Peritoneal Infusion of Oxygen Microbubbles Alters the Metabolomic Profile of the Lung and Spleen in Acute Hypoxic Exposure
Abstract
1. Introduction
2. Methods
2.1. Oxygen Microbubble (OMB) Preparation
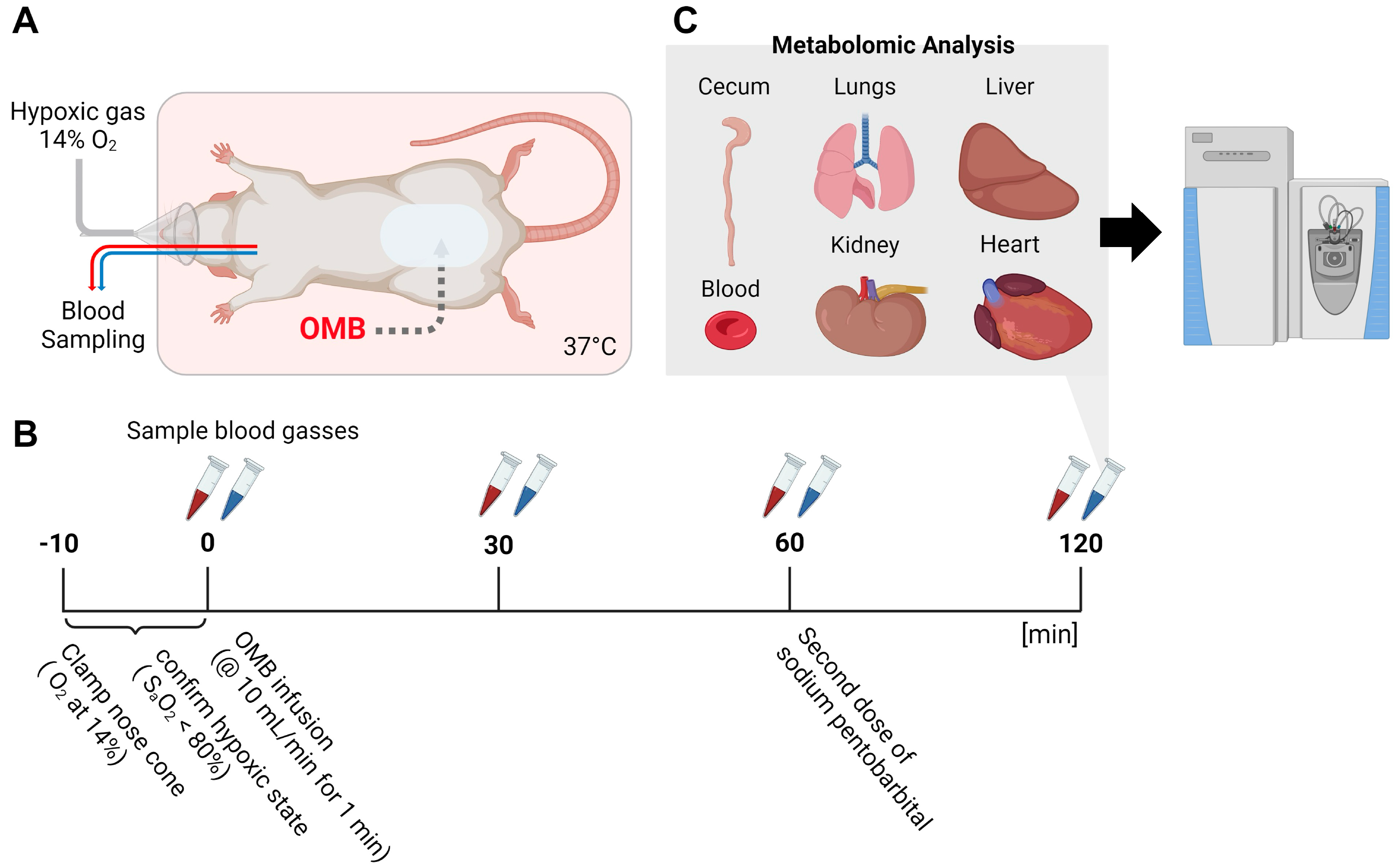
2.2. Hypoxia Model and Treatment by Peritoneal Infusion of OMBs
2.3. Metabolomic Analysis
2.4. Statistical Analysis
3. Results
3.1. Morphological Changes in Intraperitoneal OMBs
3.2. OMBs Influence Arterial PO2 and Oxygen Saturation
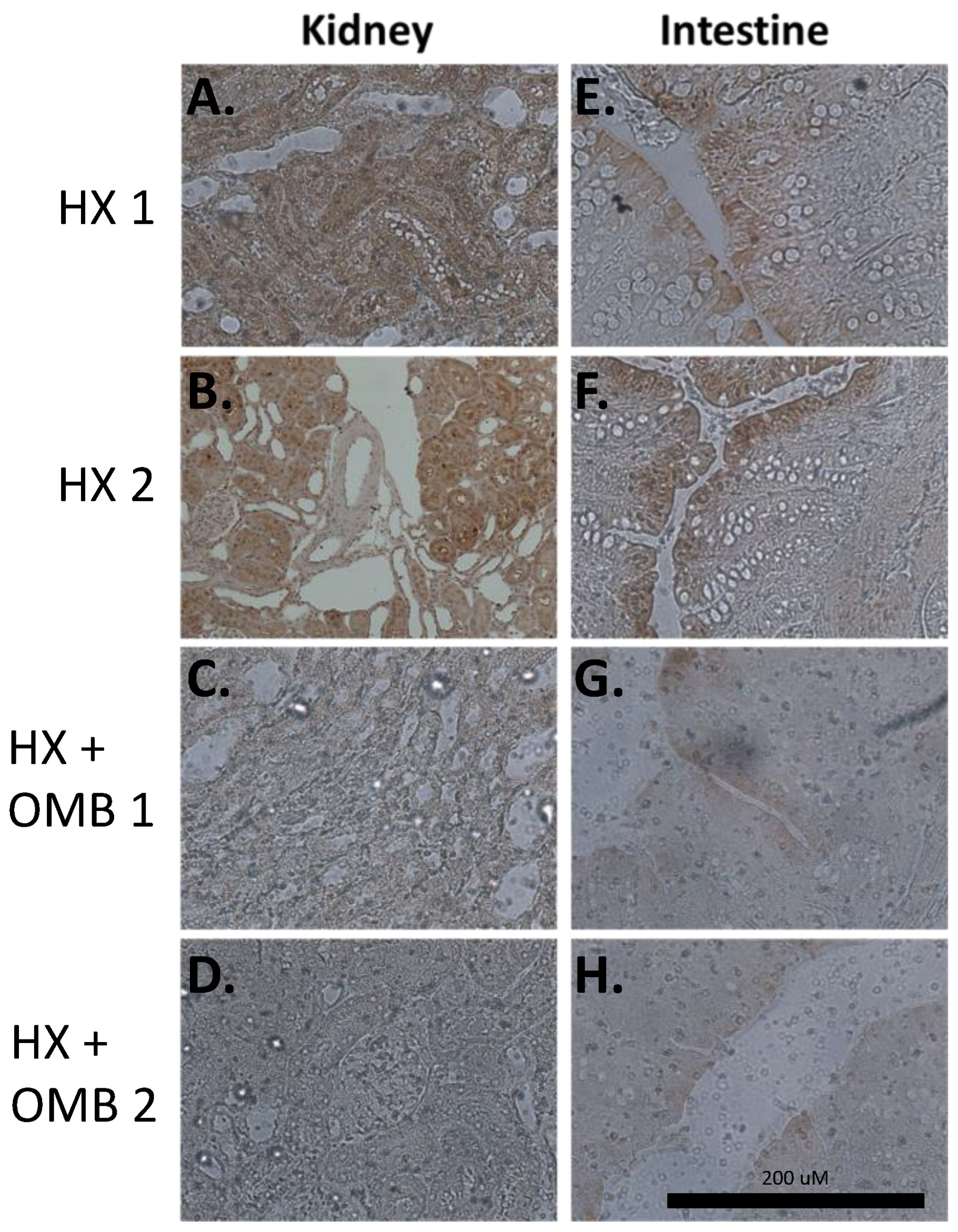
3.3. OMBs Show Histological Changes for Attenuating Microenvironment Tissue Hypoxia
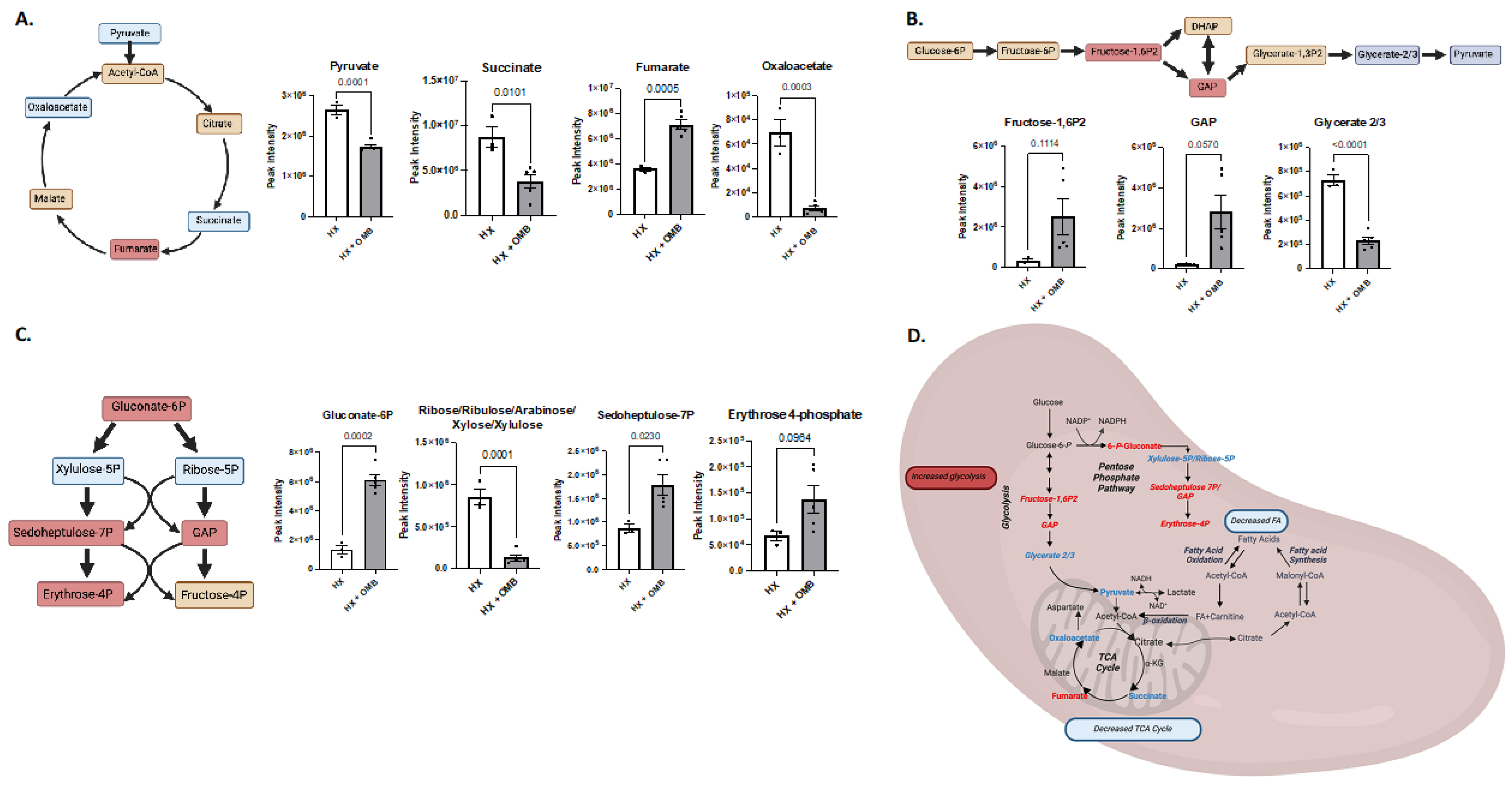
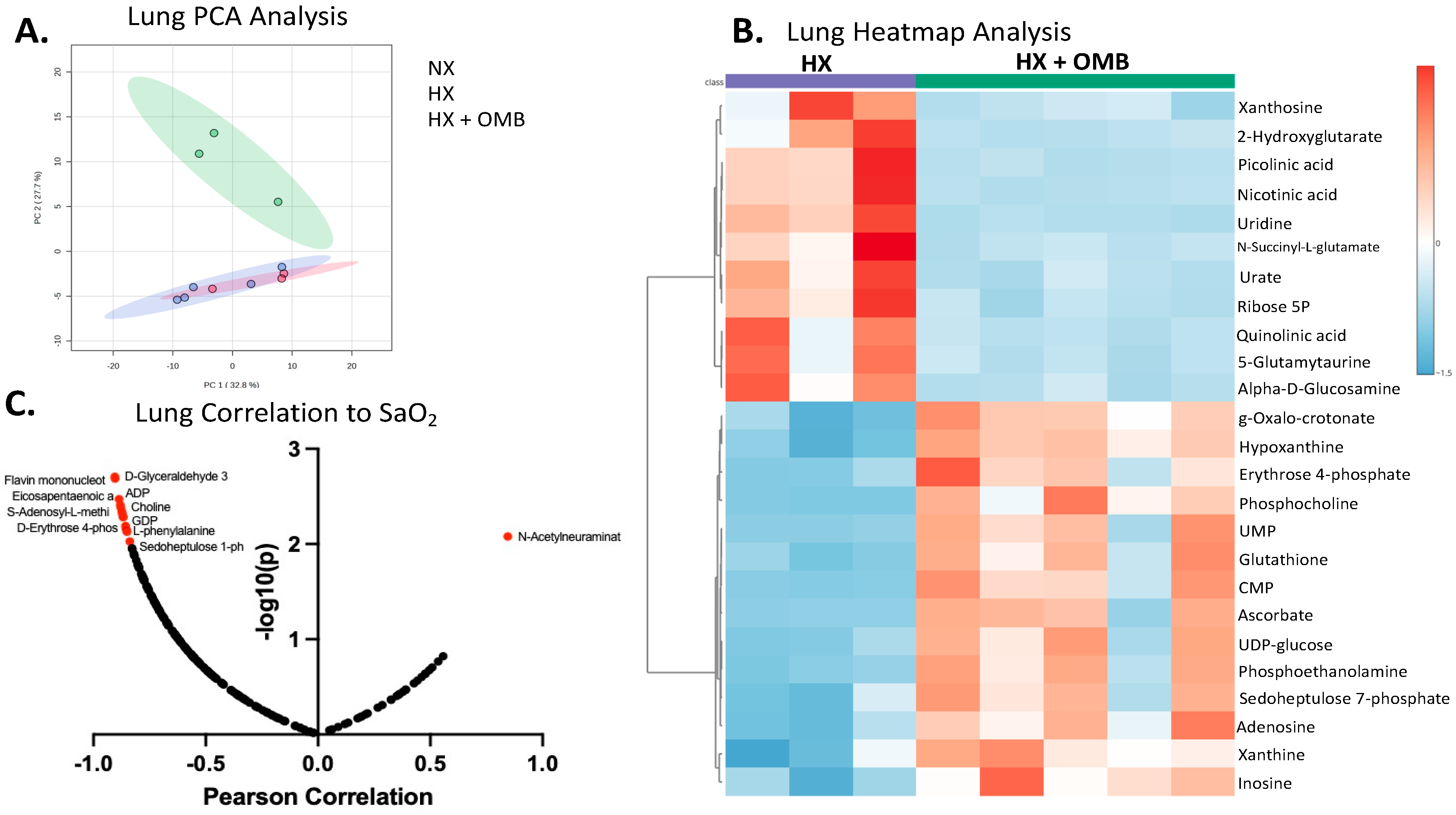
3.4. OMBs Alter Hypoxic-Induced Changes in Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grieco, D.L.; Maggiore, S.M.; Roca, O.; Spinelli, E.; Patel, B.K.; Thille, A.W.; Barbas, C.S.V.; de Acilu, M.G.; Cutuli, S.L.; Bongiovanni, F.; et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021, 47, 851–866. [Google Scholar] [CrossRef]
- Mountford, P.A.; Leiphrakpam, P.D.; Weber, H.R.; McCain, A.; Scribner, R.T.; Duarte, E.M.; Chen, J.; Noe, D.; Borden, M.A.; Buesing, K.L. Colonic oxygen microbubbles augment systemic oxygenation and CO2 removal in a porcine smoke inhalation model of severe hypoxia. Intensive Care Med. Exp. 2023, 11, 35. [Google Scholar] [CrossRef]
- Saguil, A.; Fargo, M.V. Acute Respiratory Distress Syndrome: Diagnosis and Management. Am. Fam. Physician 2020, 101, 730–738. [Google Scholar] [PubMed Central]
