1. Introduction
The Gram-negative bacterium
Escherichia coli remains one of the most widely used expression systems and continues to be the organism of choice in both laboratory experiments and early-stage commercial developments. Its longstanding dominance persists because of its versatility and reliability, making it the preferred platform for initial experimentation in comparison with other expression systems. The growth conditions for
E. coli cultures are cost-effective, and its genetics are extensively understood, enabling the construction of various variants and strains with standard molecular techniques. However, some limitations are inherent to this system, including low expression levels, inclusion body formation, and protein inactivity. The doubling time of
E. coli is approximately 20 minutes in rich medium, indicating room for improvement in its growth and the creation of new strains. However, this approach also has its limitations, and research is ongoing [
1]. Nevertheless, the most effective solution might be the implementation of novel alternative hosts that offer unique features and additional advantages.
Vibrio natriegens is a Gram-negative marine bacterium that is considered to be the fastest-growing host organism to date, with a generation time of 7–10 min [
2]. It tolerates a wide pH range, its pH optimum is 7.5, and its optimum temperature cultivation in BHI medium is 37 °C [
3]. A significant milestone was reached in 2016, marked by the independent publication of two papers [
4,
5], which were well received by the biotechnology community and which created strong interest in this organism. Its genome consists of two chromosomes of 3,248,023 bp and 1,927,310 bp, respectively, that together encode 4578 open reading frames; hence, the total genome size is approximately 5.17 Mb, i.e., over 0.5 Mb larger than the
E. coli genome [
4].
V. natriegens can utilize a wide range of substrates and grow robustly on sucrose [
5], a feedstock that is both economically and environmentally beneficial. This stands in contrast to the majority of existing industrial
E. coli strains, which are unable to utilize sucrose [
6]. Moreover, at 37 °C, the generation time of
V. natriegens is 14.8 min in LB3, which is 2.1 times faster than that of
E. coli in LB (31.3 min). These results confirm
V. natriegens as the fastest known, free-living organism. With the development of various genetic tools, we can, therefore, now use this organism as an alternative host for protein production. Several different proteins have also been produced in this system, making it a suitable alternative to
E. coli [
7,
8,
9,
10,
11,
12]. Rapid growth, low cost, and high production yield are important factors that make this host organism attractive for industrial production.
During the production of recombinant proteins, a soluble protein can be obtained in several ways, including the lowering of the cultivation temperature, a change in the host organism, or even extracellular production. Such production might be more advantageous than intracellular production because we can produce toxic proteins, proteins with preserved biological activity, and correctly folded proteins with this strategy, and such production simplifies downstream processes and overall costs. Penicillin-binding proteins (PBPs) are proteins that play an important role in cell wall peptidoglycan synthesis [
13]. D-alanyl-D-alanine (D,D)-carboxypeptidases are low molecular weight PBPs that play a significant role in maintaining normal cell morphology [
14]. Overexpression of D,D-carboxypeptidases might result in the disruption of the cell wall structure, resulting in the extracellular production of the target protein. Such production has been achieved not only in
E. coli [
15], but also in
V. natriegens [
16].
Taq DNA polymerase, isolated from
Thermus aquaticus, is a widely utilized thermostable enzyme known for its thermostability and its role in DNA amplification techniques [
17]. Originally isolated in 1976 from samples taken from Yellowstone National Park, USA, Taq DNA pol consists of 832 amino acids, with a molecular weight of approximately 94 kDa and exhibits a specific activity of approximately 292,000 U/mg [
18]. It has three domains, with the N-terminal domain exhibiting exonuclease activity and the C-terminal domain being responsible for polymerase activity [
19]. Taq DNA pol replicates a 1 kbp DNA strand in under 10 s at 72 °C [
20] and has a high fidelity, with an error rate estimated to be between 3 × 10
−4 and 3 × 10
−6 errors per polymerized nucleotide [
21]. This enzyme significantly improves yield, specificity, and automation in PCR applications [
22]. Additionally, protein engineering techniques have been used to create mutant Taq DNA pol variants, such as those sensitive to cold or with enhanced functionality, offering specific solutions for various research needs [
23,
24,
25,
26].
