Antibacterial Activity for Synthesized Coumarin Derivatives and a Coumarin Component of Lime Peel (Citrus aurantifolia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Lime Peel
2.2. Coumarin Derivatives
2.3. Sample Preparation
2.3.1. Lime Peel Extraction
2.3.2. Isolation of Lime Peel Components from n-Hexane Layer
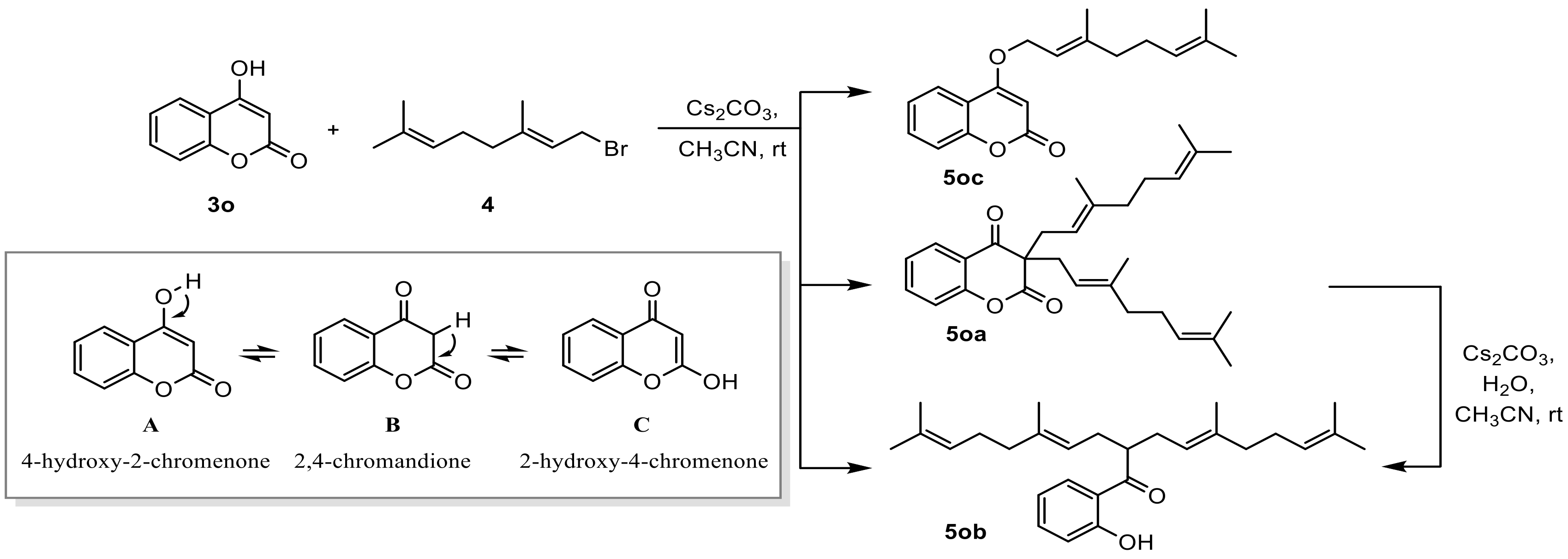
2.3.3. Synthesis of Coumarin Derivatives
2.4. Experimental Procedures
2.4.1. Compounds Separated by Lime Peel
2.4.2. Synthesis of Coumarin Derivatives and Geranyloxycoumarin Derivatives
Common Synthesis Method of Coumarin Derivatives
Common Synthesis Method of Geranyloxycoumarin Derivatives
Synthesis Method of (E)-2-((E)-3,7-Dimethylocta-2,6-dien-1-yl)-1-(2-hydroxyphenyl)-5,9-dimethyldeca-4,8-dien-1-one (5ob)
2.5. Evaluating the Antibacterial Activity
2.5.1. Test Strain
2.5.2. Microbial Culture
2.5.3. Measurement of Antibacterial Activities
Screening Test for Coumarin Derivatives
Minimum Inhibitory Concentration (MIC)
3. Results and Discussion
3.1. Synthesis
3.2. Measurement of Antibacterial Activities of Coumarin Derivatives
3.2.1. Screening Test for Coumarin Derivatives, Geranyloxycoumarin Derivatives, and Lime Peel
3.2.2. Minimum Inhibitory Concentration (MIC) of Coumarin Derivatives
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.A.; Mahmoud, A.H.; Ibrahim, B.M.M.; Bari, A.; Villinger, A. Synthesis and Evaluation of New Coumarin Derivatives as Antioxidant, Antimicrobial, and Anti-Inflammatory Agents. Molecules 2020, 25, 3251–3279. [Google Scholar] [CrossRef]
- Tanimoto, A.; Witaicenis, A.; Caruso, Í.; Piva, H.M.R.; Araujo, G.C.; Moraes, F.R.; Fossey, M.C.; Cornélio, M.L.; Souza, F.P.; Stasi, L.C.D. 4-Methylesculetin, a natural coumarin with intestinal anti-inflammatory activity, elicits a glutathione antioxidant response by different mechanisms. J. Chem. Biol. Interact. 2020, 315, 108876. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S. A Study on the Efficacy of the Coumarine Derivatives with Anti-Inflammatory Activity in the Trifoliate Orange Extract. J. Korean Oil Chem. Soc. 2012, 29, 610–617. [Google Scholar] [CrossRef]
- Curini, M.; Epifano, F.; Maltese, F.; Marcotullio, M.C.; Tubaro, A.; Altinier, G.; Gonzales, S.P.; Rodriguez, J.C. Synthesis and anti-inflammatory activity of natural and semisynthetic geranyloxycoumarins. Bioorganic Med. Chem. 2004, 14, 2241–2243. [Google Scholar] [CrossRef] [PubMed]
- Stasi, L.C.D. Coumarin Derivatives in Inflammatory Bowel Disease. Molecules 2021, 26, 422. [Google Scholar] [CrossRef]
