Wavelet Coherence Analysis of Post-Stroke Intermuscular Coupling Modulated by Myoelectric-Controlled Interfaces
Abstract
1. Introduction
2. Methods
2.1. Participants
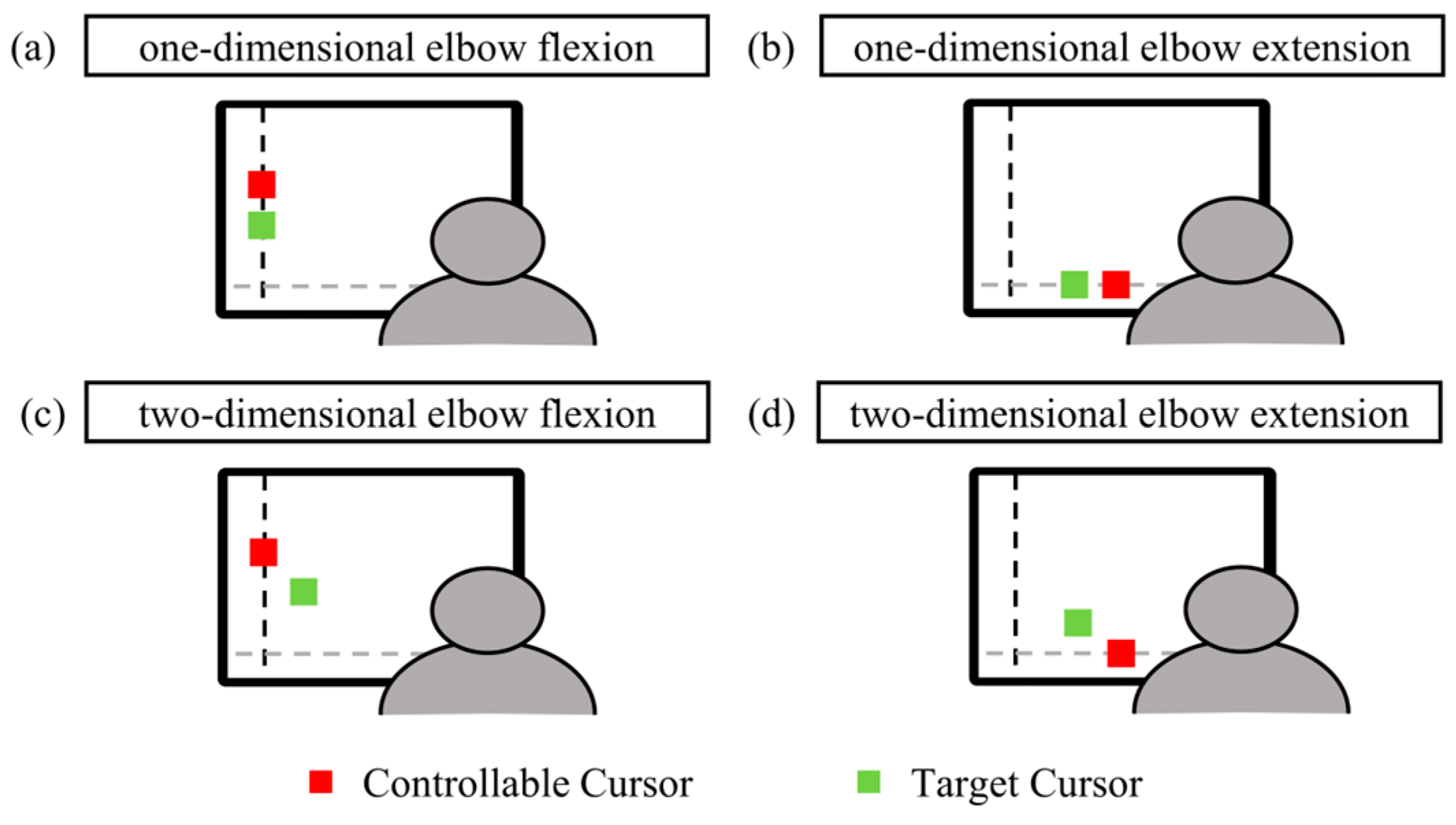
2.2. Experimental Procedure
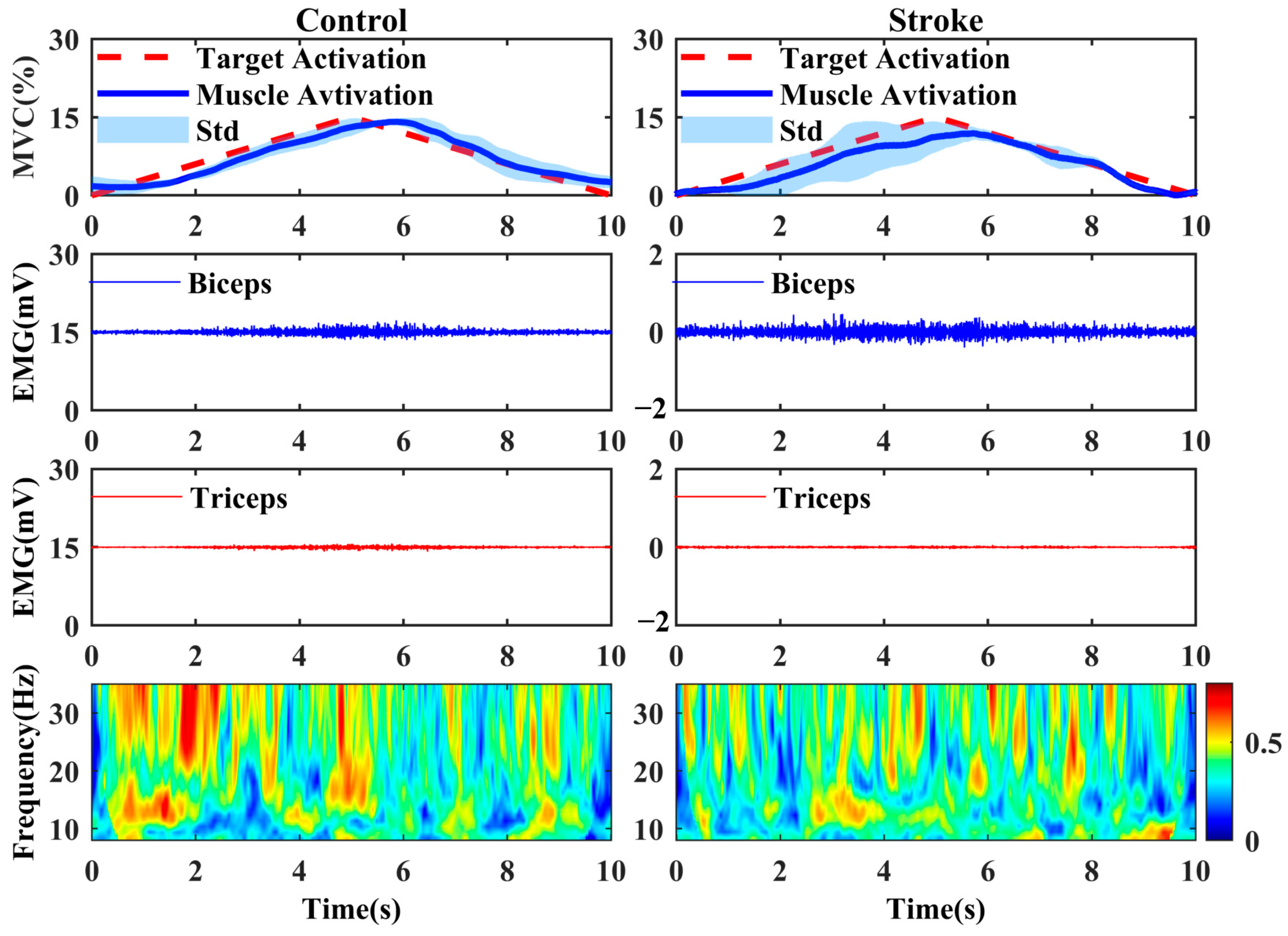
2.3. Wavelet Coherence
2.4. Statistical Analysis
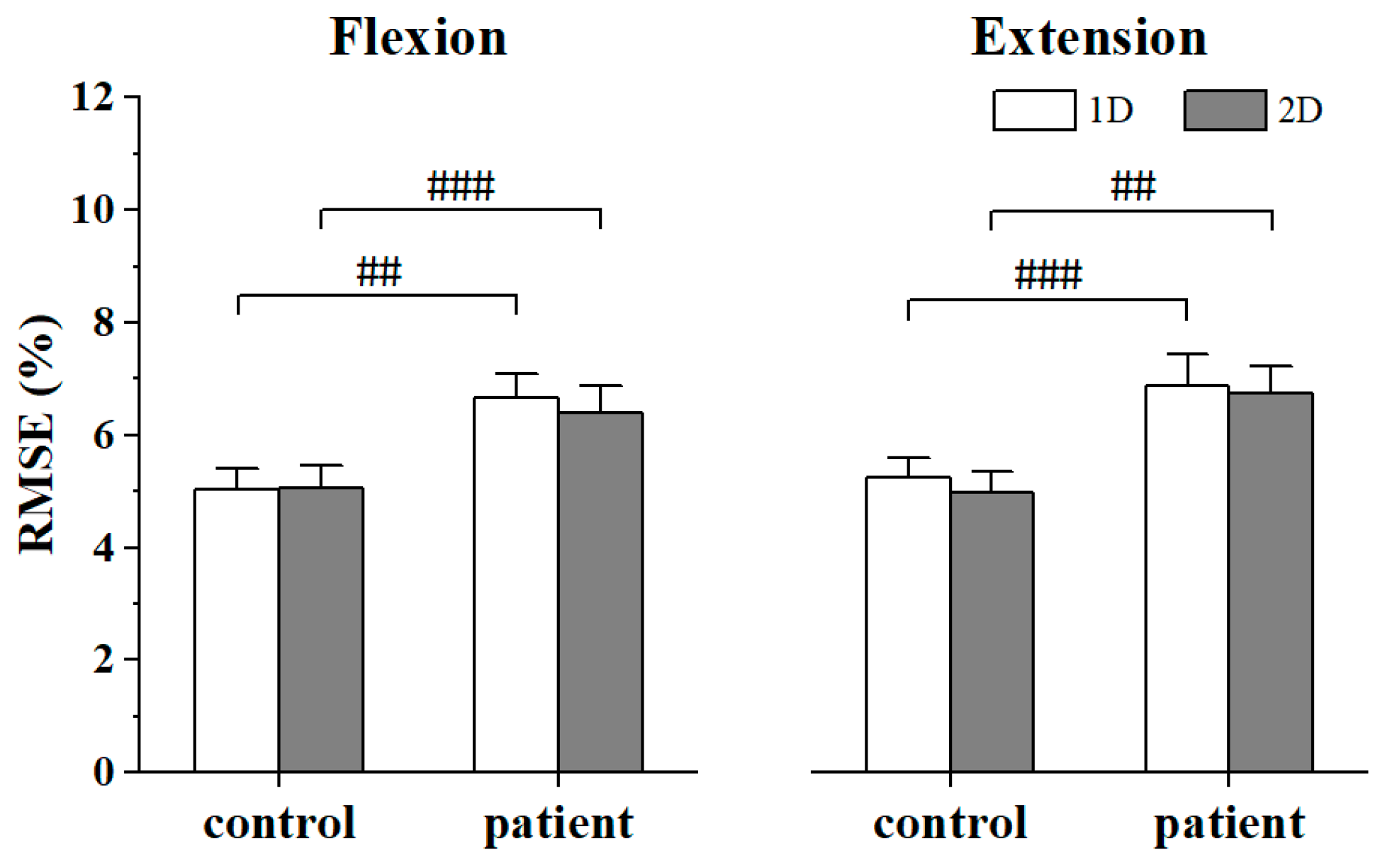
3. Results
4. Discussion
4.1. Effect of Stroke on the Intermuscular Coupling of the Synergistic Muscles
4.2. Effect of MCI Dimensionality to the Intermuscular Coupling in Alpha and Beta Band
4.3. Clinical Implication and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, S.H. Optimal feedback control and the neural basis of volitional motor control. Nat. Rev. Neurosci. 2004, 5, 534–546. [Google Scholar] [CrossRef]
- Zhu, L.L.; Lindenberg, R.; Alexander, M.P.; Schlaug, G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010, 41, 910–915. [Google Scholar] [CrossRef]
- Kirker, S.G.B.; Simpson, D.S.; Jenner, J.R.; Wing, A.M. Stepping before standing: Hip muscle function in stepping and standing balance after stroke. J. Neurol. Neurosurg. Psychiatry 2000, 68, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Hidler, J.M.; Carroll, M.; Federovich, E.H. Strength and coordination in the paretic leg of individuals following acute stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Solomon, J.M.; Shah, A.; Blanchette, A.K.; Feldman, A.G. Activation of elbow extensors during passive stretch of flexors in patients with post-stroke spasticity. Clin. Neurophysiol. 2018, 129, 2065–2074. [Google Scholar] [CrossRef]