- Cirovic, A.; Denic, A.; Clarke, B.L.; Vassallo, R.; Cirovic, A.; Landry, G.M. A hypoxia-driven occurrence of chronic kidney disease and osteoporosis in COPD individuals: New insights into environmental cadmium exposure. Toxicology 2022, 482, 153355. [Google Scholar] [CrossRef]
- Lodge, K.M.; Vassallo, A.; Liu, B.; Long, M.; Tong, Z.; Newby, P.R.; Agha-Jaffar, D.; Paschalaki, K.; Green, C.E.; Belchamber, K.B.R.; et al. Hypoxia Increases the Potential for Neutrophil-mediated Endothelial Damage in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2022, 205, 903–916. [Google Scholar] [CrossRef]
- Gayen, S.; Dachert, S.; Lashari, B.H.; Gordon, M.; Desai, P.; Criner, G.J.; Cardet, J.C.; Shenoy, K. Critical Care Management of Severe Asthma Exacerbations. J. Clin. Med. 2024, 13, 859. [Google Scholar] [CrossRef]
- El Alam, S.; Pena, E.; Aguilera, D.; Siques, P.; Brito, J. Inflammation in Pulmonary Hypertension and Edema Induced by Hypobaric Hypoxia Exposure. Int. J. Mol. Sci. 2022, 23, 12656. [Google Scholar] [CrossRef]
- Pena, E.; El Alam, S.; Siques, P.; Brito, J. Oxidative Stress and Diseases Associated with High-Altitude Exposure. Antioxidants 2022, 11, 267. [Google Scholar] [CrossRef]
- Korbecki, J.; Siminska, D.; Gassowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef]
- Hernansanz-Agustin, P.; Choya-Foces, C.; Martinez-Ruiz, A. Measurement of Superoxide Production in Acute Hypoxia by Fixed-Cell Microscopy. Methods Mol. Biol. 2021, 2202, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; An, S.H.; Gwark, J.Y.; Park, H.B. Antioxidant effects on hypoxia-induced oxidative stress and apoptosis in rat rotator cuff fibroblasts. Eur. Cell Mater. 