- Okuyama, S.; Morita, M.; Kaji, M.; Amakura, Y.; Yoshimura, M.; Shimamoto, K.; Ookido, Y.; Nakajima, M.; Furukawa, Y. Auraptene Acts as an Anti-Inflammatory Agent in the Mouse Brain. Molecules 2015, 20, 20230–20239. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.L.T.; Kim, D.C.; Ko, W.M.; Ha, T.M.; Nhiem, N.X.; Yen, P.H.; Tai, B.H.; Truong, L.H.; Long, V.N.; Gioi, T.; et al. Antiinflammatory coumarins from Paramignya trimera. J. Pharm. Biol. 2017, 55, 1195–1201. [Google Scholar] [CrossRef]
- Kirsch, G.; Abdelwahab, A.B.; Chaimbault, P. Natural and Synthetic Coumarins with Effects on Inflammation. Molecules 2016, 21, 1322. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.L.; Kim, H.J.; Hwang, K.M.; Pae, H.O.; Yun, Y.G.; Chung, H.T.; Kim, Y.C. Anti-Inflammatory Effect of Ethanol Extract of Angelica uchiyamana in Activated Murine RAW 264.7 macrophages. J. Korean Med. Soc. Herb. Formula Study 2002, 10, 189–197. [Google Scholar]
- Khatib, A.; Kim, M.Y.; Chung, S.K. Anti-inflammatory Activities of Cinanamomum burmanni BI. J. Food Sci. Biotechnol. 2005, 4, 223–227. [Google Scholar]
- Grovera, J.; Jachak, S.M. Coumarins as privileged scaffold for anti-inflammatory drug development. J. RSC Adv. 2015, 49, 14. [Google Scholar] [CrossRef]
- Qin, H.L.; Zhang, Z.W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef]
- Arshad, A.; Osman, H.; Bagley, M.C.; Lam, C.K.; Mohamad, S.; Zahariluddin, A.S.M. Synthesis and antimicrobial properties of some new thiazolyl coumarin derivatives. Eur. J. Med. Chem. 2011, 46, 3788–3794. [Google Scholar] [CrossRef] [PubMed]
- Lipeeva, A.V.; Zakharov, D.O.; Burova, L.G.; Frolova, T.S.; Baev, D.S.; Shirokikh, I.V.; Evstropov, A.N.; Sinitsyna, O.I.; Tolsikova, T.G.; Shults, E.E. Design, Synthesis and Antibacterial Activity of Coumarin-1,2,3-triazole Hybrids Obtained from Natural Furocoumarin Peucedanin. Molecules 2019, 24, 2126. [Google Scholar] [CrossRef]
- Stojković, D.L.; Jevtić, V.V.; Vuković, N.; Vukić, M.; Čanović, P.; Zarić, M.M.; Mišić, M.M.; Radovanović, D.M.; Baskić, D.; Trifunović, S.R. Synthesis, characterization, antimicrobial and antitumor reactivity of new palladium (II) complexes with methionine and tryptophane coumarin derivatives. J. Mol. Struct. 2018, 1157, 425–433. [Google Scholar] [CrossRef]
- López-Rojas, P.; Janeczko, M.; Kubiński, K.; Amesty, Á.; Masłyk, M.; Estévez-Braun, A. Synthesis and Antimicrobial Activity of 4-Substituted 1,2,3-Triazole-Coumarin Derivatives. Molecule 2018, 23, 199. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Avila, J.G.; Martínez, A.; Serrato, B.; Calderón-Mugica, J.C.; Salgado-Garciglia, R. Antifungal and antibacterial activities of Mexican tarragon (Tagetes lucida). J. Agric. Food Chem. 2006, 54, 3521–3527. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M. The Antibacterial Effects and Mechanism of Several Botanical Compounds against Ralstonia solanacearum. Master ’s Thesis, Southwest University, Chongqing, China, 2015. [Google Scholar]