- Kisiel-Sajewicz, K.; Fang, Y.; Hrovat, K.; Yue, G.H.; Siemionow, V.; Sun, C.K.; Jaskólska, A.; Jaskólski, A.; Sahgal, V.; Daly, J.J. Weakening of synergist muscle coupling during reaching movement in stroke patients. Neurorehabilit. Neural Repair 2011, 25, 359–368. [Google Scholar] [CrossRef]
- Tian, N.; Chen, Y.; Sun, W.; Liu, H.; Wang, X.; Yan, T.; Song, R. Investigating the stroke- and aging-related changes in global and instantaneous intermuscular coupling using cross-fuzzy entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1573–1582. [Google Scholar] [CrossRef]
- Yu, H.R.; Xu, W.L.; Zhuang, Y.; Tong, K.Y.; Song, R. Wavelet coherence analysis of muscle coupling during reaching movement in stroke. Comput. Biol. Med. 2021, 131, 104263. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yu, X.-M.; Shao, Q.-N.; Wang, C.; Yang, H.; Huang, S.-J.; Niu, W.-X. Muscle fatigue enhance beta band EMG-EMG coupling of antagonistic muscles in patients with post-stroke spasticity. Front. Bioeng. Biotechnol. 2020, 8, 1007. [Google Scholar] [CrossRef]
- Luo, J.; Sun, W.; Wu, Y.; Liu, H.; Wang, X.; Yan, T.; Song, R. Characterization of the coordination of agonist and antagonist muscles among stroke patients, healthy late middle-aged and young controls using a myoelectric-controlled interface. J. Neural Eng. 2018, 15, 056015. [Google Scholar] [CrossRef]
