Differential Back Muscle Flexion–Relaxation Phenomenon in Constrained versus Unconstrained Leg Postures
Abstract
1. Introduction
2. Materials and Methods
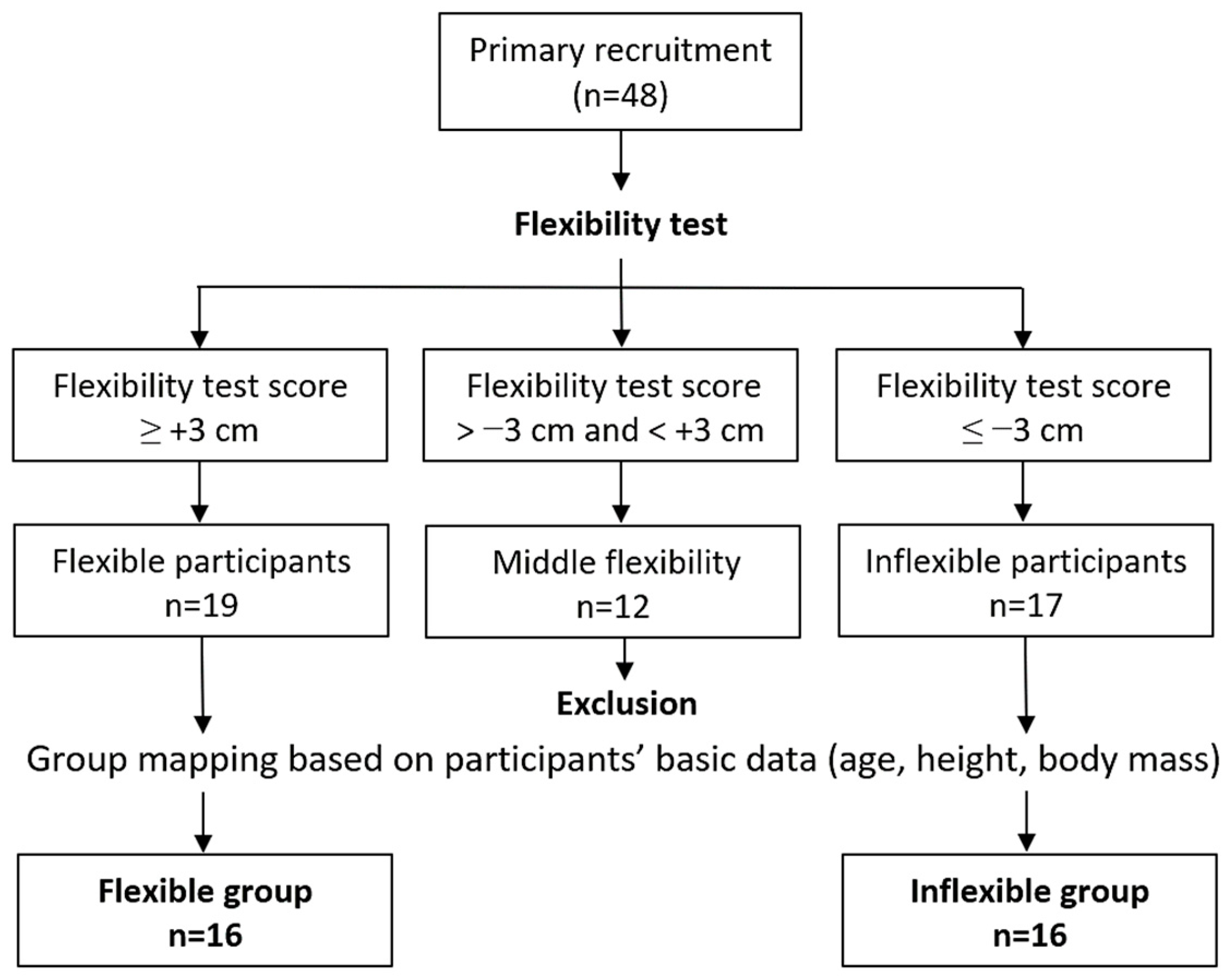
2.1. Participants
2.2. Electromyography
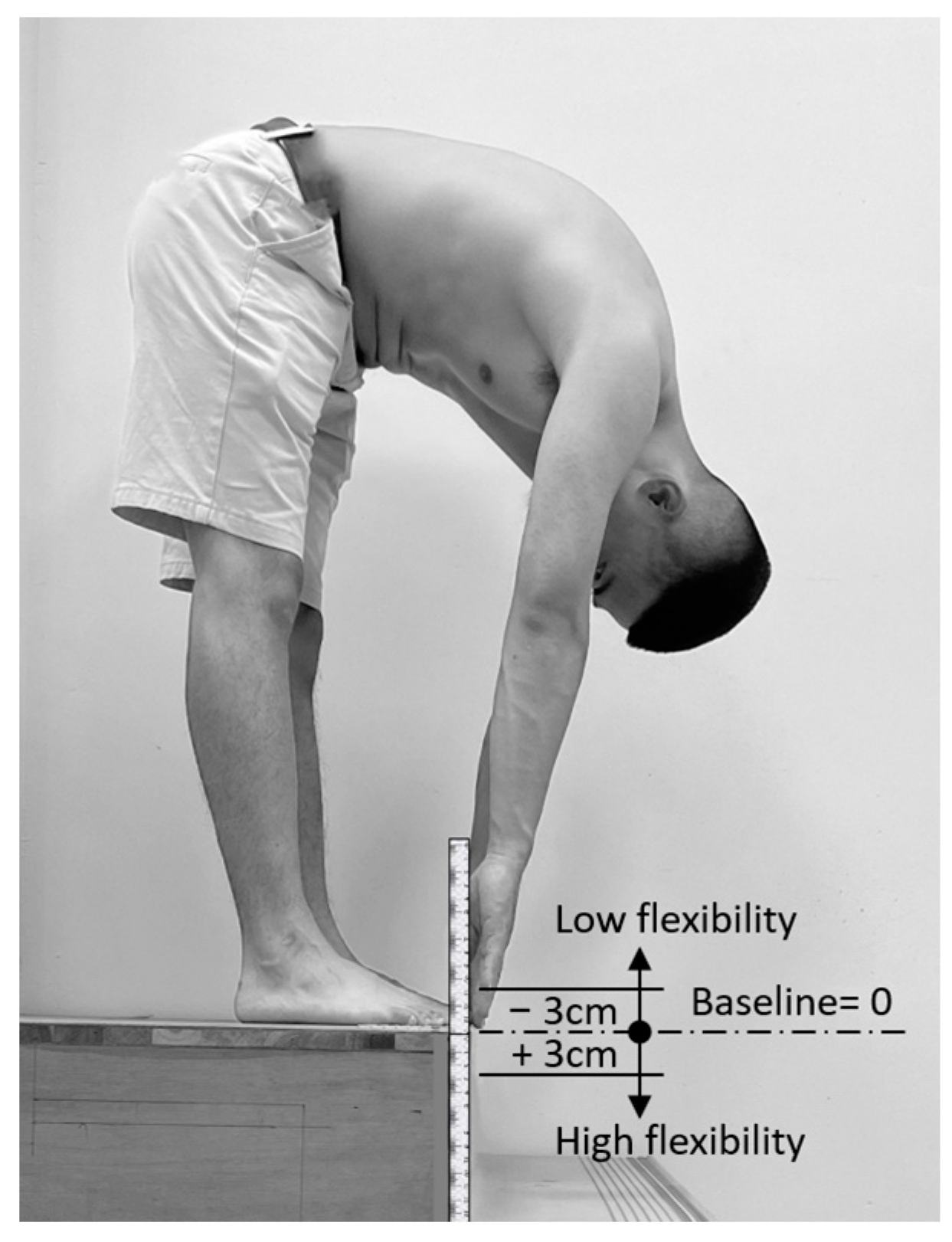
2.3. Lumbar Spine Curvature Measurements
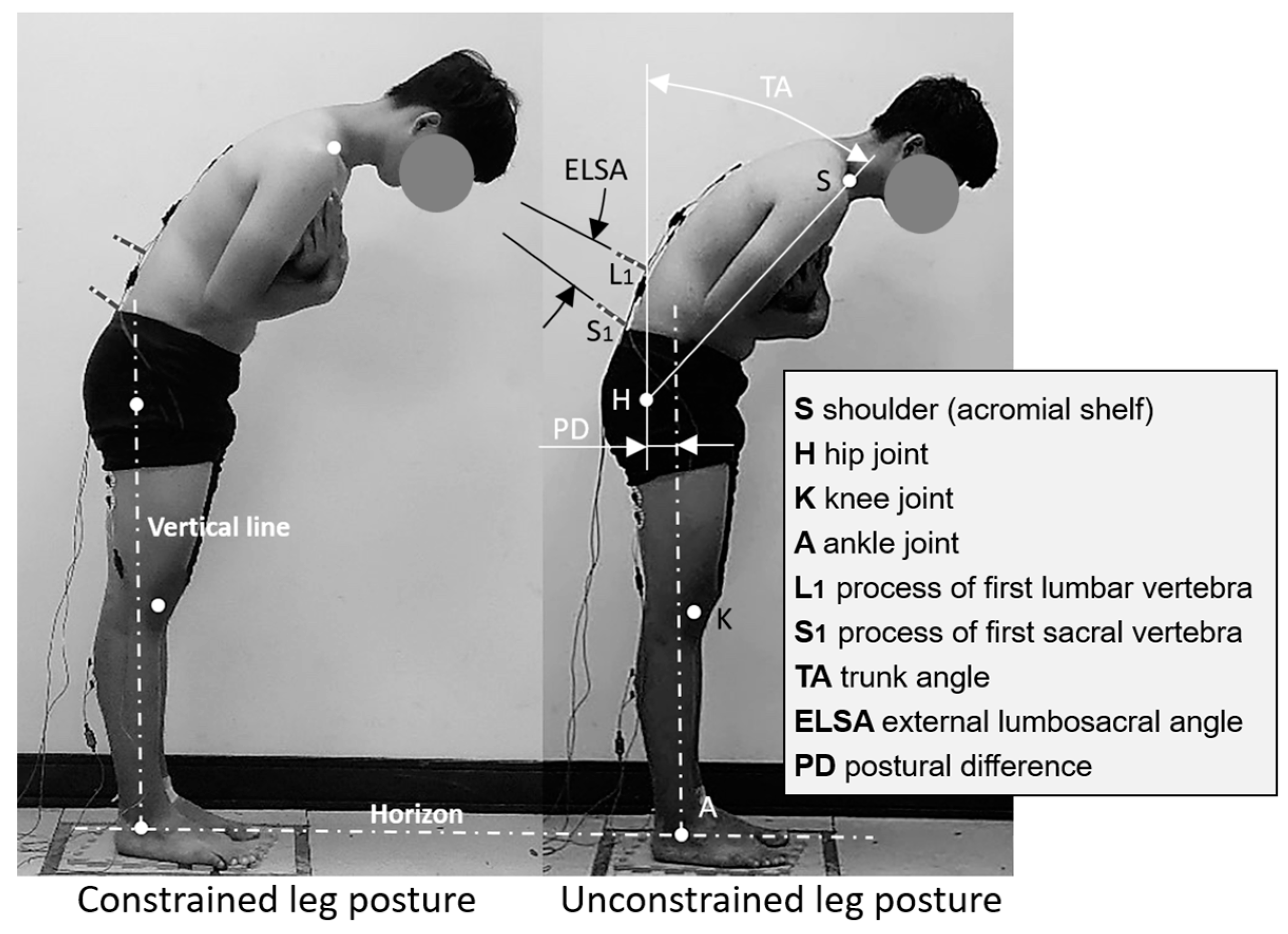
2.4. Experimental Design and Procedure
2.5. Statistical Analysis
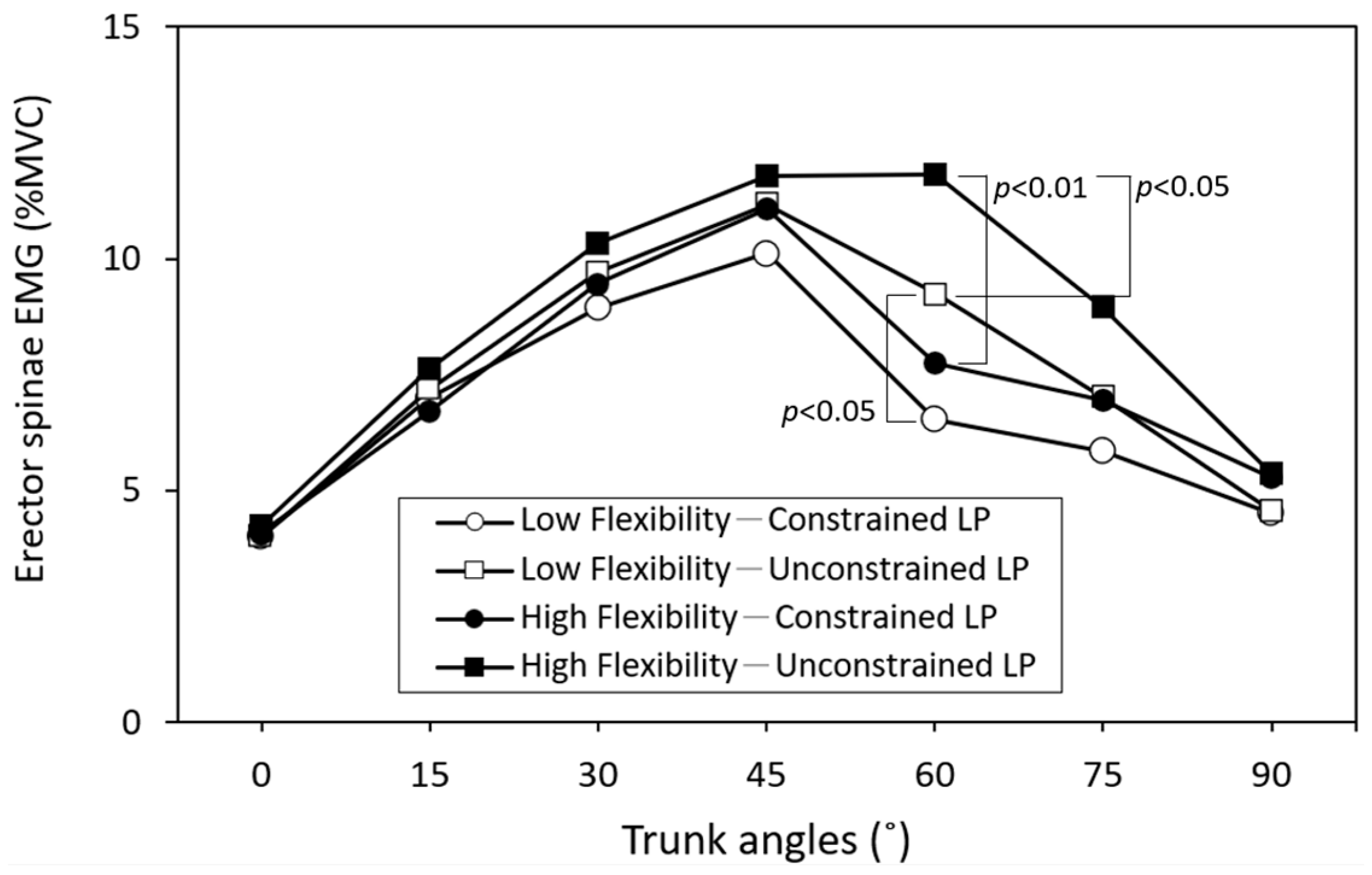
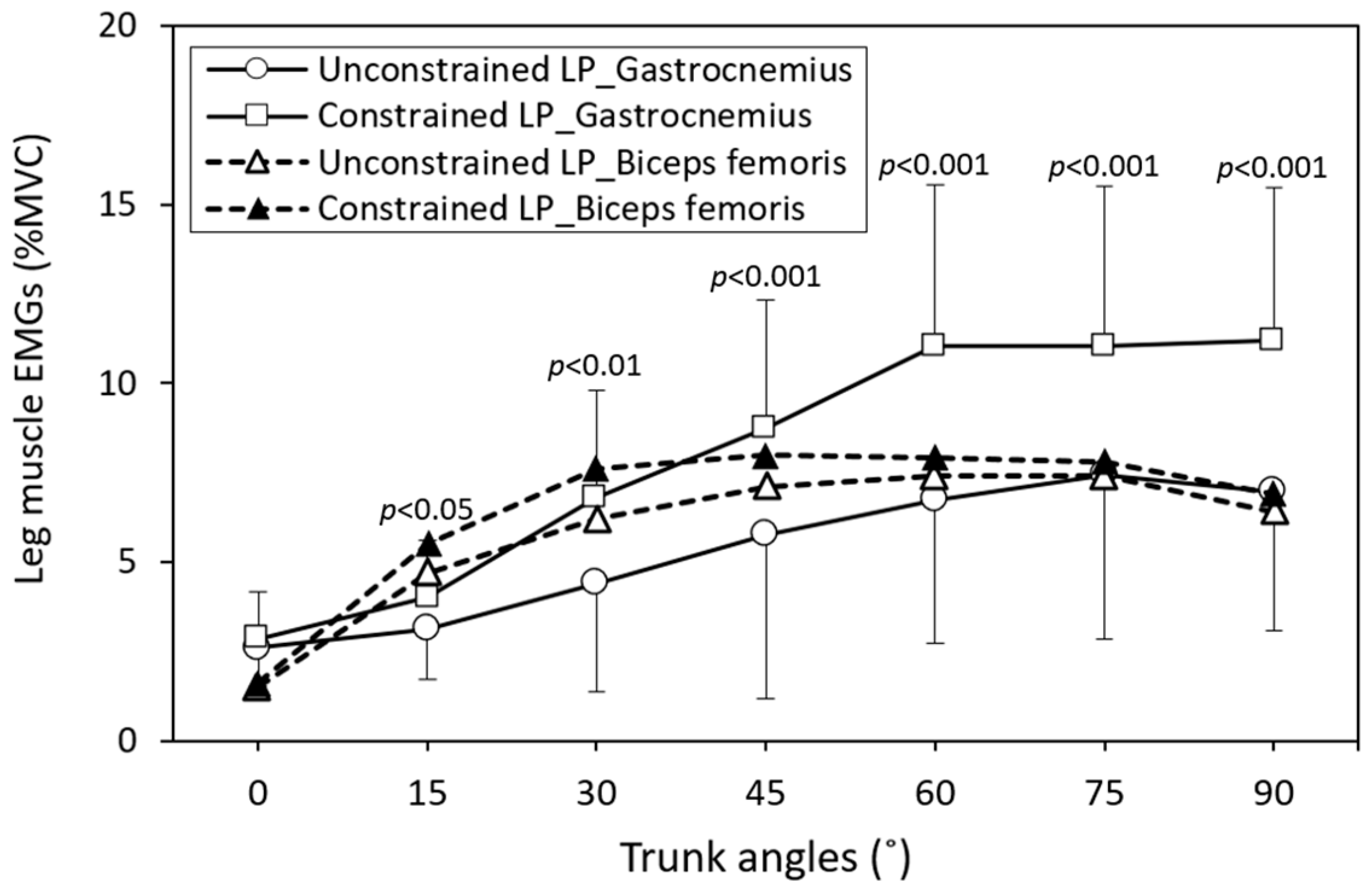
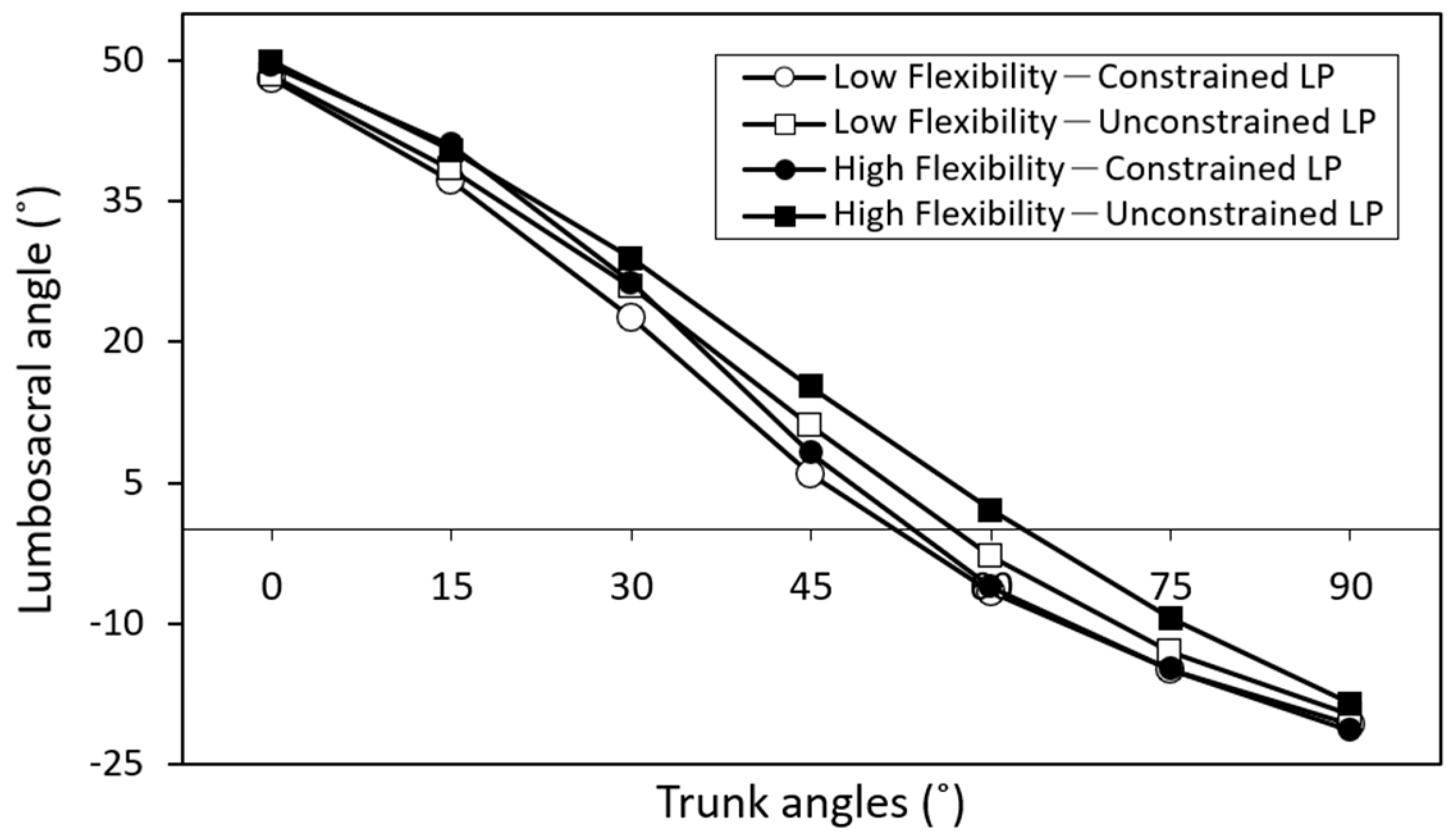
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keyserling, W.M. Workplace risk factors and occupational musculoskeletal disorders, part 1: A review of biomechanical and psychophysical research on risk factors associated with low-back pain. Am. Ind. Hyg. Assoc. J. 2000, 61, 39–50. [Google Scholar] [CrossRef]
- Shin, G.; D’souza, C.; Liu, Y.H. Creep and fatigue development in the low back in static flexion. Spine 2009, 34, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Baril-Gingras, G.; Lortie, M. The handling of objects other than boxes: Univariate analysis of handling techniques in a large transport company. Ergonomics 1995, 38, 905–925. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Shu, Y.; Li, Z.; Jiang, Z.; Mirka, G. Influence of knee angle and individual flexibility on the flexion–relaxation response of the low back musculature. J. Electromyogr. Kinesiol. 2004, 14, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Plamondon, A.; Delisle, A.; Bellefeuille, S.; Denis, D.; Gagnon, D.; Larivière, C.; IRSST MMH Research Group. Lifting strategies of expert and novice workers during a repetitive palletizing task. Appl. Ergon. 2014, 45, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Kamat, S.R.; Zula, N.M.; Rayme, N.S.; Shamsuddin, S.; Husain, K. The ergonomics body posture on repetitive and heavy lifting activities of workers in aerospace manufacturing warehouse. IOP Conf. Ser. Mater. Sci. Eng. 2017, 210, 012079. [Google Scholar] [CrossRef]
- Floyd, W.F.; Silver, P.H.S. Function of erector spinae in flexion of the trunk. Lancet 1951, 257, 133–134. [Google Scholar] [CrossRef]