2021, 41, 680–693. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Huang, W. MicroRNA-369 attenuates hypoxia-induced cardiomyocyte apoptosis and inflammation via targeting TRPV3. Braz. J. Med. Biol. Res. 2021, 54, e10550. [Google Scholar] [CrossRef]
- Midha, A.D.; Zhou, Y.; Queliconi, B.B.; Barrios, A.M.; Haribowo, A.G.; Chew, B.T.; Fong, C.O.; Blecha, J.E.; VanBrocklin, H.; Seo, Y.; et al. Organ-specific fuel rewiring in acute and chronic hypoxia redistributes glucose and fatty acid metabolism. Cell Metab. 2023, 35, 504–516.e505. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E.J.; Mohan, V.; Kheir, J.; Borden, M.A. Phospholipid-stabilized microbubble foam for injectable oxygen delivery. Langmuir 2010, 26, 15726–15729. [Google Scholar] [CrossRef]
- Kheir, J.N.; Scharp, L.A.; Borden, M.A.; Swanson, E.J.; Loxley, A.; Reese, J.H.; Black, K.J.; Velazquez, L.A.; Thomson, L.M.; Walsh, B.K.; et al. Oxygen gas-filled microparticles provide intravenous oxygen delivery. Sci. Transl. Med. 2012, 4, 140ra188. [Google Scholar] [CrossRef]
- Black, K.J.; Lock, A.T.; Thomson, L.M.; Cole, A.R.; Tang, X.; Polizzotti, B.D.; Kheir, J.N. Hemodynamic Effects of Lipid-Based Oxygen Microbubbles via Rapid Intravenous Injection in Rodents. Pharm. Res. 2017, 34, 2156–2162. [Google Scholar] [CrossRef]
- Feshitan, J.A.; Legband, N.D.; Borden, M.A.; Terry, B.S. Systemic oxygen delivery by peritoneal perfusion of oxygen microbubbles. Biomaterials 2014, 35, 2600–2606. [Google Scholar] [CrossRef]