- Colamarino, E.; de Seta, V.; Masciullo, M.; Cincotti, F.; Mattia, D.; Pichiorri, F.; Toppi, J. Corticomuscular and intermuscular coupling in simple hand movements to enable a hybrid brain–computer interface. Int. J. Neural Syst. 2021, 31, 2150052. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.; Kwon, H.J.; Shin, J.H.; Roh, J.; Park, H.S. Feasibility of isokinetic training to modify coupling of upper limb muscle synergy activation in stroke-affected upper limb. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar]
- Dewald, J.P.A.; Pope, P.S.; Given, J.D.; Buchanan, T.S.; Rymer, W.Z. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 1995, 118, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.C.; Fitts, S.S.; Kraft, G.H.; Nutter, P.B.; Mj, T.; Lm, R. Co-contraction in the hemiparetic forearm: Quantitative EMG evaluation. Arch. Phys. Med. Rehabil. 1988, 69, 348–351. [Google Scholar] [PubMed]
- Liu, J.B.; Wang, J.X.; Tan, G.S.; Sheng, Y.X.; Chang, H.; Xie, Q.; Liu, H.H. Correlation evaluation of functional corticomuscular coupling with abnormal muscle synergy after stroke. IEEE Trans. Biomed. Eng. 2021, 68, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.F.; Gibbs, J.; Halliday, D.M.; Harrison, L.M.; James, L.M.; Mayston, M.J.; Stephens, J.A. Changes in EMG coherence between long and short thumb abductor muscles during human development. J. Physiol. 