- Solomonow, M.; Baratta, R.V.; Banks, A.; Freudenberger, C.; Zhou, B.H. Flexion-relaxation response to static lumbar flexion in males and females. Clin. Biomech. 2003, 18, 273–279. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.M.; Brown, S. Creep responses of the lumbar spine to prolonged full flexion. Clin. Biomech. 1992, 7, 43–46. [Google Scholar] [CrossRef]
- Shin, G.; Mirka, G.A. An in vivo assessment of the low back response to prolonged flexion: Interplay between active and passive tissues. Clin. Biomech. 2007, 22, 965–971. [Google Scholar] [CrossRef]
- Kang, S.H.; Mirka, G.A. Creep deformation of viscoelastic lumbar tissue during sustained submaximal trunk flexion postures. J. Biomech. 2023, 115, 111647. [Google Scholar] [CrossRef] [PubMed]
- Depalle, B.; Qin, Z.; Shefelbine, S.J.; Buehler, M.J. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J. Mech. Behav. Biomed. Mater. 2015, 52, 1–13. [Google Scholar] [CrossRef]
- Zwambag, D.P.; Brown, S.H. Experimental validation of a novel spine model demonstrates the large contribution of passive muscle to the flexion relaxation phenomenon. J. Biomech. 2020, 102, 109431. [Google Scholar] [CrossRef] [PubMed]
- Gouteron, A.; Tabard-Fougere, A.; Bourredjem, A.; Casillas, J.M.; Armand, S.; Genevay, S. The flexion relaxation phenomenon in nonspecific chronic low back pain: Prevalence, reproducibility and flexion–extension ratios. A systematic review and meta-analysis. Eur. Spine J. 2022, 31, 136–151. [Google Scholar] [CrossRef]
- Alessa, F.; Ning, X. Changes of lumbar posture and tissue loading during static trunk bending. Hum. Mov. Sci. 2018, 57, 59–68. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lin, W.C.; Liao, Y.H.; Lin, C.J. Effect of individual flexibility and knee posture on the back muscle flexion-relaxation phenomenon. Int. J. Ind. Ergon. 2018, 68, 82–88. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lin, W.C.; Liao, Y.H.; Chen, Y.; Kang, P.Y. Changing the pattern of the back-muscle flexion–relaxation phenomenon through flexibility training in relatively inflexible young men. PLoS ONE 2021, 16, e0259619. [Google Scholar] [CrossRef]