The reverse transcriptases are a special group of DNA polymerases and have three enzymatic activities: (1) they employ RNA as a template for the synthesis of a complementary DNA strand, generating an RNA/DNA hybrid molecule (RNA-dependent DNA polymerase activity, RDDP); (2) they can use a DNA template to create a complementary DNA strand (DNA-dependent DNA polymerase activity, DDDP); (3) they degrade RNA strands annealed to DNA (RNase H activity). Such transcriptases are important enzymes in the fields of molecular biology, genetics, and medicine. Laboratory research is thus focused on the availability of efficient and functional reverse transcriptases with various mutations and improved properties and their subsequent production for various biotechnological applications, such as RT-PCR, qPCR, cDNA cloning, and RNA sequencing. Reverse transcriptase M-MLV was discovered in 1970 by Edward Scolnick [
27] at the beginning of the boom in reverse transcriptase studies. M-MLV reverse transcriptase, unlike most reverse transcriptases, is an enzyme with only one subunit. Its molecular mass is approximately 75 kDa, and its length is 671 amino acids. It shares a similar structure with other DNA polymerases in this family, as it consists of three separate domains: palm, thumb, and fingers. In addition, as a reverse transcriptase with RNase H activity, it contains an RNase H domain with its own active site and a terminal domain. The crystal structure of the N-terminal fragment was solved by Georgians et al. in 1995 [
28], and the structure of the whole enzyme was clarified in 2004 by Das and Georgiadis [
29].
In this study, we focus on V. natriegens strains under various growth conditions in comparison with E. coli. We introduce V. natriegens as an attractive expression host for the production of industrially relevant proteins, namely Taq DNA polymerase and M-MLV reverse transcriptase. Advanced and innovative expression systems have the potential to drive down further the production costs of enzymes.
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
Table 1 provides a comprehensive list of all the strains used here. We utilized two strains of
V. natriegens. The first was the genetically engineered Vmax™ Express strain of
V. natriegens, featuring an IPTG-inducible T7 RNA polymerase cassette and a knockout of extracellular nucleases for enhanced gene expression. For comparisons, we employed a strain of
E. coli BL21(DE3) containing the same cassette. The second was the strain of
V. natriegens (
V. natriegens PF) engineered by Pfeifer et al. [
30] to contain deletions in predicted prophage loci, impacting its robustness and production stability.
V. natriegens PF lacks a T7 polymerase cassette, and so for comparison with
E. coli, we used the BL21 strain, which is a widely used non-T7 expression
E. coli strain.
2.2. Construction of Recombinant Plasmids
Mutant M-MLV reverse transcriptase was amplified by PCR from the pUC57 plasmid with a commercially synthesized gene for mutant M-MLV (GenScript, Piscataway, NJ, USA). The point mutations were designed according to Baranauskas et al. [
31] and provide higher processivity and thermostability of this enzyme. We inserted sequences for restriction sites of chosen restrictases by specific primers on both sides of the gene coding for M-MLV to be able to insert it in the right orientation into the pJexpress404 vector (Fwd_M-MLV: AAAACATATGATGGCGGGTACGTTGAACATCGATGAACATCGCC, Rev_M-MLV: AAAACTCGAGCAGCAGCGTGCTTGTATCCGGTGTCTCATAATCGC). After PCR amplification of M-MLV, the PCR product and pJexpress404 were digested with NdeI and XhoI (New England Biolabs
®) and ligated by T4 DNA Ligase (New England Biolabs
®). Mutant Taq DNA polymerase was cloned from the pUC57 plasmid with a commercially synthesized gene for mutant Taq pol (GenScript). The point mutations were designed as given in the literature [
24,
25] in order to provide a cold-sensitive mutant with tolerance to various PCR inhibitors. Recombinant expression plasmid was prepared by digestion of pUC57-mutTaqDNApol and pET28a with the restriction endonuclease enzymes BamHI and Hind III (New England Biolabs
®), with subsequent ligation by T4 DNA Ligase (New England Biolabs
®). Both recombinant plasmids were verified by sequencing, and we transformed both plasmids into
E. coli and
V. natriegens strains.
2.3. Transformation
Strains Vmax™ Express and BL21(DE3) were transformed with the designed plasmid pET28a—mutTaqDNApol (
Table 2). BL21 and PF were transformed with the designed plasmid pJexpress404-mutM-MLV reverse transcriptase (
Table 2). Chemically competent cells BL21(DE3) and BL21 were prepared in advance by using CaCl
2 and MgCl
2 treatment. Cells were cultured to OD600 = 0.4–0.5. After this optical density (OD) was reached, cells were chilled on ice for 10 min. The culture was centrifuged at 2000×
g, 4 °C, for 10 min, and the sediment was gently suspended in 5 mL of 100 mM iced MgCl
2. The suspension was centrifuged again under the same conditions, and the obtained sediment was resuspended in 1.5 mL of 100 mM iced CaCl
2 and incubated on ice for 1.5 h. After incubation, 500 µL of 50% glycerol (
v/
v) was added, and the suspension was aliquoted. Plasmid DNA (100–150 ng) was added to a 50 µL aliquot of cells, after which the mixture was subjected to a heat shock at 42 °C for 45 s, followed by a chilling step on ice. After the addition of LB growth medium, the mixture was incubated at 37 °C for 90 min. Transformed cells were plated on LB agar plates with glucose and the respective antibiotic (100 µg/mL ampicillin, 50 µg/mL kanamycin) and incubated overnight. For the transformation of
V. natriegens strains, we opted for a modified electroporation protocol as described by Weinstock et al. [
5].
