Mathematical Modeling of the Gastrointestinal System for Preliminary Drug Absorption Assessment
Abstract
1. Introduction
2. Materials and Methods
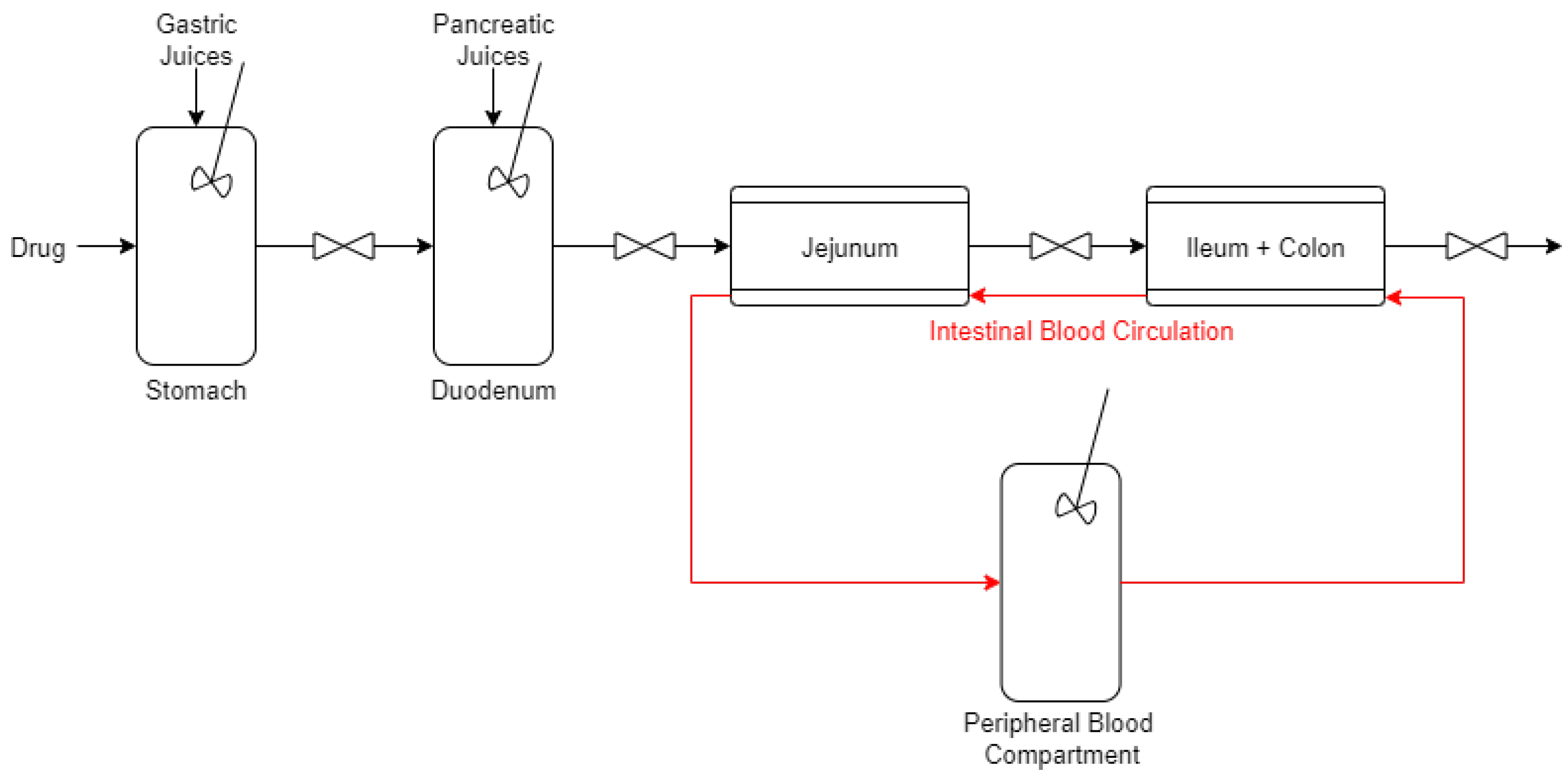
2.1. Stomach and Duodenum
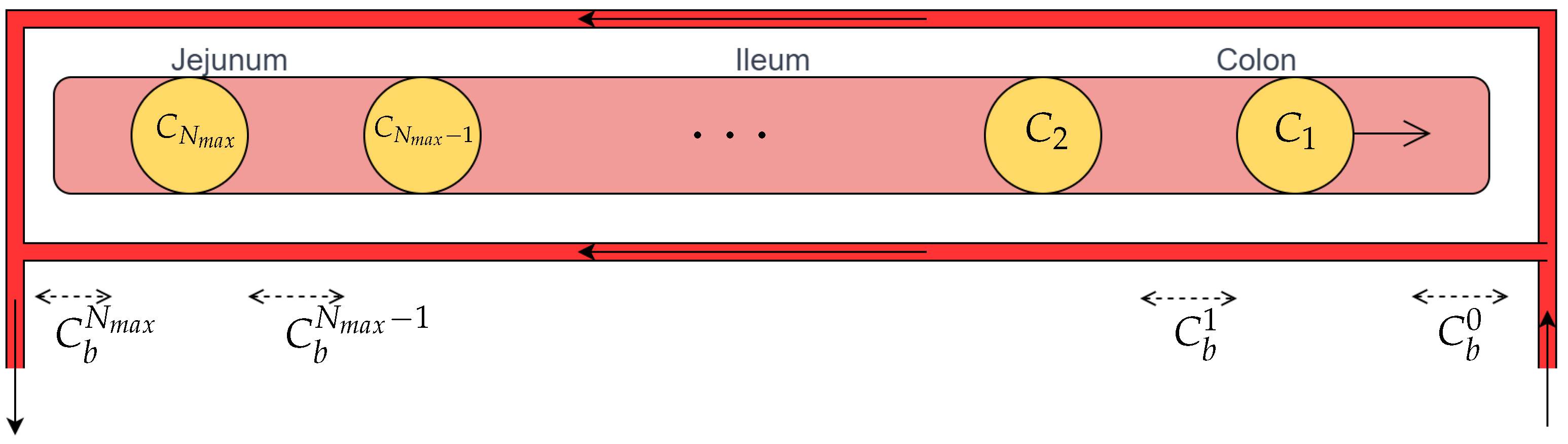
2.2. Jejunum and Ileum–Colon
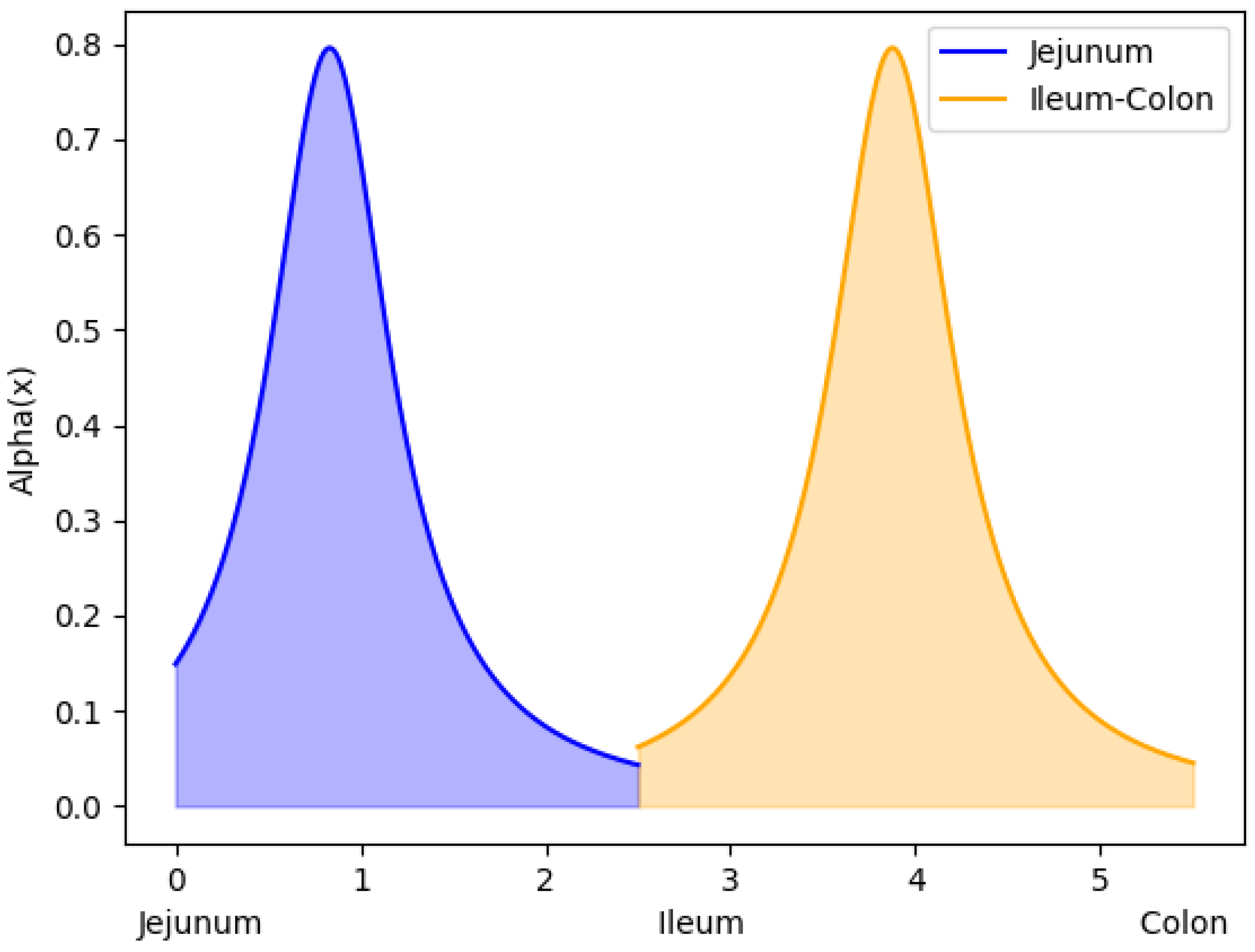
2.3. Parameters Definition and Estimation
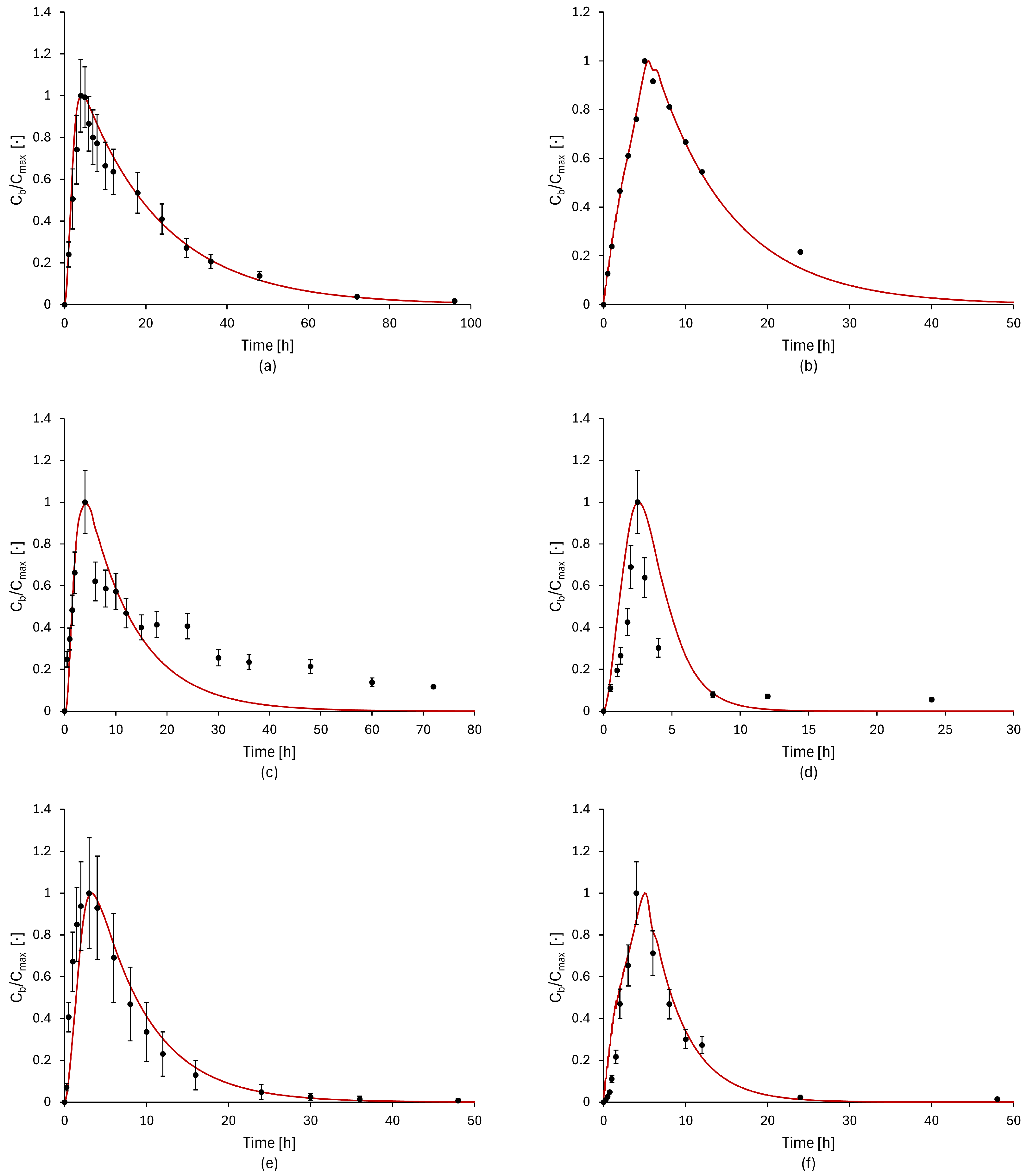
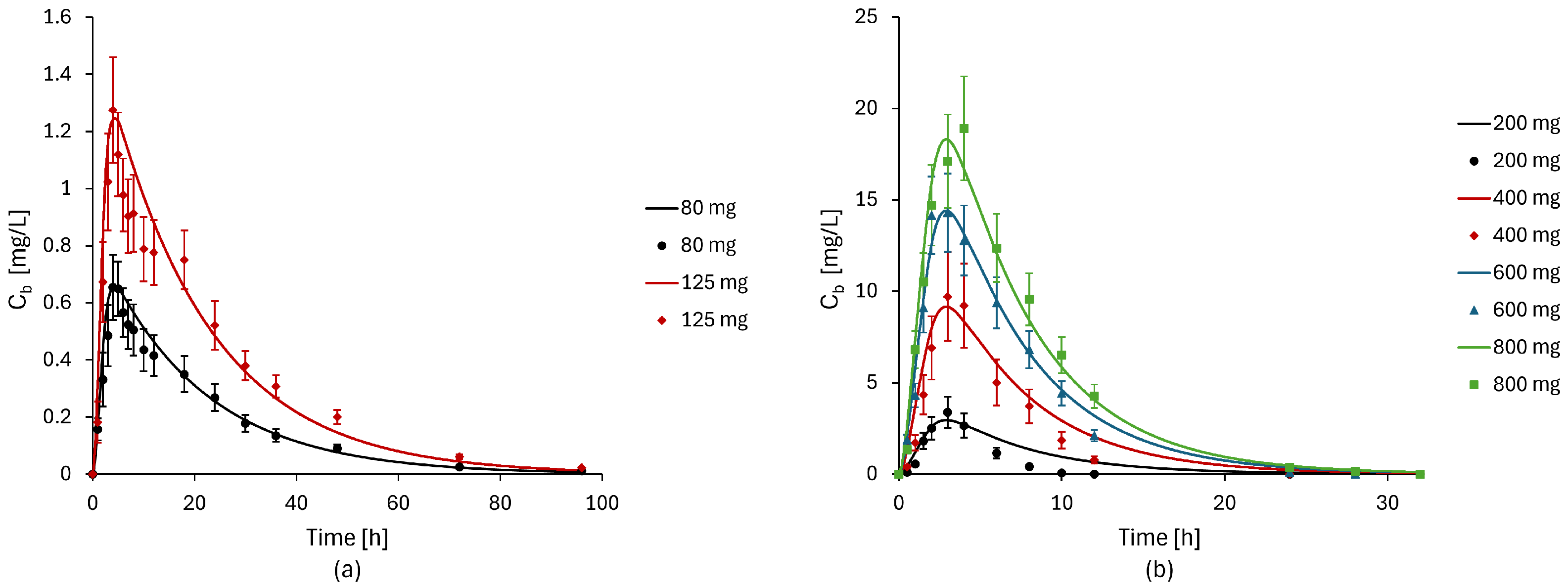
2.4. Model Validation
3. Results
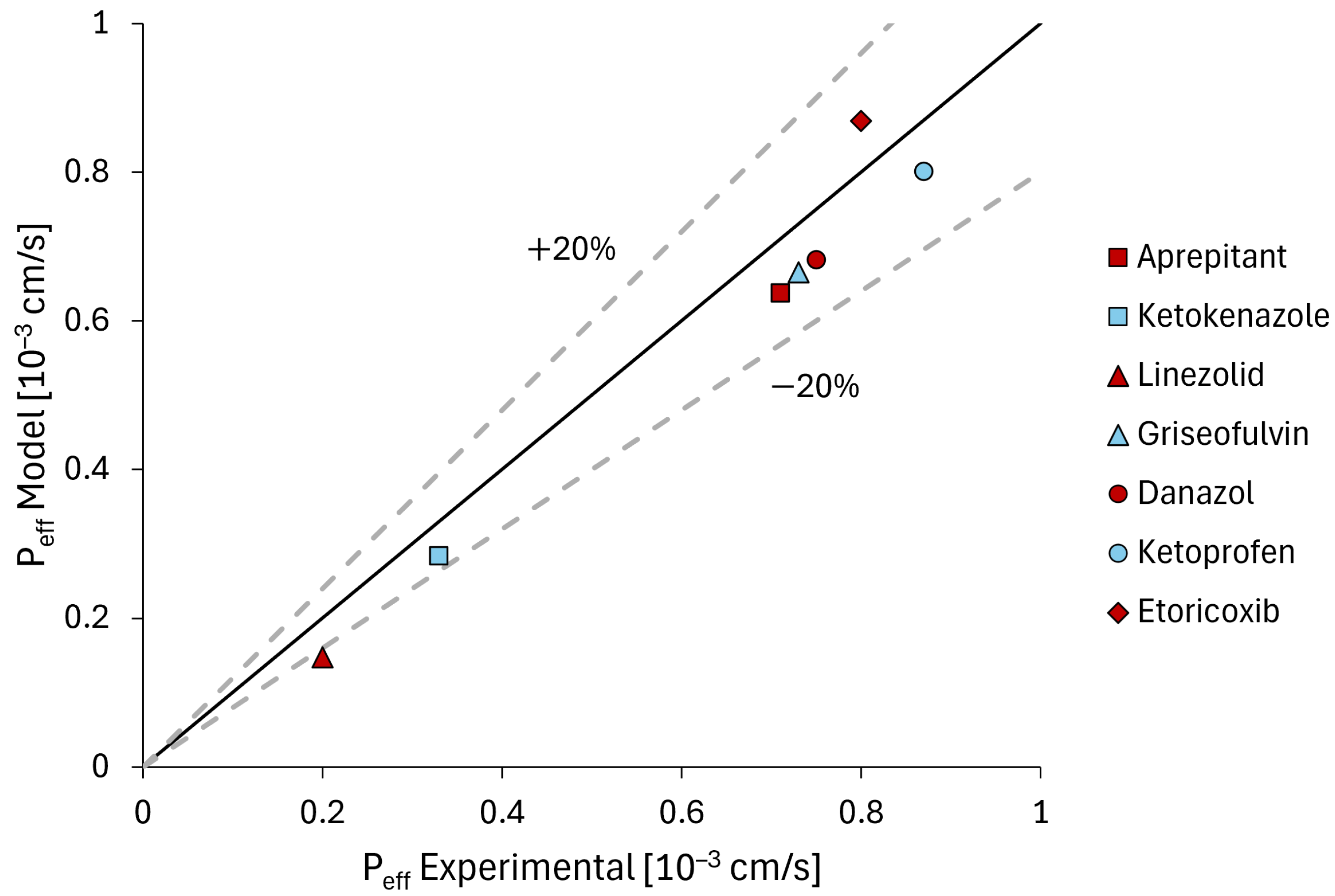
3.1. Parameter Estimation Results
3.2. Model Validation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal absorption study: Challenges and absorption enhancement strategies in improving oral drug delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef]
- Stillhart, C.; Vučićević, K.; Augustijns, P.; Basit, A.W.; Batchelor, H.; Flanagan, T.R.; Gesquiere, I.; Greupink, R.; Keszthelyi, D.; Koskinen, M.; et al. Impact of gastrointestinal physiology on drug absorption in special populations—An UNGAP review. Eur. J. Pharm. Sci. 2020, 147, 105280. [Google Scholar] [CrossRef] [PubMed]
- Kostewicz, E.S.; Aarons, L.; Bergstrand, M.; Bolger, M.B.; Galetin, A.; Hatley, O.; Jamei, M.; Lloyd, R.; Pepin, X.; Rostami-Hodjegan, A.; et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur. J. Pharm. Sci. 2014, 57, 300–321. [Google Scholar] [CrossRef] [PubMed]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.G.; Gross, J. Physiologically based pharmacokinetic models for anticancer drugs. Cancer Chemother. Pharmacol. 1979, 2, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Himmelstein, K.; Lutz, R. A review of the applications of physiologically based pharmacokinetic modeling. J. Pharmacokinet. Biopharm. 1979, 7, 127–145. [Google Scholar] [CrossRef]