- Olson, M.; Solomonow, M.; Li, L. Flexion–relaxation response to gravity. J. Biomech. 2006, 39, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Eungpinichpong, W.; Buttagat, V.; Areeudomwong, P.; Pramodhyakul, N.; Swangnetr, M.; Kaber, D.; Puntumetakul, R. Effects of restrictive clothing on lumbar range of motion and trunk muscle activity in young adult worker manual material handling. Appl. Ergon. 2013, 44, 1024–1032. [Google Scholar] [CrossRef]
- Shamsi, M.; Ahmadi, A.; Mirzaei, M.; Jaberzadeh, S. Effects of static stretching and strengthening exercises on flexion relaxation ratio in patients with LBP: A randomized clinical trial. J. Bodyw. Mov. Ther. 2022, 30, 196–202. [Google Scholar] [CrossRef]
- Gouteron, A.; Tabard-Fougère, A.; Moissenet, F.; Bourredjem, A.; Rose-Dulcina, K.; Genevay, S.; Laroche, D.; Armand, S. Sensitivity and specificity of the flexion and extension relaxation ratios to identify altered paraspinal muscles’ flexion relaxation phenomenon in nonspecific chronic low back pain patients. J. Electromyogr. Kinesiol. 2023, 68, 102740. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Analyses of myo-electrical silence of erectors spinae. J. Biomech. 2001, 34, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.G.; Yoo, W.G. Effects of the wearing of tight jeans on lumbar and hip movement during trunk flexion. J. Phys. Ther. Sci. 2012, 24, 659–661. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.L.; Lin, W.C.; Chen, Y.; Wen, Y.W.; Tsai, T.L.; Yan, S.Q. Effect of wearing jeans on the back muscle flexion-relaxation phenomenon. Int. J. Ind. Ergon. 2020, 76, 102938. [Google Scholar] [CrossRef]
- Pollock, C.L.; Ivanova, T.D.; Hunt, M.A.; Garland, S.J. Motor unit recruitment and firing rate in medial gastrocnemius muscles during external perturbations in standing in humans. J. Neurophysiol. 2014, 112, 1678–1684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Behm, D.G.; Burry, S.M.; Greeley, G.E.; Poole, A.C.; MacKinnon, S.N. An unstable base alters limb and abdominal activation strategies during the flexion relaxation response. J. Sports Sci. Med. 2006, 5, 323–332. [Google Scholar] [PubMed Central]