V. natriegens cells were grown in BHIv2 medium to OD600 = 0.5. The bacterial culture was incubated on ice for 15 min. Cells were harvested at 4000×
g at 4 °C for 10 min. The bacterial pellet was washed with electroporation buffer (680 mM sucrose, 7 mM K
2HPO
4, pH 7.2) and centrifuged at 4000×
g at 4 °C for 10 min, for a total of three times. The pellet was resuspended in residual electroporation buffer, and the volume was adjusted to OD600 = 19. The mixture was aliquoted and stored at −80 °C. After the addition of 50–200 ng plasmid DNA to the cells, the mixture was transferred to an electroporation cuvette with a gap width of 0.1 cm (Bio-Rad). For the transformation, we used the following parameters: 800 V, 25 µF, 200 Ω (Bio-Rad). Following transformation, cells were recovered in LB3 medium supplemented with 680 mM sucrose and shaken at 28 °C for at least 2 h. The mixture was then spread on the LB3 agar plates enriched with 100 μg/mL ampicillin and 1% sucrose. The plates were cultivated at 37 °C overnight.
For the two-plasmid system, we first transformed the cells with an expression plasmid and then prepared competent cells from the recombinants, which were subsequently transformed with a second plasmid (pRSFDuet-T5/T7-PBP5/6).
2.4. Protein Production in Erlenmeyer Flasks
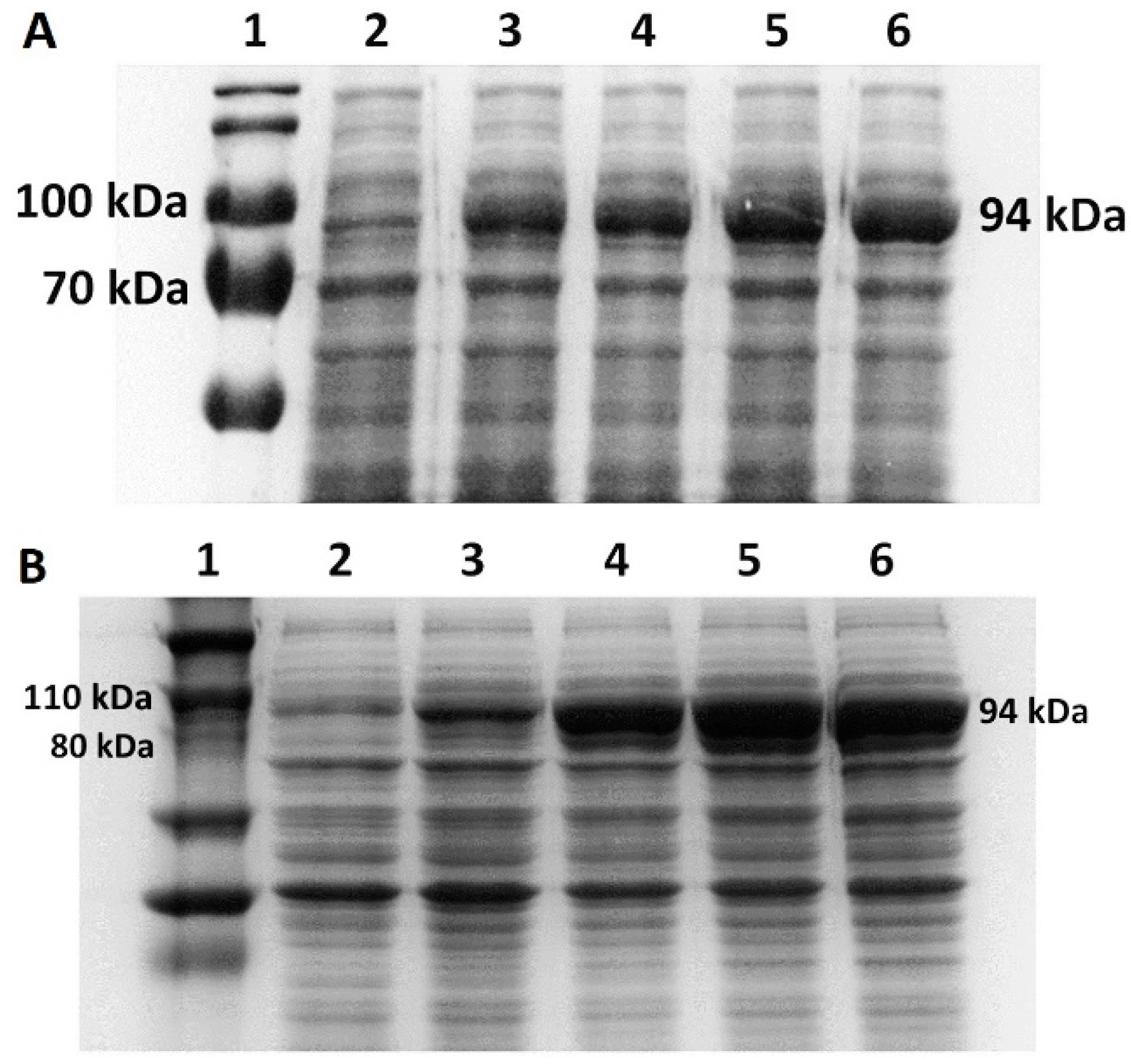
An overnight culture of E. coli BL21 (DE3)/BL21 and two strains of V. natriegens (Vmax™ Express/PF) cells carrying a plasmid with mutant Taq DNA polymerase/M-MLV reverse transcriptase was inoculated at a ratio of 1:100 into 50 mL of LB medium with the addition of 0.1% glucose (v/v) or 1% glycerol (v/v). The cell culture was allowed to grow to give OD600 = 0.5–1 when using LB/LB3 medium and OD600 = 2 when using the richer TB medium (with the addition of 1.5% NaCl (w/v)). OD was measured using a SmartSpecTM Plus instrument (Bio-Rad, Hercules, CA, USA). We induced expression by adding the inducer IPTG at a final concentration