An Exosome-Laden Hydrogel Wound Dressing That Can Be Point-of-Need Manufactured in Austere and Operational Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Exosome Isolation and Lyophilization
2.2. Exosome Characterization
2.3. Transmission Electron Microscopy
2.4. Mass Spectroscopy of Exosomes
2.5. Exosome Staining
2.6. Alginate Cellulose Biomaterial Ink Formulation
2.7. Biomaterial Ink Characterization
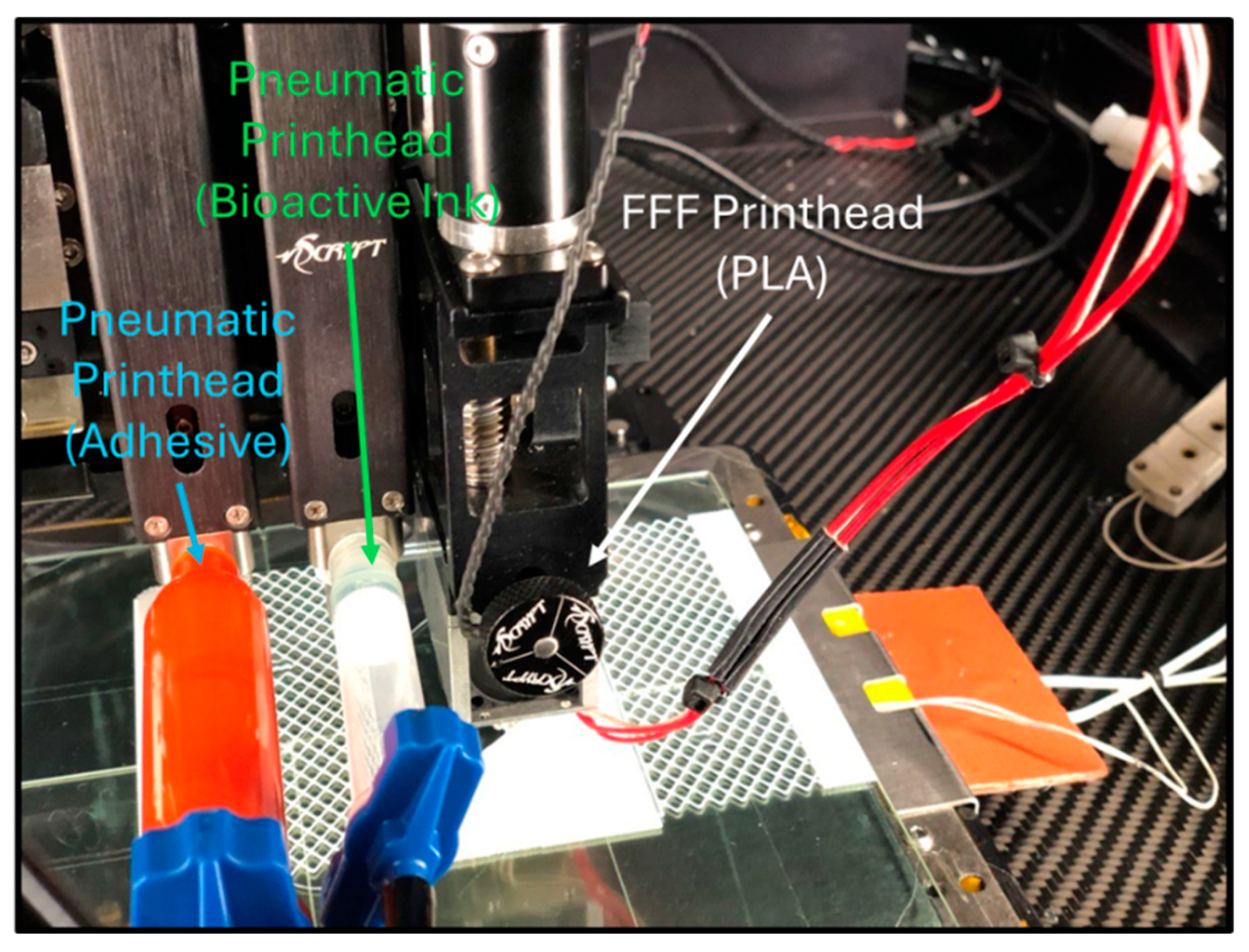
2.8. Bioactive Dressing Printing on a Commercial Laboratory Bioprinter
2.9. Mechanical Testing
2.10. EV Release from Bioprinted Dressing
2.11. Logistics and Transportation of Materials for Austere Bioprinting Exercise
2.12. Bioactive Bandage Printing on a Ruggedized 3D Printer
3. Results and Discussion
3.1. Exosome Isolation, Lyophilization and Characterization
3.2. Development of an Alginate/CMC Biomaterial Ink
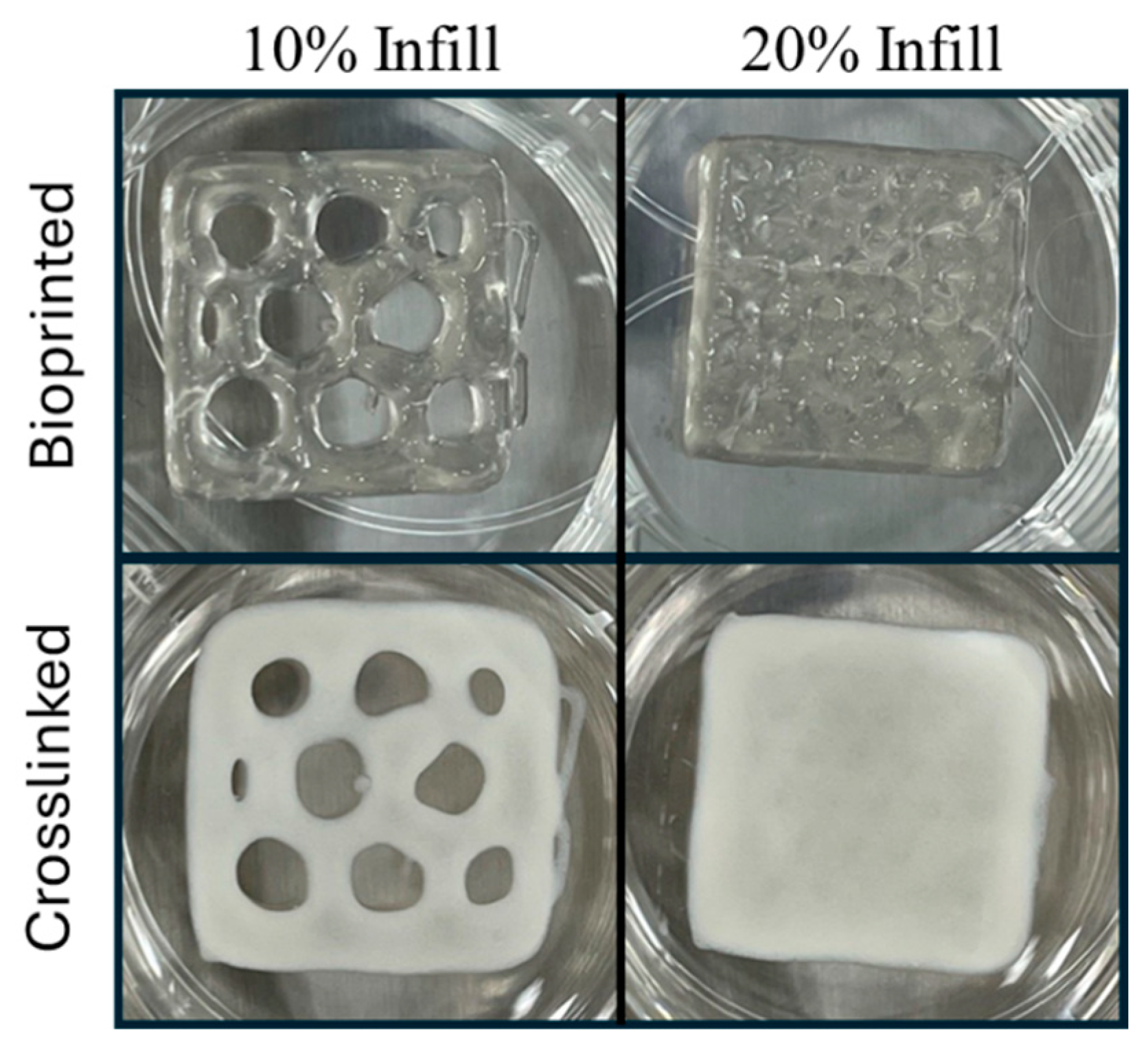
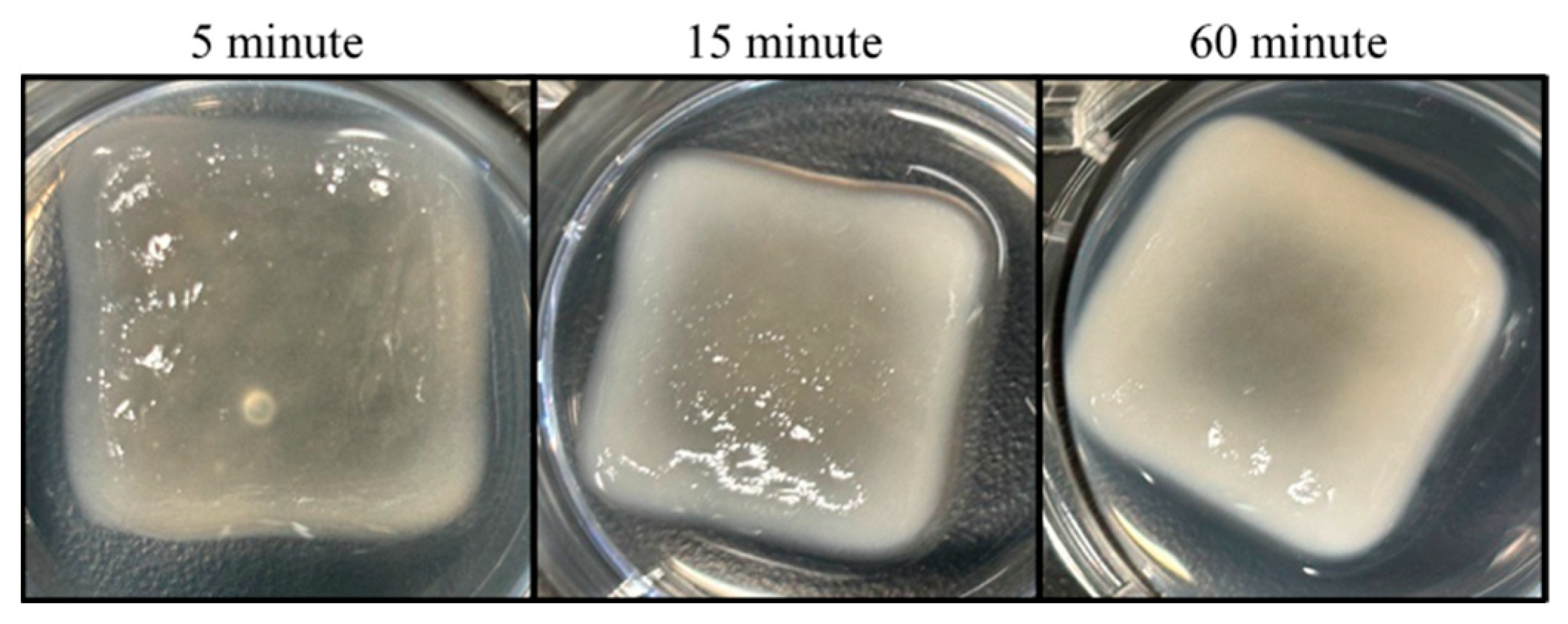
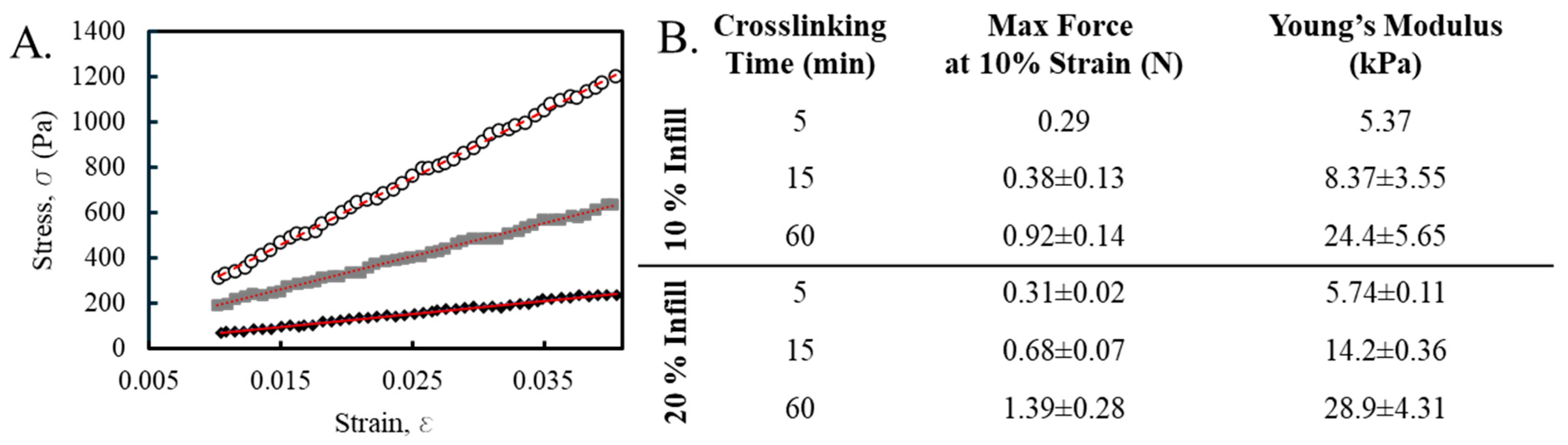
3.3. Mechanical Evaluation of Printed Dressings
3.4. Three-Dimensional Printing of EV-Laden Bio-Ink
3.5. EV Release from 3D Printed Dressing
3.6. Three-Dimensional Printing EV Dressing with a Ruggedized Printer
3.7. Three-Dimensional Printing of a Bioactive Wound Dressing in an Austere Location
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, O.N., 3rd; Nelson, M.; Estabrooke, I.; Sopko, N.; Swanson, E.W. Successful Treatment of War Zone Traumatic Lower Extremity Wound With Exposed Tendons Using an Autologous Homologous Skin Construct. Cureus 2020, 12, e7952. [Google Scholar] [CrossRef]
- Larson, D.; Carlsson, A.H.; Valdera, F.A.; Burgess, M.; Leatherman, L.; Shaffer, L.; Christy, R.J.; Nuutila, K. Evaluation of Topical Off-the-Shelf Therapies to Reduce the Need to Evacuate Battlefield-Injured Warfighters. Mil. Med. 2023, 189, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Wild, H.; Stewart, B.T.; LeBoa, C.; Stave, C.D.; Wren, S.M. Epidemiology of Injuries Sustained by Civilians and Local Combatants in Contemporary Armed Conflict: An Appeal for a Shared Trauma Registry Among Humanitarian Actors. World J. Surg. 2020, 44, 1863–1873. [Google Scholar] [CrossRef]