- Descarreaux, M.; Lafond, D.; Cantin, V. Changes in the flexion-relaxation response induced by hip extensor and erector spinae muscle fatigue. BMC Musculoskel. Dis. 2010, 11, 112. [Google Scholar] [CrossRef]
- Kim, M.H.; Yoo, W.G. Comparison of the hamstring muscle activity and flexion-relaxation ratio between asymptomatic persons and computer work-related low back pain sufferers. J. Phys. Ther. Sci. 2013, 25, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Ulrey, B.L.; Fathallah, F.A. Effect of a personal weight transfer device on muscle activities and joint flexions in the stooped posture. J. Electromyogr. Kinesiol. 2013, 23, 195–205. [Google Scholar] [CrossRef]
- Nikzad, S.; Pirouzi, S.; Taghizadeh, S.; Hemmati, L. Relationship between hamstring flexibility and extensor muscle activity during a trunk flexion task. J. Chiropr. Med. 2020, 19, 21–27. [Google Scholar] [CrossRef]
- Shamsi, M.; Mirzaei, M.; Hopayian, K. A controlled clinical trial investigating the effects of stretching and compression exercises on electromyography of calf muscles in chronic LBP patients with a deep gluteal syndrome. BMC Sports Sci. Med. Rehabil. 2024, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Netter, F.H. Atlas of Human Anatomy; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Laird, R.A.; Keating, J.L.; Kent, P. Subgroups of lumbo-pelvic flexion kinematics are present in people with and without persistent low back pain. BMC Musculoskel. Dis. 2018, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Shahvarpour, A.; Preuss, R.; Sullivan, M.J.; Negrini, A.; Larivière, C. The effect of wearing a lumbar belt on biomechanical and psychological outcomes related to maximal flexion-extension motion and manual material handling. Appl. Ergon. 2018, 69, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mackey, S.; Barnes, J.; Pike, K.; De Carvalho, D. The relation between the flexion relaxation phenomenon onset angle and lumbar spine muscle reflex onset time in response to 30 min of slumped sitting. J. Electromyogr. Kinesiol. 2021, 58, 102545. [Google Scholar] [CrossRef] [PubMed]