- Chita, R.S.; Jyotirmaya, S.; Monalisa, M.; Debananda, L.; Pratap, K.S.; Budheswar, D. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar] [CrossRef]
- Guerra, F.Q.S.; Araújo, R.S.A.; Sousa, J.P.; Silva, V.A.; Pereira, F.O.; Mendonça-Junior, F.J.B.; Barbosa-Filho, J.M.; Pereira, J.A.; Lima, E.O. A new coumarin derivative, 4-acetatecoumarin, with antifungal activity and association study against Aspergillus spp. Braz. J. Microbiol. 2018, 49, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pelayo, C.; Martínez-Quiñones, J.; Gil, J.; Durango, D. Coumarins from the peel of citrus grown in Colombia: Composition, elicitation and antifungal activity. J. Heliyon 2019, 15, e01937. [Google Scholar] [CrossRef] [PubMed]
- Kurdelas, R.R.; Lima, B.; Tapia, A.; Feresin, G.E.; Sierra, M.G.; Rodríguez, M.V.; Zacchino, S.; Enriz, R.D.; Freile, M.L. Antifungal activity of extracts and prenylated coumarins isolated from Baccharis darwinii Hook & Arn. (Asteraceae). Molecules 2010, 15, 4898–4907. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, R.S.A.; Guerra, F.Q.S.; Lima, E.D.O.; De Simone, C.A.; Tavares, J.F.; Scotti, L.; Scotti, M.T.; De Aquino, T.M.; De Moura, R.O.; Mendonça, F.J.B.; et al. Synthesis, structure-activity relationships (SAR) and in silico studies of coumarin derivatives with antifungal activity. Int. J. Mol. Sci. 2013, 14, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.Q.S.; de Araújo, R.S.A.; de Sousa, J.P.; de Oliveira Pereira, F.; Mendonça-Junior, F.J.B.; Barbosa-Filho, J.M.; de Oliveira Lima, E. Evaluation of Antifungal Activity and Mode of Action of New Coumarin Derivative, 7-Hydroxy-6-nitro-2H-1-benzopyran-2-one, against Aspergillus spp. Evid. Based Complement. Altern. Med. 2015, 925096, 8. [Google Scholar] [CrossRef]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anticancer. Agents 2002, 5, 29–46. [Google Scholar] [CrossRef]
- Manolov, I.; Kostova, I.; Netzeva, T.; Konstantinov, S.; Karaivanova, M. Cytotoxic activity of cerium complex with coumarin derivatives Molecular modeling of the ligands. Archiv. Pharm. Med. Chem. 2000, 333, 93–98. [Google Scholar] [CrossRef]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef] [PubMed]
- Gardelly, M.; Trimech, B.; Belkacem, M.A.; Harbach, M.; Abdelwahed, S.; Mosbah, A.; Bouajila, J.; Ben Jannet, H. Synthesis of novel diazaphosphinanes coumarin derivatives with promoted cytotoxic and anti-tyrosinase activities. Bioorganic Med. Chem. Lett. 2016, 26, 2450–2454. [Google Scholar] [CrossRef]
- You, C.X.; Yang, K.; Wang, C.F.; Zhang, W.J.; Wang, Y.; Han, J.; Fan, L.; Du, S.S.; Geng, Z.F.; Deng, Z.W. Cytotoxic Compounds Isolated from Murraya tetramera Huang. Molecules 2014, 19, 13225–13234. [Google Scholar] [CrossRef] [PubMed]