- Hwang, J.M. Time is tissue. Want to save millions in wound care? Start early: A QI project to expedite referral of high-risk wound care patients to specialised care. BMJ Open Qual. 2023, 12, e002206. [Google Scholar] [CrossRef]
- Shirakami, E.; Yamakawa, S.; Hayashida, K. Strategies to prevent hypertrophic scar formation: A review of therapeutic interventions based on molecular evidence. Burns Trauma 2020, 8, tkz003. [Google Scholar] [CrossRef] [PubMed]
- Monstrey, S.; Middelkoop, E.; Vranckx, J.J.; Bassetto, F.; Ziegler, U.E.; Meaume, S.; Teot, L. Updated scar management practical guidelines: Non-invasive and invasive measures. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1017–1025. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. A 2020, 108, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res. A 2017, 105, 1457–1468. [Google Scholar] [CrossRef]
- Distler, T.; Schaller, E.; Steinmann, P.; Boccaccini, A.R.; Budday, S. Alginate-based hydrogels show the same complex mechanical behavior as brain tissue. J. Mech. Behav. Biomed. Mater. 2020, 111, 103979. [Google Scholar] [CrossRef]
- Ovington, L.G. Advances in wound dressings. Clin. Dermatol. 2007, 25, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Beele, H.; Meuleneire, F.; Nahuys, M.; Percival, S.L. A prospective randomised open label study to evaluate the potential of a new silver alginate/carboxymethylcellulose antimicrobial wound dressing to promote wound healing. Int. Wound J. 2010, 7, 262–270. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef]
- Golan, M.E.; Stice, S.L. Extracellular vesicle lyophilization for enhanced distribution to the point of care. Extracell. Vesicle 2024, 3, 100041. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Richter, M.; Heinrich, E.; Koch, M.; Fuhrmann, G.; Friess, W. Enhancing the Stabilization Potential of Lyophilization for Extracellular Vesicles. Adv. Healthc. Mater. 2022, 11, 2100538. [Google Scholar] [CrossRef]