- Ippersiel, P.; Preuss, R.; Fillion, A.; Jean-Louis, J.; Woodrow, R.; Zhang, Q.; Robbins, S.M. Inter-joint coordination and the flexion-relaxation phenomenon among adults with low back pain during bending. Gait Posture 2021, 85, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Kordi Yoosefinejad, A.; Motealleh, A.; Ghofrani-Jahromi, M. Comparison of flexion relaxation phenomenon between female yogis and matched non-athlete group. BMC Sports Sci. Med. Rehabil. 2022, 14, 14. [Google Scholar] [CrossRef]
- Li, Y.; Pei, J.; Li, C.; Wu, F.; Tao, Y. The association between different physical activity levels and flexion-relaxation phenomenon in women: A cross-sectional study. BMC Sports Sci. Med. Rehabil. 2023, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Jung, H.S. Hand dominance and hand use behaviour reported in a survey of 2437 Koreans. Ergonomics 2009, 52, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Schorderet, C.; Hilfiker, R.; Allet, L. The role of the dominant leg while assessing balance performance. A systematic review and meta-analysis. Gait Posture 2021, 84, 66–78. [Google Scholar] [CrossRef]
- Ayala, F.; de Baranda, P.S.; Croix, M.D.S.; Santonja, F. Reproducibility and criterion-related validity of the sit and reach test and toe touch test for estimating hamstring flexibility in recreationally active young adults. Phys. Ther. Sport 2012, 13, 219–226. [Google Scholar] [CrossRef]
- Stegeman, D.; Hermens, H. Standards for Surface Electromyography: The European Project Surface EMG for Noninvasive Assessment of Muscles (SENIAM); Roessingh Research and Development: Enschede, The Netherlands, 2007; pp. 108–112. [Google Scholar]
- Vera-Garcia, F.J.; Moreside, J.M.; McGill, S.M. MVC techniques to normalize trunk muscle EMG in healthy women. J. Electromyogr. Kinesiol. 2010, 20, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mathiassen, S.E.; Winkel, J.; Hägg, G.M. Normalization of surface EMG amplitude from the upper trapezius muscle in ergonomic studies—A review. J. Electromyogr. Kinesiol. 1995, 5, 197–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lee, Y.H. A noninvasive protocol for the determination of lumbosacral vertebral angle. Clin. Biomech. 1997, 12, 185–189. [Google Scholar] [CrossRef]