of 1 mM. Expression was carried out for 4 h with sampling every hour, with all samples being diluted to equal OD600 values. We centrifuged the samples for 3 min at 12,000× g on a MiniSpin centrifuge (Eppendorf, Hamburg, Germany), and the cell cultures obtained after 4 h from the start of expression induction were centrifuged for 10 min, 4 °C at 7690× g (UNIVERSAL 320R, Hettich, Tuttlingen, Germany).
2.5. Cell Disruption
We resuspended the pellet obtained after the shaking flask expression in a 15 mL homogenizer buffer solution. We used three different solutions, the compositions of which are listed in
Table 3. Subsequently, we sonicated the cells by using the Sono puls device (Bandelin) for 8 min and 15 s, with pulses of 15 s and cooling intervals of 45 s at an amplitude of 30%. The cell suspension was then centrifuged at 7690×
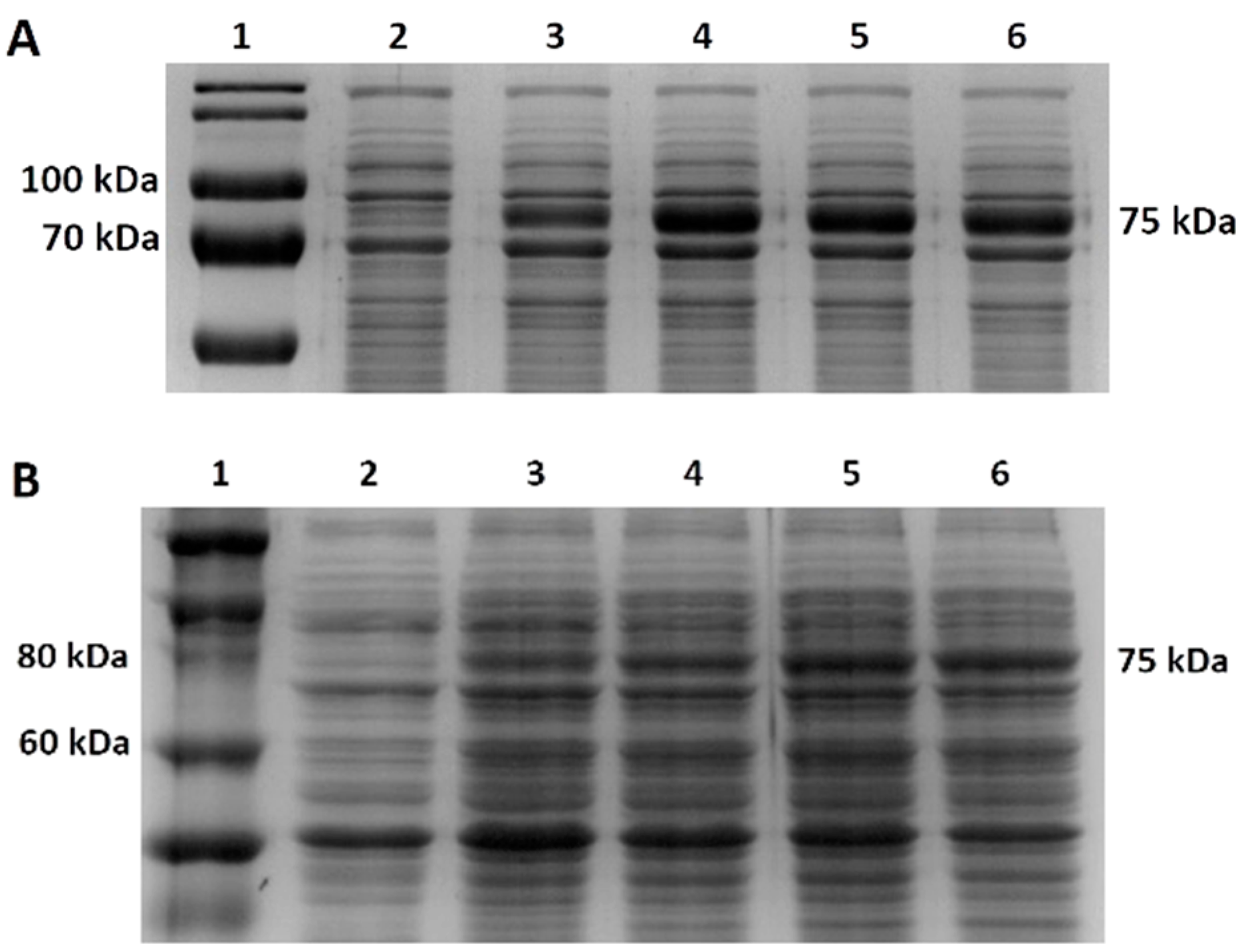
g for 15 min at 4 °C. We analyzed the pellet and supernatant using SDS-PAGE and Western blot.