- Baik, K.U.; Ahn, B.Z. Cytotoxic Activities of some Geranylated Flavones against L1210 Cell. J. Yakhak Hoeji 1988, 32, 125–128. [Google Scholar]
- Lee, J.H.; Lee, J.H.; Kim, H.K.; Kim, E.G.; Cho, S.H. Synthesis of Coumarin Analogues and their Antitumor Activity. J. YAKHAK HOEJI 2006, 50, 339–344. [Google Scholar]
- Prashanth, T.; VijayAvin, B.R.; Thirusangu, P.; Ranganatha, V.L.; Prabhakar, B.T.; Narendra, J.N.; Chandra, S.; Khanum, S.A. Synthesis of coumarin analogs appended with quinoline and thiazole moiety and their apoptogenic role against murine ascitic carcinoma. J. Biomed. Pharmacother. 2019, 4, 108707. [Google Scholar] [CrossRef] [PubMed]
- Peperidou, A.; Bua, S.; Bozdag, M.; Hadjipavlou-Litina, D.; Supuran, C.T. Novel 6- and 7-Substituted Coumarins with Inhibitory Action against Lipoxygenase and Tumor-Associated Carbonic Anhydrase IX. Molecules 2018, 23, 153. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, K.; Ponczek, M.B.; Owczarek, J.; Guga, P.; Budzisz, E. Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives. Molecules 2020, 25, 1465. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Seidel, H.; Pötzsch, B.; Watzka, M. New insight in therapeutic anticoagulation by Coumarin derivatives. Hamostaseologie 2008, 28, 44–50. [Google Scholar] [PubMed]
- Marques, A.D.S.; Lin, C.T. Molecular complexes of IQ and 4-hydroxycoumarin: A mutagen-anti-mutagen system. J.Photochem. Photobiol. 2004, 74, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chool, B.Y.; Song, K.S. UVB-Shielding Effects of para-Coumaric Acid. Cosmet. Sci. Korea 2012, 38, 263–273. [Google Scholar] [CrossRef]
- Jung, O.U.; Lee, S.S. Preparation of Dye Sensitized Solar Cell Using Coumarin Dyes Extracted from Plants. Korea Chem. Eng. Res. 2013, 51, 157–161. [Google Scholar] [CrossRef]
- Sumi, H.; Eonjoo, R. Synthesis of Geranyloxycoumarin Derivatives under Mild Conditions Using Cs2CO3. J. Turk. Chem. Soc. Chem. A 2022, 9, 57–66. [Google Scholar] [CrossRef]
- Iranshahi, M.; Jabbari, A.; Orafaie, A.; Mehri, R.; Zeraatkar, S.; Ahmadi, T.; Alimardani, M.; Sadeghian, H. Synthesis and SAR studies of o-prenylated coumarins as potent 15-lipoxygenase inhibitors. Eur. J. Med. Chem. 2012, 57, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, T.M.; Zarubaev, V.V.; Orshanskaya, I.R.; Kadyrova, R.A.; Sannikova, V.A.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F. Anti-influenza activity of monoterpene-containing substituted coumarins. Bioorganic Med. Chem. Lett. 2017, 27, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Ghulam, R. A Concise Introduction of Perkin Reaction. Org. Chem. Curr. Res. 2018, 7, 191–194. [Google Scholar] [CrossRef]