- Colloca, C.J.; Hinrichs, R.N. The biomechanical and clinical significance of the lumbar erector spinae flexion-relaxation phenomenon: A review of literature. J. Manip. Physiol. Ther. 2005, 28, 623–631. [Google Scholar] [CrossRef]
- Murillo, C.; Martinez-Valdes, E.; Heneghan, N.R.; Liew, B.; Rushton, A.; Sanderson, A.; Falla, D. High-density electromyography provides new insights into the flexion relaxation phenomenon in individuals with low back pain. Sci. Rep. 2019, 9, 15938. [Google Scholar] [CrossRef] [PubMed]
- Nordin, M.; Weiner, S.S.; Lindh, M. Biomechanics of the Lumbar Spine. In Basic Biomechanics of the Musculoskeletal System; Nordin, M., Frankel, V., Eds.; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2001; pp. 256–284. [Google Scholar]
- Schultz, A.B.; Hadespeck-Grib, K.; Sinkora, G.; Warwick, D.N. Quantitative studies of flexion–relaxation phenomenon in the back muscles. J. Orthop. Res. 1985, 3, 189–197. [Google Scholar] [CrossRef]
- Porta, M.; Pau, M.; Orrù, P.F.; Nussbaum, M.A. Trunk flexion monitoring among warehouse workers using a single inertial sensor and the influence of different sampling durations. Int. J. Environ. Res. Public Health 2020, 17, 7117. [Google Scholar] [CrossRef] [PubMed]
- Skals, S.; Bláfoss, R.; Andersen, M.S.; de Zee, M.; Andersen, L.L. Manual material handling in the supermarket sector. Part 1: Joint angles and muscle activity of trapezius descendens and erector spinae longissimus. Appl. Ergon. 2021, 92, 103340. [Google Scholar] [CrossRef]
- Cornbleet, S.L.; Woolsey, N.B. Assessment of hamstring muscle length in school-aged children using the sit-and-reach test and the inclinometer measure of hip joint angle. Phys. Ther. 1996, 76, 850–855. [Google Scholar] [CrossRef]
| Items | Low Flexibility (n = 16) | High Flexibility (n = 16) | ||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| Age (years) | 21.6 (1.4) | 19–24 | 21.6 (1.5) | 20–24 |