2007, 579, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Santoso, S.; Powers, E.J.; Bengtson, R.D.; Ouroua, A. Time-series analysis of nonstationary plasma fluctuations using wavelet transforms. Rev. Sci. Instrum. 1997, 68, 898–901. [Google Scholar] [CrossRef]
- Qassim, Y.T.; Cutmore, T.R.H.; James, D.A.; Rowlands, D.D. Wavelet coherence of EEG signals for a visual oddball task. Comput. Biol. Med. 2013, 43, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Marsden, J.F.; Brown, P.; Salenius, S. Involvement of the sensorimotor cortex in physiological force and action tremor. Neuroreport 2001, 12, 1937–1941. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, M.; Tao, H.; Song, X.; Shao, Q.; Wang, C.; Yang, H.; Niu, W.; Chen, Y. A comparison of muscle activation and concomitant intermuscular coupling of antagonist muscles among bench presses with different instability degrees in untrained men. Front. Physiol. 2022, 13, 940719. [Google Scholar] [CrossRef]
- Farmer, S.F.; Bremner, F.D.; Halliday, D.M.; Rosenberg, J.R.; Stephens, J.A. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J. Physiol. 1993, 470, 127–155. [Google Scholar] [CrossRef]
- Vallbo, A.B.; Wessberg, J. Organization of motor output in slow finger movements in man. J. Physiol. 1993, 469, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Kilner, J.M.; Baker, S.N.; Salenius, S.; Jousmäki, V.; Hari, R.; Lemon, R.N. Task-dependent modulation of 15-30 Hz coherence between rectified emgs from human hand and forearm muscles. J. Physiol. 1999, 516 Pt 2, 559–570. [Google Scholar] [CrossRef]
