ET AL. [34] | 3D | DMEM/F12 | FBS | Pneumacult | Penicillin, Streptomycin | Pronase |
| LOKANATHAN ET AL. [42] | 3D | DMEM, DKSFM | FBS | DMEM, DKSFM | N/A | Collagenase H |
| MANNA ET AL. [43] | 3D | Pneumacult | BSA | Pneumacult | Penicillin, Streptomycin | N/A |
| LEUNG ET AL. [61] | 3D | BEGM | No | Pneumacult, BEGM | N/A | Protease, DNase Pronase |
| LEE ET AL. [45] | 3D | DMEM | FBS | Pneumacult, BEGM, AECGM, LHC-8 | Penicillin, Streptomycin | N/A |
| KREFT ET AL. [31] | 3D | AECGM | N/A | AECGM | N/A | N/A |
| BERGOUGNAN ET AL. [44] | 3D | DMEM/F12 | FBS | DMEM, AECGM | Penicillin, Streptomycin, Gentamycin | N/A |
| EVERMAN ET AL. [68] | 3D | DMEM, BEGM | BSA | Pneumacult | Penicillin, Streptomycin, Gentamycin | N/A |
| JIANG ET AL. [62] | 3D | BEGM | FBS | BEGM | Penicillin, Streptomycin | Protease |
| GIANOTTI ET AL. [21] | 3D | LHC-basal medium | ABS | DMEM | Penicillin, Streptomycin | Protease |
| SCHAGEN ET AL. [46] | 3D | BEGM | N/A | B-ALI | N/A | N/A |
| SCHOGLER ET AL. [47] | 3D | BEGM | N/A | Pneumacult | Primocin | N/A |
| BROADBENT ET AL. [33] | 3D | DMEM | FBS | AECGM | Penicillin, Streptomycin | N/A |
| BUTLER ET AL. [48] | 3D | DMEM, BEGM | FBS | BEGM | Penicillin, Streptomycin, Gentamycin | N/A |
| RAREDON ET AL. [63] | 3D | B-ALI | N/A | B-ALI | Penicillin, Streptomycin | N/A |
| HUSSAIN ET AL. [49] | 2D | AECGM | No | - | Penicillin, Streptomycin | N/A |
| MULLER ET AL. [50] | 3D | BEGM | No | Pneumacult | N/A | DNase |
| FULCHER ET AL. [64] | 3D | BEGM | BSA | DMEM, LHC basal medium | Penicillin, Streptomycin, Gentamycin | DNase, Protease |
| ZHAO ET AL. [51] | 3D | DMEM/F12 | FBS | B-ALI | Penicillin, Streptomycin | Protease |
| PRYTHERCH ET AL. [65] | 3D | BEGM | N/A | DMEM, BEGM | Penicillin, Streptomycin | N/A |
| RANDELL ET AL. [54] | 3D | BEGM | BSA | DMEM, LHC basal medium | Penicillin, Streptomycin, Gentamycin | Protease |
| EVEN-TZUR ET AL. [52] | 3D | BEGM | No | DMEM, LHC basal medium | N/A | Pronase |
| NORRUDDIN ET AL. [53] | 2D | DMEM/F12, DKSFM | FBS | - | N/A | Collagenase Protease |
| CHOE ET AL. [58] | 3D | BEGM | BSA | BEGM | Penicillin, Streptomycin | N/A |
| WIDDICOMBE ET AL. [67] | 2D | DMEM/LHC-9 | BSA | - | Penicillin, Streptomycin, Gentamycin | Protease |
| BALS ET AL. [66] | 3D | MEM | N/A | DMEM/F12 | Penicillin, streptomycin, tobramycin, ceftazidime, imipenem—cilastin | Protease |
| PAQUETTE ET AL. [59] | 3D | DMEM/F12 | FBS | DMEM | Penicillin, Gentamycin | Collagenase H |
| YOO ET AL. [32] | 2D | BEGM | BSA | - | Penicillin, Streptomycin | Pronase |
| ROSS ET AL. [55] | 3D | DMEM | No | - | Penicillin, Streptomycin | Pronase |
| WERNER ET AL. [57] | 2D | DMEM | FBS | - | Penicillin, Streptomycin | Protease |
| STEINSVAG ET AL. [56] | 2D | DMEM | FBS | - | Penicillin, Streptomycin | N/A |
| LECHNER ET AL. [60] | 2D | LHC-9 | No | - | Gentamycin | N/A |
| REFERENCE | GOBLET CELLS MARKER | CILIATED CELLS MARKER | TIGHT JUNCTION MARKER | CELL VIABILITY MARKER | COMPLETE AND HANDLEABLE MUCOSA |
|---|---|---|---|---|---|
| GOLEC ET AL. [22] | N/A | N/A | N/A | No | No |
| MICHI ET AL. [34] | MUC5Ac | B-tubulin | ZO-1 | Lactate Dehydrogenase Propidium Iodide | No |
| LOKANATHAN ET AL. [42] | N/A | N/A | N/A | No | No |
| MANNA ET AL. [43] | MUC5Ac | Acetylated tubulin | N/A | No | No |