- Khalil, F.; Läer, S. Physiologically based pharmacokinetic modeling: Methodology, applications, and limitations with a focus on its role in pediatric drug development. Biomed. Res. Int. 2011, 2011, 907461. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.M.; Sinko, P.J. Physiologically-based pharmacokinetic simulation modelling. Adv. Drug Deliv. Rev. 2002, 54, 433–451. [Google Scholar] [CrossRef]
- Nestorov, I. Whole body pharmacokinetic models. Clin. Pharmacokinet. 2003, 42, 883–908. [Google Scholar] [CrossRef]
- Nestorov, I. Whole-body physiologically based pharmacokinetic models. Expert Opin. Drug Metab. Toxicol. 2007, 3, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Bois, F.Y. Physiologically based modelling and prediction of drug interactions. Basic Clin. Pharmacol. Toxicol. 2010, 106, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Parrott, N.; Lave, T. Applications of physiologically based absorption models in drug discovery and development. Mol. Pharm. 2008, 5, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Gospavic, R.; Knoll, P.; Mirzaei, S.; Popov, V. Physiologically based pharmacokinetic (PBPK) model for biodistribution of radiolabeled peptides in patients with neuroendocrine tumours. Asia Ocean. J. Nucl. Med. Biol. 2016, 4, 90. [Google Scholar]
- Bischoff, K.B. Some fundamental considerations of the applications of pharmacokinetics to cancer chemotherapy. Cancer Chemother. Rep. 1975, 59, 777–793. [Google Scholar] [PubMed]
- Brochot, C.; Tóth, J.; Bois, F.Y. Lumping in pharmacokinetics. J. Pharmacokinet. Pharmacodyn. 2005, 32, 719–736. [Google Scholar] [CrossRef]
- Nestorov, I.A.; Aarons, L.J.; Arundel, P.A.; Rowland, M. Lumping of whole-body physiologically based pharmacokinetic models. J. Pharmacokinet. Biopharm. 1998, 26, 21–46. [Google Scholar] [CrossRef]