- He, X.; Yan, Z.; Hu, X.; Zuo, Y.; Jiang, C.; Jin, L.; Shang, Y. FeCl3-Catalyzed Cascade Reaction: An Efficient Approach to Functionalized Coumarin Derivatives. Synth. Commun. 2014, 44, 1507–1514. [Google Scholar] [CrossRef]
- Nirajkumar, H.J.; Sachin, S.S.; Nishant, K.R.; Dnyaneshwar, R.S.; Ramdas, A.P. Heterogeneously Catalyzed Pechmann Condensation Employing the Tailored Zn0.925Ti0.075O NPs: Synthesis of Coumarin. ACS Omega 2019, 4, 8522–8527. [Google Scholar] [CrossRef]
- Abraham, G.G.; David, M.; Aparicio, S.; Hidemí, A.M.; Abraham, G.R.; Luis, F.R.; Cuauhtémoc, A.S.; Cárlos, E.; Lobato, G.; Nancy, R.C. Synthesis of 3-carboxylated Coumarins by Knoevenagel Condensation and Exploratory Anti-inflammatory Activity Evaluation by in vivo model. Am. J. Org. Chem. 2016, 6, 17–28. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, H.D. Recent advances in the synthesis of coumarin derivatives via Knoevenagel condensation: A review. Synth. Commun. 2014, 44, 2756–2788. [Google Scholar] [CrossRef]
- Shue, Y.J.; Shyh-Chyun, Y. Activator-free and one-pot C-allylation by simple palladium catalyst in water. Tetrahedron Lett. 2012, 53, 1380–1384. [Google Scholar] [CrossRef]
- Orhan, I.E.; Deniz, F.S.S.; Salmas, R.E.; Durdagi, S.; Epifano, F.; Genovese, S.; Fiorito, S. Combined molecular modeling and cholinesterase inhibition studies on some natural and semisynthetic O-alkylcoumarin derivatives. Bioorg. Chem. 2019, 84, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Venturella, P.; Bellino, A.; Luisa, M.M. Synthesis of terpenoid coumarins, an approach to the synthesis of Piloselliodan. Gazz. Chim. Ital. 1982, 112, 433–434. [Google Scholar]
- Cravotto, G.; Nano, G.; Palmisano, G.; Tagliapietra, S. 4-Hydroxycoumarin and Related Systems: Sitoselectivity of the Mitsunobu Reaction with Prenyl Alcohols. Heterocycles 2003, 60, 1351–1358. [Google Scholar] [CrossRef]
- Karataş, M.O.; Noma, S.S.S.; Gürses, C.; Balcıoğlu, S.; Ateş, B.; Alıcı, B.; Çakır, Ü. Water Soluble Coumarin Quaternary Ammonium Chlorides: Synthesis and Biological Evaluation. Chem. Biodivers. 2020, 17, e2000258. [Google Scholar] [CrossRef]
 | |||
|---|---|---|---|
| No. | Phenol (1) | Product (3) | Yield (%) |
| 1 | 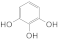 1a 1a |  3a 3a | 72 |
| 2 | 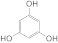 1b 1b |  3b 3b | 66 |
| 3 | 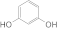 1c 1c | 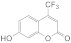 3c 3c | 78 |
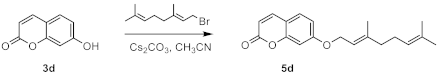 | ||
|---|---|---|
| Entry | Conditions | Yield (%) |
| 1 | TEA, acetone, rt, 12 h | Degradation |
| 2 | K2CO3, acetone, rt, 5 h | 35 |
| 3 | K2CO3, acetone, rt, 26 h | 62 |
| 4 | K2CO3, acetone, reflux, 1 h | 73 |
| 5 | K2CO3, CH3CN, reflux, 1 h | 74 |
| 6 | Cs2CO3, CH3CN, rt, 3 h | 93 |