- Barnhill, J.; Gaston, J.D.; Deffenbaugh, P.I.; Wagner, L.; Liacouras, P.C.; Ho, V.B. Additive Manufacturing for Fabrication of Point-of-Care Therapies in Austere Environments. Mil. Med. 2023, 188, e1847–e1853. [Google Scholar] [CrossRef]
- Wisdom, C.; Chartrain, N.; Blaize-Wise, K.; Klarmann, G.J.; Gilchrist, K.H.; Ho, V.B. Point-of-Need Additive Manufacturing in Austere Arctic Environments: An Evaluation of Medical Logistics Requirements and Capabilities Demonstration. Bioengineering 2024, 11, 232. [Google Scholar] [CrossRef]
- Bastawrous, S.; Wu, L.; Liacouras, P.C.; Levin, D.B.; Ahmed, M.T.; Strzelecki, B.; Amendola, M.F.; Lee, J.T.; Coburn, J.; Ripley, B. Establishing 3D Printing at the Point of Care: Basic Principles and Tools for Success. Radiographics 2022, 42, 451–468. [Google Scholar] [CrossRef]
- El Baradie, K.B.Y.; Nouh, M.; O’Brien Iii, F.; Liu, Y.; Fulzele, S.; Eroglu, A.; Hamrick, M.W. Freeze-Dried Extracellular Vesicles from Adipose-Derived Stem Cells Prevent Hypoxia-Induced Muscle Cell Injury. Front. Cell Dev. Biol. 2020, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Milojevic, M.; Harih, G.; Vihar, B.; Vajda, J.; Gradisnik, L.; Zidaric, T.; Stana Kleinschek, K.; Maver, U.; Maver, T. Hybrid 3D Printing of Advanced Hydrogel-Based Wound Dressings with Tailorable Properties. Pharmaceutics 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Klarmann, G.J.; Piroli, M.E.; Loverde, J.R.; Nelson, A.F.; Li, Z.; Gilchrist, K.H.; Gaston, J.D.; Ho, V.B. 3D printing a universal knee meniscus using a custom collagen ink. Bioprinting 2023, 31, e00272. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.A.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 2017, 13, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Coumans, F.A.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Susa, F.; Limongi, T.; Borgione, F.; Peiretti, S.; Vallino, M.; Cauda, V.; Pisano, R. Comparative Studies of Different Preservation Methods and Relative Freeze-Drying Formulations for Extracellular Vesicle Pharmaceutical Applications. ACS Biomater. Sci. Eng. 2023, 9, 5871–5885. [Google Scholar] [CrossRef]