| Height (cm) | 172.8 (4.7) | 166–180 | 173 (4.5) | 161–178 |
| Body mass (kg) | 67 (7.8) | 52–77 | 68.1 (7.5) | 49–78 |
| Acromial height (cm) | 142 (4.7) | 135–150 | 141.9 (4.2) | 135–151 |
| Hip height (cm) | 88.4 (4.6) | 78–95 | 87.0 (3.6) | 79–93 |
| Knee height (cm) | 47.8 (2.4) | 44–52 | 47.5 (1.8) | 44–50 |
| Flexibility (cm) | −12.1 (8.3) | −3–−34 | 9.2 (5.9) | 3–25 |
| Variables | Responses | DF | SS | MS | F | p | Power |
|---|---|---|---|---|---|---|---|
| Flexibility | Lumbar erector spinae | 1 | 66 | 66 | 7.2 | <0.01 | 0.763 |
| Biceps femoris | 1 | 45 | 45 | 8.6 | <0.01 | 0.835 | |
| Gastrocnemius | 1 | 119 | 119 | 9.5 | <0.01 | 0.869 | |
| Lumbosacral angle | 1 | 506 | 506 | 9.7 | <0.01 | 0.876 | |
| Leg posture | Lumbar erector spinae | 1 | 107 | 107 | 11.6 | <0.001 | 0.926 |
| Biceps femoris | 1 | 45 | 45 | 8.5 | <0.01 | 0.830 | |
| Gastrocnemius | 1 | 759 | 759 | 60.7 | <0.001 | 1.000 | |
| Lumbosacral angle | 1 | 901 | 901 | 17.3 | <0.001 | 0.986 | |
| Trunk angle | Lumbar erector spinae | 6 | 2007 | 335 | 36.4 | <0.001 | 1.000 |
| Biceps femoris | 6 | 1646 | 274 | 52.6 | <0.001 | 1.000 | |
| Gastrocnemius | 6 | 2625 | 437 | 35.0 | <0.001 | 1.000 | |
| Lumbosacral angle | 6 | 234,761 | 39,127 | 753.3 | <0.001 | 1.000 | |
| Flexibility × Leg posture | Lumbar erector spinae | 1 | 4 | 4 | 0.4 | 0.505 | 0.102 |
| Biceps femoris | 1 | <1 | <1 | <0.1 | 0.995 | 0.050 | |
| Gastrocnemius | 1 | 2 | 2 | 0.2 | 0.687 | 0.069 | |
| Lumbosacral angle | 1 | 41 | 41 | 0.8 | 0.374 | 0.144 | |
| Flexibility × Trunk angle | Lumbar erector spinae | 6 | 37 | 6 | 0.7 | 0.668 | 0.270 |
| Biceps femoris | 6 | 43 | 7 | 1.4 | 0.222 | 0.539 | |
| Gastrocnemius | 6 | 19 | 3 | 0.3 | 0.956 | 0.120 | |
| Lumbosacral angle | 6 | 105 | 18 | 0.3 | 0.917 | 0.146 | |
| Leg posture × Trunk angle | Lumbar erector spinae | 6 | 116 | 19 | 2.1 | <0.05 | 0.757 |
| Biceps femoris | 6 | 17 | 3 | 0.6 | 0.770 | 0.221 | |
| Gastrocnemius | 6 | 226 | 38 | 3.0 | <0.01 | 0.907 | |
| Lumbosacral angle | 6 | 479 | 80 | 1.5 | 0.165 | 0.593 | |