2.6. SDS-PAGE Electrophoresis
For the electrophoretic separation of proteins under denaturing conditions in polyacrylamide gel, we carried out SDS-PAGE according to Laemmli et al. [
32] within the apparatus from Hoefer™ (Mighty Small™ II Mini Vertical Electrophoresis Systems, MA, USA). We prepared a 12% separation gel and a 4% application gel. After assembling the apparatus, we applied an 8 μL sample and 3.5 μL size standard PageRuler
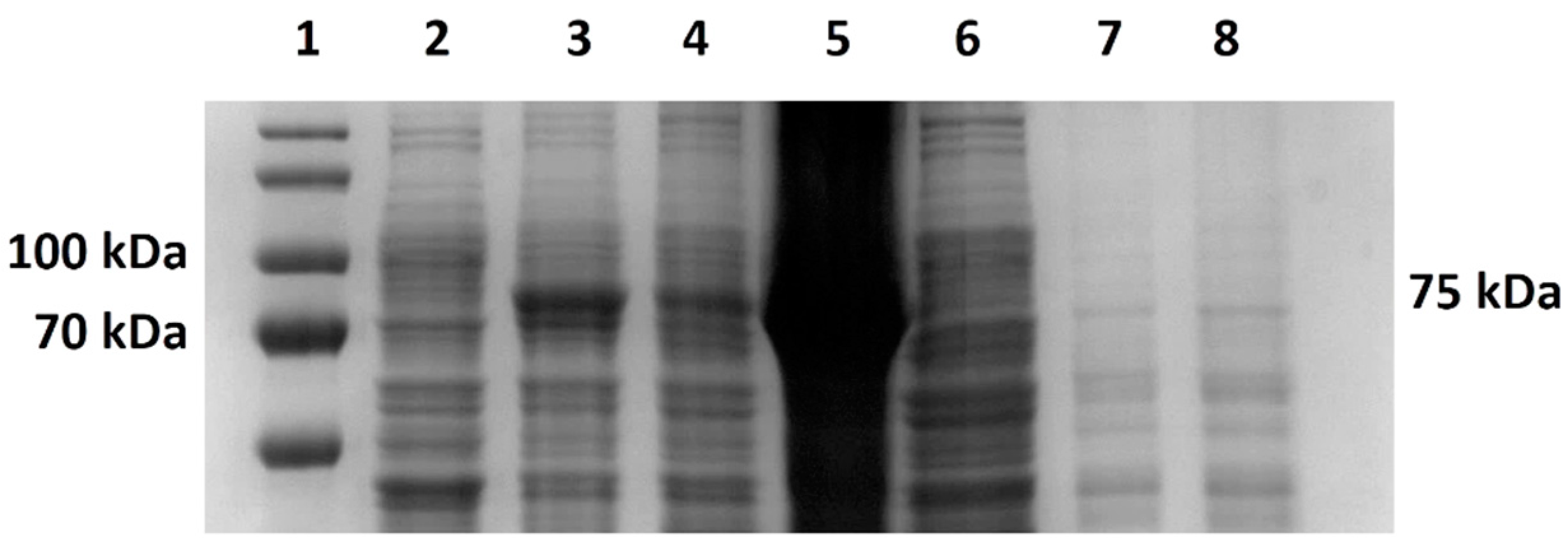
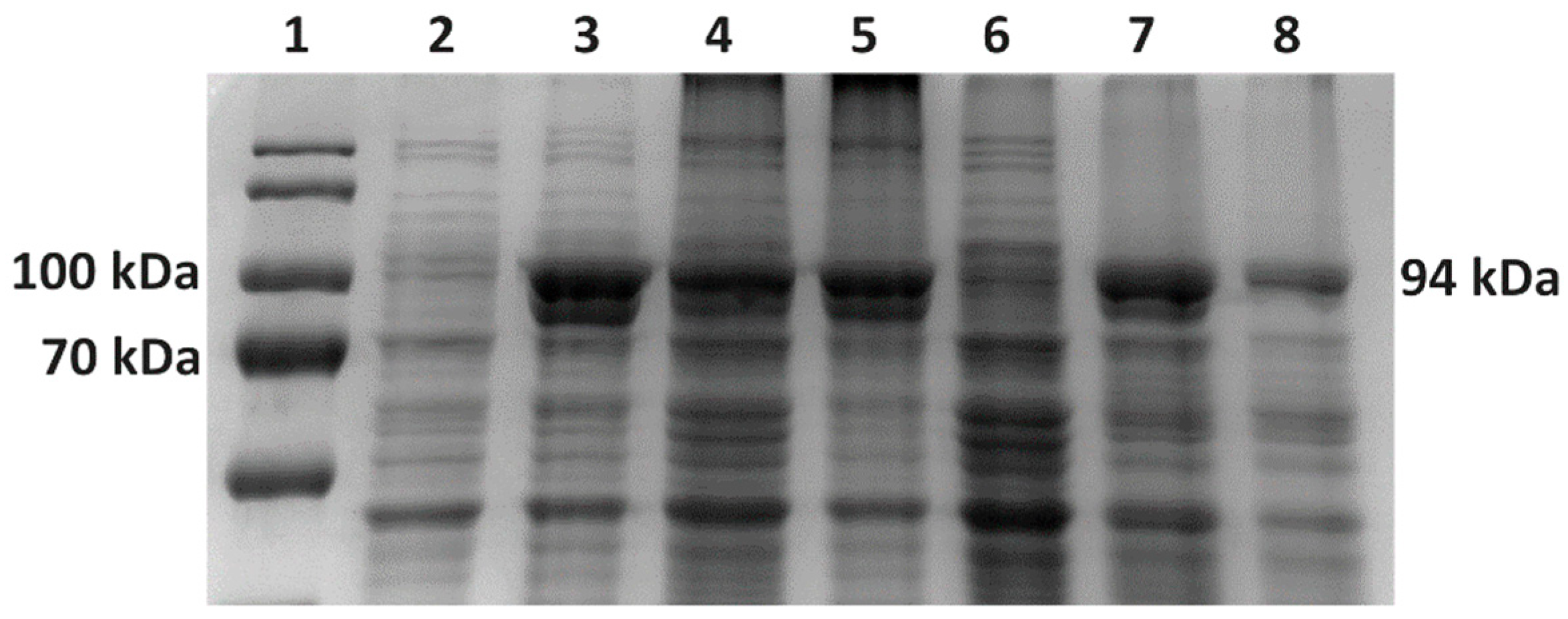
TM Prestained Protein Ladder 26616 (Thermofisher, Waltham, MA, USA) or Novex™ Sharp Pre-stained Protein Standard (Invitrogen, Waltham, MA, USA). Electrophoretic separation was run in 1× concentrated electrode buffer solution. We set the voltage to the maximum, with the current set to 20 mA per gel for 90 min. After electrophoretic separation, we stained the gel with a staining solution (Coomassie brilliant blue G-250 0.1%, ammonium sulfate 10%, phosphoric acid 2%).