- Lyu, N.; Knight, R.; Robertson, S.Y.T.; Dos Santos, A.; Zhang, C.; Ma, C.; Xu, J.; Zheng, J.; Deng, S.X. Stability and Function of Extracellular Vesicles Derived from Immortalized Human Corneal Stromal Stem Cells: A Proof of Concept Study. AAPS J. 2022, 25, 8. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665. [Google Scholar] [CrossRef]
- Teoh, J.H.; Mozhi, A.; Sunil, V.; Tay, S.M.; Fuh, J.; Wang, C.-H. 3D Printing Personalized, Photocrosslinkable Hydrogel Wound Dressings for the Treatment of Thermal Burns. Adv. Funct. Mater. 2021, 31, 2105932. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionisio, M.; Lopez, C.R.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, Q.; Han, Q.; Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2019, 10, 295–299. [Google Scholar] [CrossRef]
- Agarwal, T.; Narayana, S.N.G.H.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.M.C.; Silva, J.C.; Serro, A.P.; Cabral, J.M.S.; Sanjuan-Alberte, P.; Ferreira, F.C. 3D Bioprinting of Novel kappa-Carrageenan Bioinks: An Algae-Derived Polysaccharide. Bioengineering 2022, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Stavarache, C.; Garea, S.A.; Serafim, A.; Olaret, E.; Vlasceanu, G.M.; Marin, M.M.; Iovu, H. Three-Dimensional-Printed Sodium Alginate and k-Carrageenan-Based Scaffolds with Potential Biomedical Applications. Polymers 2024, 16, 305. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.; Tuladhar, S.; Launen, L.; Habib, A. 3D Bio-Printability of Hybrid Pre-Crosslinked Hydrogels. Int. J. Mol. Sci. 2021, 22, 13481. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Osidak, E.O.; Karalkin, P.A.; Osidak, M.S.; Parfenov, V.A.; Sivogrivov, D.E.; Pereira, F.; Gryadunova, A.A.; Koudan, E.V.; Khesuani, Y.D.; Kasyanov, V.A.; et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 31. [Google Scholar] [CrossRef] [PubMed]