- Grosse, P.; Cassidy, M.J.; Brown, P. EEG–EMG, meg–EMG and EMG–EMG frequency analysis: Physiological principles and clinical applications. Clin. Neurophysiol. 2002, 113, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Delcamp, C.; Cormier, C.; Chalard, A.; Amarantini, D.; Gasq, D. Changes in intermuscular connectivity during active elbow extension reveal a functional simplification of motor control after stroke. Front. Neurosci. 2022, 16, 940907. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.D.; Der-Yeghiaian, L.; Le, V.; Motiwala, R.R.; Cramer, S.C. Robot-based hand motor therapy after stroke. Brain 2007, 131, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Tariq, H.; Afridi, A.; Rathore, F.A. Technological advancements in stroke rehabilitation. J. Pak. Med. Assoc. 2022, 72, 1672–1674. [Google Scholar] [PubMed]
- Cancel, N.; Fischer, M.; Gulotta, L.; Koo, J.; McKittrick, S. Moderate aerobic exercise has an inconclusive effect on fine motor control. J. Adv. Stud. Sci. 2014. Available online: https://minds.wisconsin.edu/bitstream/handle/1793/80038/Moderate%20Aerobic%20Exercise%20has%20an%20Inconclusive%20Effect%20on%20Fine%20Motor%20Control.pdf?sequence=1&isAllowed=y (accessed on 5 August 2024).