- Kuepfer, L.; Niederalt, C.; Wendl, T.; Schlender, J.F.; Willmann, S.; Lippert, J.; Block, M.; Eissing, T.; Teutonico, D. Applied concepts in PBPK modeling: How to build a PBPK/PD model. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 516–531. [Google Scholar] [CrossRef]
- Demeester, C.; Robins, D.; Edwina, A.E.; Tournoy, J.; Augustijns, P.; Ince, I.; Lehmann, A.; Vertzoni, M.; Schlender, J.F. Physiologically based pharmacokinetic (PBPK) modelling of oral drug absorption in older adults—An AGePOP review. Eur. J. Pharm. Sci. 2023, 188, 106496. [Google Scholar] [CrossRef]
- Brown, R.P.; Delp, M.D.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar] [CrossRef]
- Jones, H.M.; Parrott, N.; Ohlenbusch, G.; Lavé, T. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modelling. Clin. Pharmacokinet. 2006, 45, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tong, X.; McCarver, D.G.; Hines, R.N.; Beard, D.A. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model. J. Pharmacokinet. Pharmacodyn. 2006, 33, 485–518. [Google Scholar] [CrossRef]
- Li, J.; Gwilt, P.R. The effect of age on the early disposition of doxorubicin. Cancer Chemother. Pharmacol. 2003, 51, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, G.; Charest-Tardif, G.; Truchon, G.; Tardif, R. Physiologically based modeling of n-hexane kinetics in humans following inhalation exposure at rest and under physical exertion: Impact on free 2, 5-hexanedione in urine and on n-hexane in alveolar air. J. Occup. Environ. Hyg. 2005, 2, 86–97. [Google Scholar] [CrossRef]
- Dennison, J.E.; Bigelow, P.L.; Mumtaz, M.M.; Andersen, M.E.; Dobrev, I.D.; Yang, R.S. Evaluation of potential toxicity from co-exposure to three CNS depressants (toluene, ethylbenzene, and xylene) under resting and working conditions using PBPK modeling. J. Occup. Environ. Hyg. 2005, 2, 127–135. [Google Scholar] [CrossRef]