2.7. Western Blot
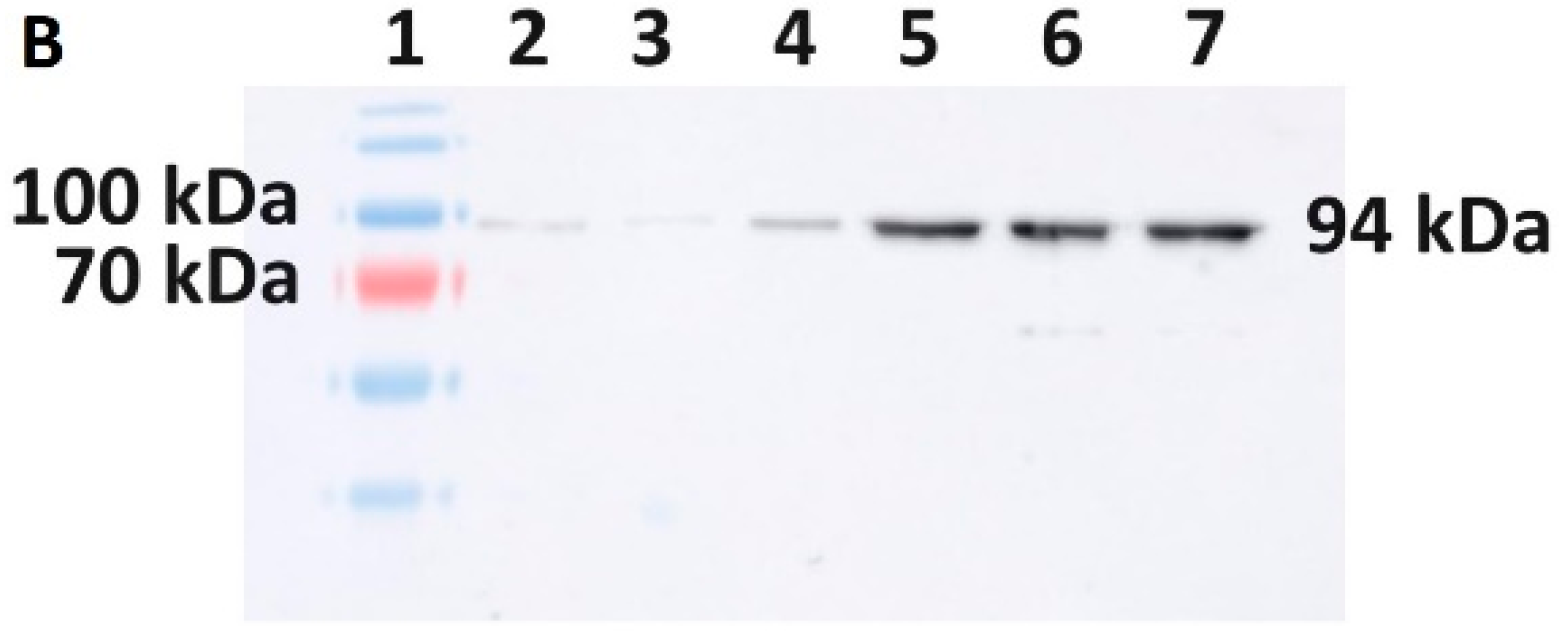
Western blot was used for the specific detection of mutant Taq DNA polymerase and mutant M-MLV reverse transcriptase. Protein samples separated on SDS-PAGE were transferred to a PVDF membrane (Bio-Rad). The semi-dry transfer was performed using a Biometra blotter. The parameters of the analysis were set to 10 V, 220 mA, 45 min. The PVDF membrane was incubated in a specific antibody solution (mouse anti-His) (Invitrogen) at a dilution of 1:1000 in a 1% skimmed milk solution at 4 °C overnight. The next day, the membrane was washed with TBST solution and incubated with anti-mouse secondary antibody solution (1:10,000) in a 1% skimmed milk solution (Goat Anti-Mouse IgG, Sigma Aldrich, Saint-Louis, MO, USA) for 1 h at room temperature. Proteins on the membrane were visualized by using a chemiluminescence solution, namely Clarity Max Western ECL Substrate kit (Bio-Rad), according to the manufacturer’s protocol. Subsequently, the signal was detected by using an ImageQuant LAS 500 chemiluminescence CCD camera (GE Healthcare, Chicago, IL, USA).