- Mohammed, R.U.R.; Zollinger, N.T.; McCain, A.R.; Romaguera-Matas, R.; Harris, S.P.; Buesing, K.L.; Borden, M.A.; Terry, B.S. Testing oxygenated microbubbles via intraperitoneal and intrathoracic routes on a large pig model of LPS-induced acute respiratory distress syndrome. Physiol. Rep. 2022, 10, e15451. [Google Scholar] [CrossRef]
- Legband, N.D.; Feshitan, J.A.; Borden, M.A.; Terry, B.S. Evaluation of peritoneal microbubble oxygenation therapy in a rabbit model of hypoxemia. IEEE Trans. Biomed. Eng. 2015, 62, 1376–1382. [Google Scholar] [CrossRef]
- Fiala, A.; Slagle, C.; Legband, N.; Aghabaglou, F.; Buesing, K.; Borden, M.; Harris, S.; Terry, B. Treatment of a Rat Model of LPS-Induced ARDS via Peritoneal Perfusion of Oxygen Microbubbles. J. Surg. Res. 2020, 246, 450–456. [Google Scholar] [CrossRef]
- Leiphrakpam, P.D.; Weber, H.R.; Foster, K.W.; Buesing, K.L. Intraperitoneal oxygen microbubble therapy: A novel approach to enhance systemic oxygenation in a smoke inhalation model of acute hypoxic respiratory failure. Surg. Open Sci. 2023, 16, 127–133. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Carpena, M.; Garcia-Oliveira, P.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Analytical Metabolomics and Applications in Health, Environmental and Food Science. Crit. Rev. Anal. Chem. 2022, 52, 712–734. [Google Scholar] [CrossRef]
- Rauckhorst, A.J.; Borcherding, N.; Pape, D.J.; Kraus, A.S.; Scerbo, D.A.; Taylor, E.B. Mouse tissue harvest-induced hypoxia rapidly alters the in vivo metabolome, between-genotype metabolite level differences, and 13C-tracing enrichments. Mol. Metab. 2022, 66, 101596. [Google Scholar] [CrossRef]