- Fayyazbakhsh, F.; Khayat, M.J.; Leu, M.C. 3D-Printed Gelatin-Alginate Hydrogel Dressings for Burn Wound Healing: A Comprehensive Study. Int. J. Bioprint. 2022, 8, 618. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simoes, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Lowe, A. An Overview of Factors Affecting the Skins Youngs Modulus. J. Aging Sci. 2016, 4, 1000156. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Wang, C.; Xu, M.; Fan, Q.; Li, C.; Zhou, X. Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases. Asian J. Pharm. Sci. 2023, 18, 100772. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Roszkowski, S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin. Exp. Med. 2024, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Prasai, A.; Jay, J.W.; Jupiter, D.; Wolf, S.E.; El Ayadi, A. Role of Exosomes in Dermal Wound Healing: A Systematic Review. J. Investig. Dermatol. 2022, 142, 662–678.e8. [Google Scholar] [CrossRef] [PubMed]
- Nooshabadi, V.T.; Khanmohamadi, M.; Valipour, E.; Mahdipour, S.; Salati, A.; Malekshahi, Z.V.; Shafei, S.; Amini, E.; Farzamfar, S.; Ai, J. Impact of exosome-loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. J. Biomed. Mater. Res. Part A 2020, 108, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Dong, R.; Tang, J.; Li, H.; Dang, J.; Zhang, Z.; Yu, Z.; Guo, B.; Yi, C. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem. Eng. J. 2022, 430, 132664. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Singh, A.; Kumar, A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 2020, 249, 120020. [Google Scholar] [CrossRef]
- Li, Q.; Gong, S.; Yao, W.; Yang, Z.; Wang, R.; Yu, Z.; Wei, M. Exosome loaded genipin crosslinked hydrogel facilitates full thickness cutaneous wound healing in rat animal model. Drug Deliv. 2021, 28, 884–893. [Google Scholar] [CrossRef]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D. Recent advances in 3D printing for wound healing: A systematic review. J. Drug Deliv. Sci. Technol. 2022, 74, 103564. [Google Scholar] [CrossRef]