- Charissou, C.; Vigouroux, L.; Berton, E.; Amarantini, D. Fatigue- and training-related changes in ‘beta’ intermuscular interactions between agonist muscles. J. Electromyogr. Kinesiol. 2016, 27, 52–59. [Google Scholar] [CrossRef]
- Charissou, C.; Amarantini, D.; Baurès, R.; Berton, E.; Vigouroux, L. Effects of hand configuration on muscle force coordination, co-contraction and concomitant intermuscular coupling during maximal isometric flexion of the fingers. Eur. J. Appl. Physiol. 2017, 117, 2309–2320. [Google Scholar] [CrossRef]
- Patel, P.; Kaingade, S.R.; Wilcox, A.; Lodha, N. Force control predicts fine motor dexterity in high-functioning stroke survivors. Neurosci. Lett. 2020, 729, 135015. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, L.L.; Lin, Y.-T.; Hu, C.-L.; Hwang, I.-S. Variations in static force control and motor unit behavior with error amplification feedback in the elderly. Front. Hum. Neurosci. 2017, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Lee, S.W.; Harris-Love, M.L.; Lum, P.S. Neural coupling between homologous muscles during bimanual tasks: Effects of visual and somatosensory feedback. J. Neurophysiol. 2017, 117, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The extraction of neural information from the surface EMG for the control of upper-limb prostheses: Emerging avenues and challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Mugler, E.M.; Tomic, G.; Singh, A.; Hameed, S.; Lindberg, E.W.; Gaide, J.; Alqadi, M.; Robinson, E.; Dalzotto, K.; Limoli, C.; et al. Myoelectric computer interface training for reducing co-activation and enhancing arm movement in chronic stroke survivors: A randomized trial. Neurorehabilit. Neural Repair 2019, 33, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Kishta, A.; Mugler, E.; Slutzky, M.W.; Roh, J. Myoelectric interface training enables targeted reduction in abnormal muscle co-activation. J. Neuroeng. Rehabil. 2022, 19, 67. [Google Scholar] [CrossRef]
- Jian, C.Y.; Deng, L.C.; Liu, H.H.; Yan, T.B.; Wang, X.Y.; Song, R. Modulating and restoring inter-muscular coordination in stroke patients using two-dimensional myoelectric computer interface: A cross-sectional and longitudinal study. J. Neural Eng. 2021, 18, 036005. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Liu, H.; Deng, L.; Wang, X.; Yan, T.; Song, R. Stroke-induced alteration in multi-layer information transmission of cortico-motor system during elbow isometric contraction modulated by myoelectric-controlled interfaces. J. Neural Eng. 2021, 18, 0460e1. [Google Scholar] [CrossRef]
- Kennedy, M.W.; Crowell, C.R.; Striegel, A.D.; Villano, M.; Schmiedeler, J.P. Relative efficacy of various strategies for visual feedback in standing balance activities. Exp. Brain Res. 2013, 230, 117–125. [Google Scholar] [CrossRef]
- Mista, C.A.; Christensen, S.W.; Graven-Nielsen, T. Modulation of motor variability related to experimental muscle pain during elbow-flexion contractions. Hum. Mov. Sci. 2015, 39, 222–235. [Google Scholar] [CrossRef]
- Missenard, O.; Mottet, D.; Perrey, S. The role of cocontraction in the impairment of movement accuracy with fatigue. Exp. Brain Res. 2008, 185, 151–156. [Google Scholar] [CrossRef]
- Gordon, K.E.; Ferris, D.P. Proportional myoelectric control of a virtual object to investigate human efferent control. Exp. Brain Res. 2004, 159, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Rhif, M.; Ben Abbes, A.; Farah, I.R.; Martínez, B.; Sang, Y. Wavelet transform application for/in non-stationary time-series analysis: A review. Appl. Sci. 2019, 9, 1345. [Google Scholar] [CrossRef]