| 7 | Cs2CO3, CH3CN, reflux, 30 min | 87 |
| 8 | Ag2CO3, CH3CN, rt, 3 h | 85 |
 | |||||||
|---|---|---|---|---|---|---|---|
| No. | Coumarin | No. | Product | No. | Coumarin | No. | Product |
| 3c | 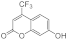 | 5c | 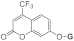 | 3k |  | 5k | 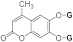 |
| 3d | 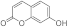 | 5d | 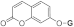 | 3l | 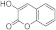 | 5l |  |
| 3e | 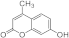 | 5e |  | 3m |  | 5m |  |
| 3f | 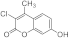 | 5f | 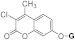 | 3n | 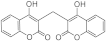 | 5od |  |
| 3g | 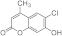 | 5g | 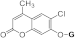 | 3o | 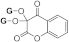 | 5oa | 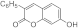 |
| 3h | 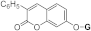 | 5h | 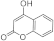 | 5oc |  | ||
| 3i |  | 5i | 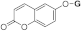 | 5od | 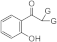 | ||
| 3j | 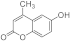 | 5j | 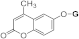 | ||||
 | |||||||
| Organism | Condition | °C |
|---|---|---|
| Bacillus cereus KCCM 11204 | ENB (yeast extract 0.25% + Brain Heart Infusion broth 1.25% + Nutrient broth 0.55%) | 30 |
| Micrococcus luteus IAM 1056 | ENB (yeast extract 0.25% + Brain Heart Infusion broth 1.25% + Nutrient broth 0.55%) | 30 |
| Enterococcus faecium KCCM 12118 | BHI (Brain Heart Infusion broth) | 37 |
| Listeria monocytogenes KCCM 40307 | BHI (Brain Heart Infusion broth) | 37 |
| Salmonella enteritidis KCCM 12021 | Nutrient broth | 37 |
| Shigella boydii KCCM 41649 | Nutrient broth | 37 |
| Escherichia coli KCCM 11835 | LB broth | 37 |
| Staphylococcus aureus subsp. aureus KCCM 40050 | LB broth | 37 |
| Test Organisms | Coumarin Derivatives | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMSO | 3a | 3b | 3c | 3d | 3e | 3f | 3g | 3h | 3i | 3j | 3k | 3l | 3n | 3o | 3p | |
| B. cereus KCCM 11204 | - | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | ++ | ++ | ++ | ++++ | - | +++ | ++++ | ++ | ++ |
| M. luteus IAM 1056 | - | ++++ | ++++ | +++ | - | + | + | - | + | - | ++ | ++ | + | +++ | - | ++ |
| L. monocytogenes KCCM 40307 | - | +++ | ++++ | ++ | - | + | + | + | + | + | + | + | ++ | ++ | ++ | + |
| E. faecium KCCM 12118 | - | +++ | +++ | ++ | - | ++ | + | - | + | + | + | + | ++ | ++ | + | + |
| S. enteritidis KCCM 12021 | - | ++ | + | ++ | - | ++ | - | - | ++ | + | ++ | ++ | +++ | - | - | ++ |
| S. boydii KCCM 41649 | - | ++ | + | ++ | - | +++ | - | - | ++ | - | ++ | ++ | +++ | - | - | +++ |