- Singh, M.; Jonnalagadda, S. Design and characterization of 3D printed, neomycin-eluting poly-L-lactide mats for wound-healing applications. J. Mater. Sci. Mater. Med. 2021, 32, 44. [Google Scholar] [CrossRef] [PubMed]
- Klinkajon, W.; Supaphol, P. Novel copper (II) alginate hydrogels and their potential for use as anti-bacterial wound dressings. Biomed. Mater. 2014, 9, 045008. [Google Scholar] [CrossRef] [PubMed]
- Mikula, K.; Skrzypczak, D.; Ligas, B.; Witek-Krowiak, A. Preparation of hydrogel composites using Ca2+ and Cu2+ ions as crosslinking agents. SN Appl. Sci. 2019, 1, 643. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Tuñón-Molina, A.; Bakshi, H.; Sabater i Serra, R.; Alfagih, I.M.; Tambuwala, M.M.; Serrano-Aroca, Á. Biocompatible Alginate Film Crosslinked with Ca2+ and Zn2+ Possesses Antibacterial, Antiviral, and Anticancer Activities. ACS Omega 2023, 8, 24396–24405. [Google Scholar] [CrossRef]
- Tordi, P.; Gelli, R.; Ridi, F.; Bonini, M. A bioinspired and sustainable route for the preparation of Ag-crosslinked alginate fibers decorated with silver nanoparticles. Carbohydr. Polym. 2024, 326, 121586. [Google Scholar] [CrossRef]
- Qin, Y. Silver-containing alginate fibres and dressings. Int. Wound J. 2005, 2, 172–176. [Google Scholar] [CrossRef]






| Group | Accession Code | Gene ID | Description (Alternative Name) | Coverage [%] | # Peptides |
|---|---|---|---|---|---|
| 1. Transmembrane or GPI-anchored proteins associated with plasma membrane and/or endosomes | P05106 | ITGB3 | Integrin beta-3 | 13 | 10 |
| P31431 | SDC4 | Syndecan-4 | 13 | 2 | |
| H7C1K4 | SDC1 | Syndecan-1 (Fragment) | 8 | 1 | |
| F8VWK8 | CD63 | Tetraspanin (Fragment) | 8 | 2 | |
| J3QRJ3 | THY1 | Thy-1 membrane glycoprotein (CD90) | 10 | 1 | |
| 2. Cytosolic proteins recovered in EVs | P07355 | ANXA2 | Annexin A2 | 59 | 15 |
| P11142 | HSPA8 | Heat shock cognate 71 kDa protein | 45 | 28 | |
| Q8WUM4 | PDCD6IP | Programmed cell death 6-interacting protein (ALIX) | 9 | 8 | |