- Espenhahn, S.; Rossiter, H.E.; van Wijk, B.C.M.; Redman, N.; Rondina, J.M.; Diedrichsen, J.; Ward, N.S. Sensorimotor cortex beta oscillations reflect motor skill learning ability after stroke. Brain Commun. 2020, 2, fcaa161. [Google Scholar] [CrossRef] [PubMed]
- Zackowski, K.M.; Dromerick, A.W.; Sahrmann, S.A.; Thach, W.T.; Bastian, A.J. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain 2004, 127, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Mordkoff, J.T.; Danek, R.H. Dividing attention between color and shape revisited: Redundant targets coactivate only when parts of the same perceptual object. Atten. Percept. Psychophys. 2011, 73, 103–112. [Google Scholar] [CrossRef]
- Young, S.J.; van Doornik, J.; Sanger, T.D. Visual feedback reduces co-contraction in children with dystonia. J. Child Neurol. 2011, 26, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fellows, S.J.; Kaus, C.; Thilmann, A.F. Voluntary movement at the elbow in spastic hemiparesis. Ann. Neurol. 1994, 36, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.H.; Zibrandtsen, I.C.; Wienecke, T.; Kjaer, T.W.; Christensen, M.S.; Nielsen, J.B.; Langberg, H. Corticomuscular coherence in the acute and subacute phase after stroke. Clin. Neurophysiol. 2017, 128, 2217–2226. [Google Scholar] [CrossRef]
- Houston, M.; Li, X.Y.; Zhou, P.; Li, S.; Roh, J.; Zhang, Y.C. Alterations in muscle networks in the upper extremity of chronic stroke survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1026–1034. [Google Scholar] [CrossRef]
- Canning, C.G.; Ada, L.; O’Dwyer, N.J. Abnormal muscle activation characteristics associated with loss of dexterity after stroke. J. Neurol. Sci. 2000, 176, 45–56. [Google Scholar] [CrossRef]
- Delcamp, C.; Gasq, D.; Cormier, C.; Amarantini, D. Corticomuscular and intermuscular coherence are correlated after stroke: A simplified motor control? Brain Commun. 2023, 5, fcad187. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Calautti, C.; Pauri, F.; Baron, J.C. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003, 2, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, W.; Yao, W.; Qi, W.; Chen, X.; Xie, B.; Xie, P. Analysis of multichannel intermuscular coupling characteristics during rehabilitation after stroke. J. Biomed. Eng. 2019, 36, 720–727. [Google Scholar]
- Houston, M.; Li, R.; Roh, J.; Zhang, Y. Altered muscle networks in post-stroke survivors. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3771–3774. [Google Scholar]
- Volkmann, J.; Joliot, M.; Mogilner, A.Y.; Ioannides, A.A.; Lado, F.A.; Fazzini, E.; Ribary, U.; Llinás, R.R. Central motor loop oscillations in parkinsonian resting tremor revealed magnetoencephalography. Neurology 1996, 46, 1359. [Google Scholar] [CrossRef] [PubMed]
- Mima, T.; Hallett, M. Corticomuscular coherence: A review. J. Clin. Neurophysiol. 1999, 16, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Power, H.A.; Norton, J.A.; Porter, C.L.; Doyle, Z.; Hui, I.; Chan, K.M. Transcranial direct current stimulation of the primary motor cortex affects cortical drive to human musculature as assessed by intermuscular coherence. J. Physiol. 2006, 577, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.M.; Zaaimi, B.; Williams, T.L.; Baker, S.N.; Baker, M.R. Beta-band intermuscular coherence: A novel biomarker of upper motor neuron dysfunction in motor neuron disease. Brain 2012, 135, 2849–2864. [Google Scholar] [CrossRef] [PubMed]
- Karbasforoushan, H.; Cohen-Adad, J.; Dewald, J.P.A. Brainstem and spinal cord mri identifies altered sensorimotor pathways post-stroke. Nat. Commun. 2019, 10, 3524. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, X.; Fox, J.; Jefferys, J.G. Interaction dynamics of neuronal oscillations analysed using wavelet transforms. J. Neurosci. Methods 2007, 160, 178–185. [Google Scholar] [CrossRef]