| Flexibility × Leg posture × Trunk angle | Lumbar erector spinae | 6 | 7 | 1 | 0.1 | 0.992 | 0.083 |
| Biceps femoris | 6 | 3 | 1 | 0.1 | 0.996 | 0.076 | |
| Gastrocnemius | 6 | 9 | 2 | 0.1 | 0.994 | 0.080 | |
| Lumbosacral angle | 6 | 104 | 17 | 0.3 | 0.920 | 0.144 |
| Trunk Angle (°) | Low Flexibility | Duncan Groups | High Flexibility | Duncan Groups | Difference |
|---|---|---|---|---|---|
| 0 | 48.1 (5.8) | A | 49.6 (8.3) | A | 1.5 |
| 15 | 37.8 (6.5) | B | 40.6 (7.3) | B | 2.8 |
| 30 | 24.2 (6.8) | C | 27.6 (7.0) | C | 3.4 * |
| 45 | 8.5 (6.6) | D | 11.7 (6.5) | D | 3.2 * |
| 60 | −4.8 (7.0) | E | −2.0 (8.7) | E | 2.8 |
| 75 | −14.0 (6.1) | F | −12.2 (7.6) | F | 1.8 |
| 90 | −20.3 (7.1) | G | −20.0 (7.4) | G | 0.3 |
| Trunk Angle (°) | Constrained Leg Posture | Duncan Groups | Unconstrained Leg Posture | Duncan Groups | Difference |
|---|---|---|---|---|---|
| 0 | 48.7 (7.4) | A | 49.0 (6.9) | A | 0.3 |
| 15 | 39.1 (7.9) | B | 39.4 (6.1) | B | 0.3 |
| 30 | 24.4 (7.3) | C | 27.3 (6.8) | C | 2.9 |
| 45 | 7.1 (8.0) | D | 13.1 (7.6) | D | 6.0 ** |
| 60 | −6.4 (7.4) | E | −0.4 (7.4) | E | 6.0 ** |
| 75 | −14.9 (6.3) | F | −11.1 (7.1) | F | 3.8 * |
| 90 | −21.1 (6.8) | G | −19.2 (7.6) | G | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Liao, Y.-H. Differential Back Muscle Flexion–Relaxation Phenomenon in Constrained versus Unconstrained Leg Postures. Bioengineering 2024, 11, 736. https://doi.org/10.3390/bioengineering11070736
Chen Y-L, Liao Y-H. Differential Back Muscle Flexion–Relaxation Phenomenon in Constrained versus Unconstrained Leg Postures. Bioengineering. 2024; 11(7):736. https://doi.org/10.3390/bioengineering11070736
Chicago/Turabian StyleChen, Yi-Lang, and Ying-Hua Liao. 2024. "Differential Back Muscle Flexion–Relaxation Phenomenon in Constrained versus Unconstrained Leg Postures" Bioengineering 11, no. 7: 736. https://doi.org/10.3390/bioengineering11070736
APA StyleChen, Y.-L., & Liao, Y.-H. (2024). Differential Back Muscle Flexion–Relaxation Phenomenon in Constrained versus Unconstrained Leg Postures. Bioengineering, 11(7), 736. https://doi.org/10.3390/bioengineering11070736