| 3. Major components of non-EV co-isolated structures | J3QTR3 | RPS27A | Ubiquitin-40S ribosomal protein S27a (Fragment) | 44 | 4 |
| M0QX76 | RPS16 | 40S ribosomal protein S16 (Fragment) | 20 | 1 | |
| P02647 | APOA1 | Apolipoprotein A-I 1 | 17 | 3 | |
| P04114 | APOB | Apolipoprotein B-100 1 | 3 | 12 | |
| A0A087WWT3 | ALB | Serum albumin 1 | 89 | 56 | |
| 4. Transmembrane, lipid-bound, and soluble proteins associated with other intracellular compartments than PM/endosomes | P62805 | H4C1 | Histone H4 | 50 | 5 |
| Q5QNW6 | H2BC18 | Histone H2B type 2-F | 36 | 5 | |
| P10412 | H1-4 | Histone H1.4 | 23 | 5 | |
| P07305 | H1-0 | Histone H1.0 | 16 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisdom, E.C.; Lamont, A.; Martinez, H.; Rockovich, M.; Lee, W.; Gilchrist, K.H.; Ho, V.B.; Klarmann, G.J. An Exosome-Laden Hydrogel Wound Dressing That Can Be Point-of-Need Manufactured in Austere and Operational Environments. Bioengineering 2024, 11, 804. https://doi.org/10.3390/bioengineering11080804
Wisdom EC, Lamont A, Martinez H, Rockovich M, Lee W, Gilchrist KH, Ho VB, Klarmann GJ. An Exosome-Laden Hydrogel Wound Dressing That Can Be Point-of-Need Manufactured in Austere and Operational Environments. Bioengineering. 2024; 11(8):804. https://doi.org/10.3390/bioengineering11080804
Chicago/Turabian StyleWisdom, E. Cate, Andrew Lamont, Hannah Martinez, Michael Rockovich, Woojin Lee, Kristin H. Gilchrist, Vincent B. Ho, and George J. Klarmann. 2024. "An Exosome-Laden Hydrogel Wound Dressing That Can Be Point-of-Need Manufactured in Austere and Operational Environments" Bioengineering 11, no. 8: 804. https://doi.org/10.3390/bioengineering11080804
APA StyleWisdom, E. C., Lamont, A., Martinez, H., Rockovich, M., Lee, W., Gilchrist, K. H., Ho, V. B., & Klarmann, G. J. (2024). An Exosome-Laden Hydrogel Wound Dressing That Can Be Point-of-Need Manufactured in Austere and Operational Environments. Bioengineering, 11(8), 804. https://doi.org/10.3390/bioengineering11080804