| LEUNG ET AL. [61] | MUC5Ac | N/A | ZO-1 | No | No |
| LEE ET AL. [45] | MUC5Ac | B-tubulin | N/A | Propidium Iodide | No |
| KREFT ET AL. [31] | N/A | N/A | N/A | Trypan Blue | No |
| BERGOUGNAN ET AL. [44] | MUC5Ac | Acetylated tubulin | ZO-1 | Trypan Blue | No |
| EVERMAN ET AL. [68] | N/A | N/A | N/A | Trypan Blue | No |
| JIANG ET AL. [62] | N/A | N/A | N/A | No | No |
| GIANOTTI ET AL. [21] | MUC5Ac | B-tubulin | ZO-1 | No | No |
| SCHAGEN ET AL. [46] | MUC5Ac | Acetylated tubulin | ZO-1 | No | No |
| SCHOGLER ET AL. [47] | MUC5Ac | B-tubulin | ZO-1 | No | No |
| BROADBENT ET AL. [33] | MUC5Ac | B-tubulin | ZO-1 | Trypan Blue | No |
| BUTLER ET AL. [48] | MUC5Ac | Acetylated tubulin | N/A | No | Yes |
| RAREDON ET AL. [63] | MUC5Ac | Acetylated tubulin | ZO-1 | No | No |
| HUSSAIN ET AL. [49] | N/A | N/A | N/A | Trypan Blue | No |
| MULLER ET AL. [50] | N/A | N/A | N/A | No | No |
| FULCHER ET AL. [64] | N/A | N/A | N/A | No | No |
| ZHAO ET AL. [51] | MUC5Ac | B-tubulin | ZO-1 | No | No |
| PRYTHERCH ET AL. [65] | Periodic acid-Shift stain | N/A | ZO-1 | No | No |
| RANDELL ET AL. [54] | N/A | N/A | N/A | No | No |
| EVEN-TZUR ET AL. [52] | MUC5Ac | B-tubulin | ZO-1 | No | No |
| NORRUDDIN ET AL. [53] | MUC5Ac | Cytokeratin-18 | N/A | No | No |
| CHOE ET AL. [58] | N/A | N/A | N/A | No | No |
| WIDDICOMBE ET AL. [67] | N/A | N/A | N/A | No | No |
| BALS ET AL. [66] | N/A | N/A | N/A | No | No |
| PAQUETTE ET AL. [59] | Periodic acid-Shift stain | N/A | N/A | No | No |
| YOO ET AL. [32] | N/A | N/A | N/A | No | No |
| ROSS ET AL. [55] | N/A | N/A | N/A | No | No |
| WERNER ET AL. [57] | N/A | N/A | N/A | Trypan Blue | No |
| STEINSVAG ET AL. [56] | Periodic acid-Shift stain | N/A | N/A | No | No |
| LECHNER ET AL. [60] | N/A | N/A | N/A | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndongo Sonfack, D.J.; Tanguay Boivin, C.; Touzel Deschênes, L.; Maurand, T.; Maguemoun, C.; Berthod, F.; Gros-Louis, F.; Champagne, P.-O. Bioengineering Human Upper Respiratory Mucosa: A Systematic Review of the State of the Art of Cell Culture Techniques. Bioengineering 2024, 11, 826. https://doi.org/10.3390/bioengineering11080826
Ndongo Sonfack DJ, Tanguay Boivin C, Touzel Deschênes L, Maurand T, Maguemoun C, Berthod F, Gros-Louis F, Champagne P-O. Bioengineering Human Upper Respiratory Mucosa: A Systematic Review of the State of the Art of Cell Culture Techniques. Bioengineering. 2024; 11(8):826. https://doi.org/10.3390/bioengineering11080826
Chicago/Turabian StyleNdongo Sonfack, Davaine Joel, Clémence Tanguay Boivin, Lydia Touzel Deschênes, Thibault Maurand, Célina Maguemoun, François Berthod, François Gros-Louis, and Pierre-Olivier Champagne. 2024. "Bioengineering Human Upper Respiratory Mucosa: A Systematic Review of the State of the Art of Cell Culture Techniques" Bioengineering 11, no. 8: 826. https://doi.org/10.3390/bioengineering11080826
APA StyleNdongo Sonfack, D. J., Tanguay Boivin, C., Touzel Deschênes, L., Maurand, T., Maguemoun, C., Berthod, F., Gros-Louis, F., & Champagne, P.-O. (2024). Bioengineering Human Upper Respiratory Mucosa: A Systematic Review of the State of the Art of Cell Culture Techniques. Bioengineering, 11(8), 826. https://doi.org/10.3390/bioengineering11080826