- Clewell, H.J.; Gentry, P.R.; Covington, T.R.; Sarangapani, R.; Teeguarden, J.G. Evaluation of the potential impact of age-and gender-specific pharmacokinetic differences on tissue dosimetry. Toxicol. Sci. 2004, 79, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Espié, P.; Tytgat, D.; Sargentini-Maier, M.L.; Poggesi, I.; Watelet, J.B. Physiologically based pharmacokinetics (PBPK). Drug Metab. Rev. 2009, 41, 391–407. [Google Scholar] [CrossRef]
- Rowland, M.; Peck, C.; Tucker, G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 45–73. [Google Scholar] [CrossRef]
- Germani, M.; Crivori, P.; Rocchetti, M.; Burton, P.S.; Wilson, A.G.; Smith, M.E.; Poggesi, I. Evaluation of a basic physiologically based pharmacokinetic model for simulating the first-time-in-animal study. Eur. J. Pharm. Sci. 2007, 31, 190–201. [Google Scholar] [CrossRef]
- Björkman, S.; Wada, D.R.; Berling, B.M.; Benoni, G. Prediction of the disposition of midazolam in surgical patients by a physiologically based pharmacokinetic model. J. Pharm. Sci. 2001, 90, 1226–1241. [Google Scholar] [CrossRef]
- Andrew, M.A.; Hebert, M.F.; Vicini, P. Physiologically based pharmacokinetic model of midazolam disposition during pregnancy. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 5454–5457. [Google Scholar]
- Edginton, A.N.; Willmann, S. Physiology-based simulations of a pathological condition: Prediction of pharmacokinetics in patients with liver cirrhosis. Clin. Pharmacokinet. 2008, 47, 743–752. [Google Scholar] [CrossRef] [PubMed]