- Carneiro, T.J.; Araújo, R.; Vojtek, M.; Gonçalves-Monteiro, S.; Diniz, C.; de Carvalho, A.L.B.; Marques, M.P.M.; Gil, A.M. Multi-Organ NMR Metabolomics to Assess In Vivo Overall Metabolic Impact of Cisplatin in Mice. Metabolites 2019, 9, 279. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, Y.A.; Dhillon, R.; Dowell, J.; Denu, J.M.; Pamenter, M.E. Metabolomic Analysis of Carbohydrate and Amino Acid Changes Induced by Hypoxia in Naked Mole-Rat Brain and Liver. Metabolites 2022, 12, 56. [Google Scholar] [CrossRef]
- Nemkov, T.; Reisz, J.A.; Gehrke, S.; Hansen, K.C.; D’Alessandro, A. High-Throughput Metabolomics: Isocratic and Gradient Mass Spectrometry-Based Methods. Methods Mol. Biol. 2019, 1978, 13–26. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Zhou, G.; Pang, Z.; Lu, Y.; Ewald, J.; Xia, J. OmicsNet 2.0: A web-based platform for multi-omics integration and network visual analytics. Nucleic Acids Res. 2022, 50, W527–W533. [Google Scholar] [CrossRef]
- Kwan, J.J.; Borden, M.A. Lipid monolayer collapse and microbubble stability. Adv. Colloid Interface Sci. 2012, 183–184, 82–99. [Google Scholar] [CrossRef]
- Evans, S.M.; Kim, K.; Moore, C.E.; Uddin, I.; Capozzi, M.E.; Craft, J.R.; Sulikowski, G.A.; Jayagopal, A. Molecular probes for imaging of hypoxia in the retina. Bioconjug. Chem. 2014, 25, 2030–2037. [Google Scholar] [CrossRef]
- Bensaad, K.; Favaro, E.; Lewis, C.A.; Peck, B.; Lord, S.; Collins, J.M.; Pinnick, K.E.; Wigfield, S.; Buffa, F.M.; Li, J.-L.; et al. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014, 9, 349–365. [Google Scholar] [CrossRef]
- Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism. Cells 2019, 8, 214. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Dematteo, R.G.; Venneti, S.; Finley, L.W.; Lu, C.; Judkins, A.R.; Rustenburg, A.S.; Grinaway, P.B.; Chodera, J.D.; Cross, J.R.; et al. Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell Metab. 2015, 22, 304–311. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Brune, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef]
- Gessner, P.; Lum, J.; Frenguelli, B.G. The mammalian purine salvage pathway as an exploitable route for cerebral bioenergetic support after brain injury. Neuropharmacology 2023, 224, 109370. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, Q.; Xu, J.; Sellers, H.G.; Brown, Z.L.; Liu, Z.; Bordan, Z.; Shi, X.; Zhao, D.; Cai, Y.; et al. Purine synthesis suppression reduces the development and progression of pulmonary hypertension in rodent models. Eur. Heart J. 2023, 44, 1265–1279. [Google Scholar] [CrossRef]
- Tabata, S.; Umemura, S.; Narita, M.; Udagawa, H.; Ishikawa, T.; Tsuboi, M.; Goto, K.; Ishii, G.; Tsuchihara, K.; Ochiai, A.; et al. Metabolic Hallmarks for Purine Nucleotide Biosynthesis in Small Cell Lung Carcinoma. Mol. Cancer Res. 2024, 22, 82–93. [Google Scholar] [CrossRef]
- Sant’ana, P.G.; de Tomasi, L.C.; Murata, G.M.; Vileigas, D.F.; Mota, G.A.F.; de Souza, S.L.B.; Silva, V.L.; de Campos, L.P.; Okoshi, K.; Padovani, C.R.; et al. Hypoxia-Inducible Factor 1-Alpha and Glucose Metabolism during Cardiac Remodeling Progression from Hypertrophy to Heart Failure. Int. J. Mol. Sci. 2023, 24, 6201. [Google Scholar] [CrossRef]