- Watanabe, T.; Nojima, I.; Mima, T.; Sugiura, H.; Kirimoto, H. Magnification of visual feedback modulates corticomuscular and intermuscular coherences differently in young and elderly adults. NeuroImage 2020, 220, 117089. [Google Scholar] [CrossRef]
- Wright, Z.A.; Rymer, W.Z.; Slutzky, M.W. Reducing abnormal muscle coactivation after stroke using a myoelectric-computer interface: A pilot study. Neurorehabilit. Neural Repair 2014, 28, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Salmelin, R.; Hari, R. Characterization of spontaneous meg rhythms in healthy adults. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.R.; Croft, R.J.; Dominey, S.J.J.; Burgess, A.P.; Gruzelier, J.H. Paradox lost? Exploring the role of alpha oscillations during externally vs. Internally directed attention and the implications for idling and inhibition hypotheses. Int. J. Psychophysiol. 2003, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kranczioch, C.; Debener, S.; Maye, A.; Engel, A.K. Temporal dynamics of access to consciousness in the attentional blink. NeuroImage 2007, 37, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Puh, U.; Vovk, A.; Sevsek, F.; Suput, D. Increased cognitive load during simple and complex motor tasks in acute stage after stroke. Int. J. Psychophysiol. 2007, 63, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Meester, D.; Al-Yahya, E.; Dawes, H.; Martin-Fagg, P.; Piñon, C. Associations between prefrontal cortex activation and h-reflex modulation during dual task gait. Front. Hum. Neurosci. 2014, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.B.; Kang, N.; Misra, G.; Marble, S.; Patten, C.; Coombes, S.A. Visual feedback alters force control and functional activity in the visuomotor network after stroke. NeuroImage Clin. 2018, 17, 505–517. [Google Scholar] [CrossRef]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting cortical activity at beta-band frequencies slows movement in humans. Curr. Biol. 2009, 19, 1637–1641. [Google Scholar] [CrossRef]



| Patients | Sex | Age | Lesion Side | Type of Stroke | Months after Stroke | UE-FMA Score (0–66) |
|---|---|---|---|---|---|---|
| Patient 1 | M | 53 | L | Hemo | 1.5 | 23 |
| Patient 2 | F | 61 | R | Hemo | 18 | 47 |
| Patient 3 | F | 53 | L | Hemo | 1.5 | 35 |
| Patient 4 | M | 27 | R | Isch | 1 | 23 |
| Patient 5 | M | 25 | L | Isch | 8 | 19 |
| Patient 6 | M | 38 | R | Isch | 3 | 61 |
| Patient 7 | M | 71 | R | Hemo | 12 | 54 |
| Patient 8 | M | 52 | L | Isch | 5 | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Sun, W.; Song, R.; Xu, W. Wavelet Coherence Analysis of Post-Stroke Intermuscular Coupling Modulated by Myoelectric-Controlled Interfaces. Bioengineering 2024, 11, 802. https://doi.org/10.3390/bioengineering11080802
He X, Sun W, Song R, Xu W. Wavelet Coherence Analysis of Post-Stroke Intermuscular Coupling Modulated by Myoelectric-Controlled Interfaces. Bioengineering. 2024; 11(8):802. https://doi.org/10.3390/bioengineering11080802
Chicago/Turabian StyleHe, Xinyi, Wenbo Sun, Rong Song, and Weiling Xu. 2024. "Wavelet Coherence Analysis of Post-Stroke Intermuscular Coupling Modulated by Myoelectric-Controlled Interfaces" Bioengineering 11, no. 8: 802. https://doi.org/10.3390/bioengineering11080802
APA StyleHe, X., Sun, W., Song, R., & Xu, W. (2024). Wavelet Coherence Analysis of Post-Stroke Intermuscular Coupling Modulated by Myoelectric-Controlled Interfaces. Bioengineering, 11(8), 802. https://doi.org/10.3390/bioengineering11080802