- El-Masri, H.A.; Kenyon, E.M. Development of a human physiologically based pharmacokinetic (PBPK) model for inorganic arsenic and its mono-and di-methylated metabolites. J. Pharmacokinet. Pharmacodyn. 2008, 35, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Clewell 3rd, H.; Gentry, P.R.; Covington, T.R.; Gearhart, J.M. Development of a physiologically based pharmacokinetic model of trichloroethylene and its metabolites for use in risk assessment. Environ. Health Perspect. 2000, 108, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Vossen, M.; Sevestre, M.; Niederalt, C.; Jang, I.J.; Willmann, S.; Edginton, A.N. Dynamically simulating the interaction of midazolam and the CYP3A4 inhibitor itraconazole using individual coupled whole-body physiologically-based pharmacokinetic (WB-PBPK) models. Theor. Biol. Med Model. 2007, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wong, H. Predicting oral drug absorption: Mini review on physiologically-based pharmacokinetic models. Pharmaceutics 2017, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- CY Chow, E.; Sandy Pang, K. Why we need proper PBPK models to examine intestine and liver oral drug absorption. Curr. Drug Metab. 2013, 14, 57–79. [Google Scholar] [CrossRef]
- Sjögren, E.; Thorn, H.; Tannergren, C. In silico modeling of gastrointestinal drug absorption: Predictive performance of three physiologically based absorption models. Mol. Pharm. 2016, 13, 1763–1778. [Google Scholar] [CrossRef]
- Jamei, M.; Marciniak, S.; Feng, K.; Barnett, A.; Tucker, G.; Rostami-Hodjegan, A. The Simcyp® population-based ADME simulator. Expert Opin. Drug Metab. Toxicol. 2009, 5, 211–223. [Google Scholar] [CrossRef]
- Gobeau, N.; Stringer, R.; De Buck, S.; Tuntland, T.; Faller, B. Evaluation of the GastroPlus™ advanced compartmental and transit (acat) model in early discovery. Pharm. Res. 2016, 33, 2126–2139. [Google Scholar] [CrossRef]