- Cooper, A.J.L.; Hanigan, M.H. Metabolism of Glutathione S-Conugates: Multiple Pathways. Compr. Toxicol. 2017, 10, 363–406. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, T.; Sun, T.; Xie, F.; Jiang, X.; Huang, X.; Zhang, J.; Zhang, X.; Yu, A. Current status of spleen-mediated inflammatory response in traumatic acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Deng, R.; Tang, J.; Zhang, B.; Tang, Y.; Wang, J.K.; Li, F.; Anderson, V.M.; McNutt, M.A.; Gu, J. The spleen as a target in severe acute respiratory syndrome. FASEB J. 2006, 20, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Drakopanagiotakis, F.; Xifteri, A.; Polychronopoulos, V.; Bouros, D. Apoptosis in lung injury and fibrosis. Eur. Respir. J. 2008, 32, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Mirchandani, A.S.; Jenkins, S.J.; Bain, C.C.; Sanchez-Garcia, M.A.; Lawson, H.; Coelho, P.; Murphy, F.; Griffith, D.M.; Zhang, A.; Morrison, T.; et al. Hypoxia shapes the immune landscape in lung injury and promotes the persistence of inflammation. Nat. Immunol. 2022, 23, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Guagliardo, R.; Perez-Gil, J.; De Smedt, S.; Raemdonck, K. Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins. J. Control. Release 2018, 291, 116–126. [Google Scholar] [CrossRef]