| E. coli KCCM 11835 | - | +++ | + | + | - | ++ | - | - | ++ | - | ++ | ++ | + | - | + | ++ |
| S. aureus subsp. aureus KCCM 40050 | - | ++++ | ++++ | +++ | - | ++ | + | - | ++ | - | ++ | - | ++ | ++++ | - | ++ |
| Test Organisms | Geranyloxycoumarin Derivatives | Lime Peel | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMSO | 5c | 5d | 5e | 5f | 5g | 5h | 5i | 5j | 5k | 5l | 5m | 5m-1 | 5m-2 | 5m-3 | |
| B. cereus KCCM 11204 | - | + | ++ | + | ++ | + | + | + | + | - | + | + | + | + | + |
| M. luteus IAM 1056 | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| L. monocytogenes KCCM 40307 | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| E. faecium KCCM 12118 | - | + | + | + | + | ++ | + | + | + | + | + | + | + | + | + |
| S. enteritidis KCCM 12021 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S. boydii KCCM 41649 | - | + | - | - | - | - | - | - | - | - | - | - | - | - | + |
| E. coli KCCM 11835 | - | + | + | + | + | - | + | - | + | + | + | + | + | + | + |
| S. aureus subsp. aureus KCCM 40050 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| DMSO | B. cereus KCCM 11204 | M. luteus IAM 1056 | L. monocytogenes KCCM 40307 | E. faecium KCCM 12118 | S. enteritidis KCCM 12021 | S. boydii KCCM 41649 | E. coli KCCM 1835 | S. aureus subsp. aureus KCCM 40050 | |
|---|---|---|---|---|---|---|---|---|---|
| 3a | - | 23 | - | - | 8.1 | 30 | 46 | - | - |
| 3b | - | 1.5 | 1.5 | 1.5 | 2.9 | - | - | 2.9 | 1.5 |
| 3c | - | 3.3 | 12.7 | 3.3 | 1.7 | 6.7 | - | - | 3.3 |
| 3d | - | 44 | 30 | 7.8 | 4.2 | 7.8 | 3.6 | 7.8 | 61 |
| 3e | - | 23 | 23 | 3.6 | 7.9 | 7.9 | 7.9 | 3.3 | 50 |
| 3j | - | 42 | 28 | 19 | 14 | 19 | 3.3 | 3.3 | 7.9 |
| 3k | - | 50 | 50 | 38 | - | 50 | - | - | 10 |
| 3n | - | 27 | 3.7 | 1.2 | 3.7 | 27 | 14 | - | 2.9 |
| 3o | - | 46 | 15.1 | 7.6 | 7.8 | 3.3 | 7.8 | 7.8 | 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S. Antibacterial Activity for Synthesized Coumarin Derivatives and a Coumarin Component of Lime Peel (Citrus aurantifolia). Bioengineering 2024, 11, 752. https://doi.org/10.3390/bioengineering11080752
Hwang S. Antibacterial Activity for Synthesized Coumarin Derivatives and a Coumarin Component of Lime Peel (Citrus aurantifolia). Bioengineering. 2024; 11(8):752. https://doi.org/10.3390/bioengineering11080752
Chicago/Turabian StyleHwang, Sumi. 2024. "Antibacterial Activity for Synthesized Coumarin Derivatives and a Coumarin Component of Lime Peel (Citrus aurantifolia)" Bioengineering 11, no. 8: 752. https://doi.org/10.3390/bioengineering11080752
APA StyleHwang, S. (2024). Antibacterial Activity for Synthesized Coumarin Derivatives and a Coumarin Component of Lime Peel (Citrus aurantifolia). Bioengineering, 11(8), 752. https://doi.org/10.3390/bioengineering11080752