- Jamei, M.; Turner, D.; Yang, J.; Neuhoff, S.; Polak, S.; Rostami-Hodjegan, A.; Tucker, G. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009, 11, 225–237. [Google Scholar] [CrossRef]
- Agoram, B.; Woltosz, W.S.; Bolger, M.B. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv. Drug Deliv. Rev. 2001, 50, S41–S67. [Google Scholar] [CrossRef] [PubMed]
- Pompa, M.; Capocelli, M.; Piemonte, V. A new gastro-intestinal mathematical model to study drug bioavailability. Med Eng. Phys. 2019, 74, 106–114. [Google Scholar] [CrossRef]
- Cacace, F.; Menci, M.; Papi, M.; Piemonte, V. In-Silico Prediction of Oral Drug Bioavailability: A multi-boluses approach. Med Eng. Phys. 2021, 98, 140–150. [Google Scholar] [CrossRef]
- Gibaldi, M. Limitations of classical theories of drug absorption. In Drug Absorption; Prescott, L.F., Nimmo, W.S., Eds.; ADIS Press: Berlin/Heidelberg, Germany, 1979; pp. 1–5. [Google Scholar]
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology; Surgical Neurology International: Pittsford, NY, USA, 2017. [Google Scholar]
- Cassandras, C.G. Discrete event systems. In Modeling and Performance Analysis, Aksen Associates, Irwin; Birkhäuser Boston: Boston, MA, USA, 1993. [Google Scholar]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Sugano, K.; Okazaki, A.; Sugimoto, S.; Tavornvipas, S.; Omura, A.; Mano, T. Solubility and dissolution profile assessment in drug discovery. Drug Metab. Pharmacokinet. 2007, 22, 225–254. [Google Scholar] [CrossRef]
- Po, H.N.; Senozan, N. The Henderson-Hasselbalch equation: Its history and limitations. J. Chem. Educ. 2001, 78, 1499. [Google Scholar] [CrossRef]
- NIH. NCBI, National Center for Biotechnology Information. 2024. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 4 October 2024).