- Okabe, R.; Chen-Yoshikawa, T.F.; Yoneyama, Y.; Yokoyama, Y.; Tanaka, S.; Yoshizawa, A.; Thompson, W.L.; Kannan, G.; Kobayashi, E.; Date, H.; et al. Mammalian enteral ventilation ameliorates respiratory failure. Med 2021, 2, 773–783.e5. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisk, C.; Fan, A.; Cendali, F.I.; Kakiuchi, K.; Swindle, D.; Pak, D.I.; Tolson, R.; Grier, A.; Buesing, K.; Zaeske, S.; et al. Peritoneal Infusion of Oxygen Microbubbles Alters the Metabolomic Profile of the Lung and Spleen in Acute Hypoxic Exposure. Bioengineering 2024, 11, 761. https://doi.org/10.3390/bioengineering11080761
Lisk C, Fan A, Cendali FI, Kakiuchi K, Swindle D, Pak DI, Tolson R, Grier A, Buesing K, Zaeske S, et al. Peritoneal Infusion of Oxygen Microbubbles Alters the Metabolomic Profile of the Lung and Spleen in Acute Hypoxic Exposure. Bioengineering. 2024; 11(8):761. https://doi.org/10.3390/bioengineering11080761
Chicago/Turabian StyleLisk, Christina, Alex Fan, Francesca I. Cendali, Kenta Kakiuchi, Delaney Swindle, David I. Pak, Robert Tolson, Abby Grier, Keely Buesing, Seth Zaeske, and et al. 2024. "Peritoneal Infusion of Oxygen Microbubbles Alters the Metabolomic Profile of the Lung and Spleen in Acute Hypoxic Exposure" Bioengineering 11, no. 8: 761. https://doi.org/10.3390/bioengineering11080761
APA StyleLisk, C., Fan, A., Cendali, F. I., Kakiuchi, K., Swindle, D., Pak, D. I., Tolson, R., Grier, A., Buesing, K., Zaeske, S., D’Alessandro, A., Borden, M. A., & Irwin, D. C. (2024). Peritoneal Infusion of Oxygen Microbubbles Alters the Metabolomic Profile of the Lung and Spleen in Acute Hypoxic Exposure. Bioengineering, 11(8), 761. https://doi.org/10.3390/bioengineering11080761