- Sjögren, E.; Westergren, J.; Grant, I.; Hanisch, G.; Lindfors, L.; Lennernäs, H.; Abrahamsson, B.; Tannergren, C. In silico predictions of gastrointestinal drug absorption in pharmaceutical product development: Application of the mechanistic absorption model GI-Sim. Eur. J. Pharm. Sci. 2013, 49, 679–698. [Google Scholar] [CrossRef]
- Shekhawat, P.B.; Pokharkar, V.B. Understanding peroral absorption: Regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm. Sin. B 2017, 7, 260–280. [Google Scholar] [CrossRef]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Majumdar, A.K.; Howard, L.; Goldberg, M.R.; Hickey, L.; Constanzer, M.; Rothenberg, P.L.; Crumley, T.M.; Panebianco, D.; Bradstreet, T.E.; Bergman, A.J.; et al. Pharmacokinetics of aprepitant after single and multiple oral doses in healthy volunteers. J. Clin. Pharmacol. 2006, 46, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; Aboul-Einien, M.H.; Mohamed, O.H.; Farid, S.F. Relative bioavailability of griseofulvin lyophilized dry emulsion tablet vs. immediate release tablet: A single-dose, randomized, open-label, six-period, crossover study in healthy adult volunteers in the fasted and fed states. Eur. J. Pharm. Sci. 2008, 35, 219–225. [Google Scholar] [CrossRef]
- Welshman, I.R.; Sisson, T.A.; Jungbluth, G.L.; Stalker, D.J.; Hopkins, N.K. Linezolid absolute bioavailability and the effect of food on oral bioavailability. Biopharm. Drug Dispos. 2001, 22, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sunesen, V.H.; Vedelsdal, R.; Kristensen, H.G.; Christrup, L.; Müllertz, A. Effect of liquid volume and food intake on the absolute bioavailability of danazol, a poorly soluble drug. Eur. J. Pharm. Sci. 2005, 24, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.Y.; Lee, E.J.; Chung, S.Y.; Choi, S.O.; Kim, H.K.; Kwon, J.T.; Kang, W.; Kwon, K.I. The effects of food on the bioavailability of fenofibrate administered orally in healthy volunteers via sustained-release capsule. Clin. Pharmacokinet. 2006, 45, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Pargal, A.; Kelkar, M.G.; Nayak, P.J. The effect of food on the bioavailability of ibuprofen and flurbiprofen from sustained release formulations. Biopharm. Drug Dispos. 1996, 17, 511–519. [Google Scholar] [CrossRef]
- Daneshmend, T.K.; Warnock, D.W.; Ene, M.D.; Johnson, E.M.; Potten, M.; Richardson, M.; Williamson, P. Influence of food on the pharmacokinetics of ketoconazole. Antimicrob. Agents Chemother. 1984, 25, 1–3. [Google Scholar] [CrossRef]
- Bannwarth, B.; Lapicque, F.; Netter, P.; Monot, C.; Tamisier, J.; Thomas, P.; Royer, R. The effect of food on the systemic availability of ketoprofen. Eur. J. Clin. Pharmacol. 1988, 33, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.G.; Porras, A.G.; Matthews, C.Z.; Rose, M.J.; Woolf, E.J.; Musser, B.J.; Dynder, A.L.; Mazina, K.E.; Lasseter, K.C.; Hunt, T.L.; et al. Single-and multiple-dose pharmacokinetics of etoricoxib, a selective inhibitor of cyclooxygenase-2, in man. J. Clin. Pharmacol. 2003, 43, 268–276. [Google Scholar] [CrossRef]
- Hellström, P.M.; Grybäck, P.; Jacobsson, H. The physiology of gastric emptying. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 397–407. [Google Scholar] [CrossRef]
- Miron, Y.; Zeeman, G.; Van Lier, J.B.; Lettinga, G. The role of sludge retention time in the hydrolysis and acidification of lipids, carbohydrates and proteins during digestion of primary sludge in CSTR systems. Water Res. 2000, 34, 1705–1713. [Google Scholar] [CrossRef]
- He, X. Integration of physical, chemical, mechanical, and biopharmaceutical properties in solid oral dosage form development. In Developing Solid Oral Dosage Forms; Elsevier: Amsterdam, The Netherlands, 2009; pp. 407–441. [Google Scholar]
- Zhao, P.; Zhang, L.; Grillo, J.; Liu, Q.; Bullock, J.; Moon, Y.; Song, P.; Brar, S.; Madabushi, R.; Wu, T.; et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin. Pharmacol. Ther. 2011, 89, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Lu, C. PBPK modeling and simulation in drug research and development. Acta Pharm. Sin. B 2016, 6, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Fujii, Y.; Tabata, F.; Ito, J.; Metsugi, Y.; Kameda, A.; Akimoto, K.; Takahashi, M. Profiling and trend analysis of food effects on oral drug absorption considering micelle interaction and solubilization by bile micelles. Drug Metab. Pharmacokinet. 2011, 26, 180–191. [Google Scholar] [CrossRef] [PubMed]
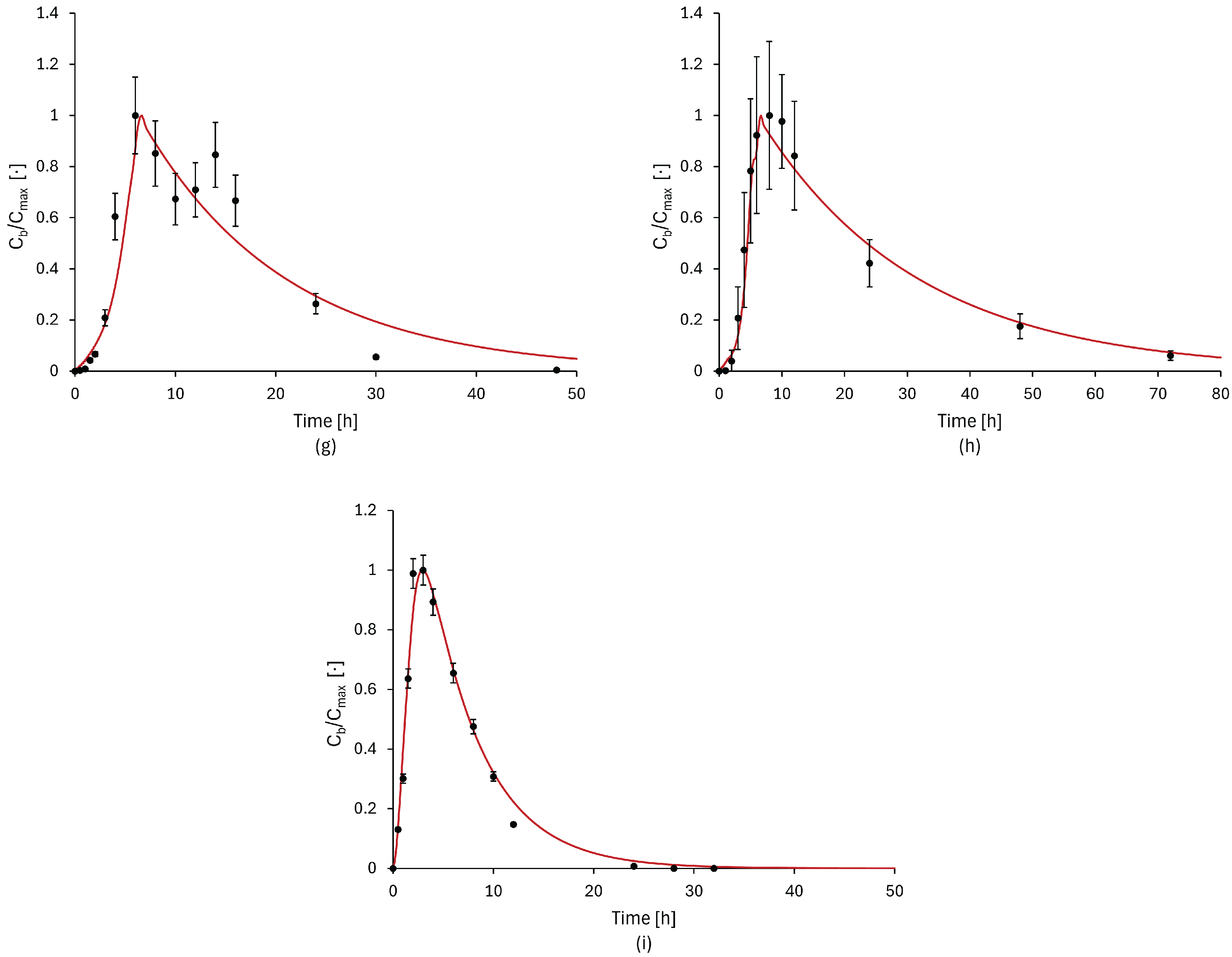
| Drug | (mol/L) | D ( /s) | (g/mL) | Reference | |
|---|---|---|---|---|---|
| Aprepitant | 0.63 | 1.51 | 9.15 | [53] | |
| Griseofulvin | 0.7 | 1.38 | 17.7 | [53] | |
| Linezolid | 0.67 | 1.12 | 1.8 | [52] | |
| Danazol | 0.68 | 1.21 | 4.7 | [53] | |
| Fenofibrate | 0.66 | 1.18 | 4.7 | [53] | |
| Ibuprofen | 0.61 | 1.6 | 5.3 | [52] | |
| Ketokenazole | 0.66 | 1.38 | 6.5 | [53] | |
| Ketoprofen | 0.7 | 1.6 | 4.45 | [52] | |
| Etoricoxib | 0.59 | 1.41 | 4.96 | [52] |
| Drug | Reference |
|---|---|
| Aprepitant | [56] |
| Griseofulvin | [57] |
| Linezolid | [58] |
| Danazol | [59] |
| Fenofibrate | [60] |
| Ibuprofen | [61] |
| Ketokenazole | [62] |
| Ketoprofen | [63] |
| Etoricoxib | [64] |
| Symbol | Parameter | Value | Reference |
|---|---|---|---|
| Peripheral circulation duration | 90 s | [47] | |
| Emptying frequency | Hz | [44] | |
| Blood velocity in the intestine | m/s | [44] | |
| u | Average bolus velocity in the intestine | 1 m/h | [44] |
| Blood flow rate in the intestine | L/s | [47] | |
| Length of the jejunum | 2 m | [47] | |
| Length of the ileum | 4 m | [47] | |
| Length of the colon | m | [47] | |
| Blood volume in peripheral circulation | 4 L | [44] | |
| Intestine radius | m | [47] |
| Drug | [ /s] | [ /s] | [ ] |
|---|---|---|---|
| Aprepitant | 5.157 | 5.100 | 2.012 |
| Ketokenazole | 3.506 | 1.311 | 8.095 |
| Griseofulvin | 5.858 | 4.949 | 4.695 |
| Linezolid | 1.184 | 1.195 | 5.352 |
| Irbesartan | 6.860 | 5.994 | 5.036 |
| Danazol | 7.325 | 4.029 | 10.287 |
| Fenofibrate | 104.031 | 73.810 | 0.836 |
| Ibuprofen | 286.936 | 18.827 | 3.074 |
| Ketoprofen | 7.000 | 6.000 | 25.343 |
| Etoricoxib | 6.993 | 6.988 | 2.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, A.; Itaj, F.; Cacace, F.; Piemonte, V. Mathematical Modeling of the Gastrointestinal System for Preliminary Drug Absorption Assessment. Bioengineering 2024, 11, 813. https://doi.org/10.3390/bioengineering11080813
D’Ambrosio A, Itaj F, Cacace F, Piemonte V. Mathematical Modeling of the Gastrointestinal System for Preliminary Drug Absorption Assessment. Bioengineering. 2024; 11(8):813. https://doi.org/10.3390/bioengineering11080813
Chicago/Turabian StyleD’Ambrosio, Antonio, Fatjon Itaj, Filippo Cacace, and Vincenzo Piemonte. 2024. "Mathematical Modeling of the Gastrointestinal System for Preliminary Drug Absorption Assessment" Bioengineering 11, no. 8: 813. https://doi.org/10.3390/bioengineering11080813
APA StyleD’Ambrosio, A., Itaj, F., Cacace, F., & Piemonte, V. (2024). Mathematical Modeling of the Gastrointestinal System for Preliminary Drug Absorption Assessment. Bioengineering, 11(8), 813. https://doi.org/10.3390/bioengineering11080813