2.8. Protein Precipitation
The sample containing mutant Taq DNA polymerase was initially heated at 70 °C for 20 min to remove thermolabile proteins. After this heating step, we centrifuged the suspension at 12,000×
g at 4 °C (UNIVERSAL 320R, Hettich) and then precipitated the protein by using a modified protocol according to Pluthero [
33] with ammonium sulfate. We added (NH
4)
2SO
4 to the sample at a concentration of 176 g/L and mixed it on ice for 1 h. After centrifuging the suspension, we resuspended the sediment in a sonication buffer A and dialyzed it.
2.9. Dialysis
To remove low molecular weight substances from the sample after protein precipitation and affinity chromatography, we chose dialysis. The sample was transferred into a SnakeSkin Dialysis Tubing bag, 10K MWCO, 16 mm (Thermo Fisher Scientific, Waltham, MA, USA), and dialyzed against sonication buffer A. The dialysis was performed at 4 °C with constant stirring.
2.10. Affinity Chromatography
Purification of the samples was carried out by high-pressure affinity chromatography on a ÄKTA Avant 25 (GE Healthcare) with 1 mL HisTrapTM HP (GE Healthcare), which contained Ni SepharoseTM affinity medium for the capture and purification of affinity-tagged histidine proteins (6× His-tag). The column was washed with water and equilibration buffer (50 mM Tris HCl, pH 8.0, 0.5 M NaCl). The sample was applied to the column via a pump, and the uncaptured fraction was collected by an automated fraction collector. The bound proteins on the column were eluted using an elution solution (50 mM Tris HCl pH 8.0, 0.5 M NaCl, 0.5 M imidazole). The fraction containing the elution was dialyzed against sonication buffer A.
2.11. Reverse Transcription
RNA encoding green fluorescent protein (GFP) at a concentration of 50 mg/L was used as the template. The positive control was a sample containing commercial reverse transcriptase from the QuantiFast SYBR Green RT-PCR Kit. Reverse transcription was carried out in a LabCycler BASIC, SensoQuest at 42 °C for 60 min.
2.12. PCR
The RNA transcribed into cDNA was amplified by PCR, which was performed in a Labcycler Gradient Thermocycler (SensoQuest, Göttingen, Germany). The protocol specified by the manufacturer of the Taq DNA Polymerase (New England Biolabs®) (denaturation at 95 °C for 20 s, annealing at 55 °C for 30 s, and polymerization at 68 °C for 60 s, in 30 cycles) was used for amplification.
4. Discussion
E. coli remains the most widely used host organism in the production of recombinant proteins. Several approaches have been developed to improve protein production, such as codon optimization [
34], the screening and creation of new strains [
1], and the use of tags or chaperones [
35]. Nevertheless, a lack of protein production, the formation of inclusion bodies [
36], or the contamination of the culture with phages [
37] often occurs. Therefore, the use of an alternative expression system, such as the Gram-negative bacterium
V. natriegens, is advisable. It has a doubling time of <10 min [
2], which makes it the fastest useable growing bacterium and thus a highly attractive expression host [
5]. The explanation for the high levels of biosynthesis might lie partly with its number of ribosomes since
V. natriegens has 115,000 per cell in the exponential phase, whereas
E. coli has 70,000–90,000 [
38].
The primary objective of our study has been to evaluate the production efficiency of mutant Taq DNA polymerase and mutant M-MLV reverse transcriptase under controlled conditions in two host organisms: E. coli and V. natriegens. Another goal has been to determine the optimal organism for the production of these specific proteins. Additionally, our aim has been to assess whether different expression conditions, including media composition and temperature, influence the yield of the target proteins.
The selection of a suitable culture medium is an important factor for protein production, with several essential components such as a carbon source, nitrogen, essential salts, and minerals being required. In general, three types of media are available: chemically defined, semi-defined, and complex. The selection of the appropriate medium is important to achieve optimum cell density. Changing the conditions of cultivation is the simplest modification for altering bacterial growth. The most suitable medium for
E. coli is LB medium and a simple change to richer media can lead to an increase in production [
39]. Media change was successful in the case of the Taq DNA polymerase when its production in
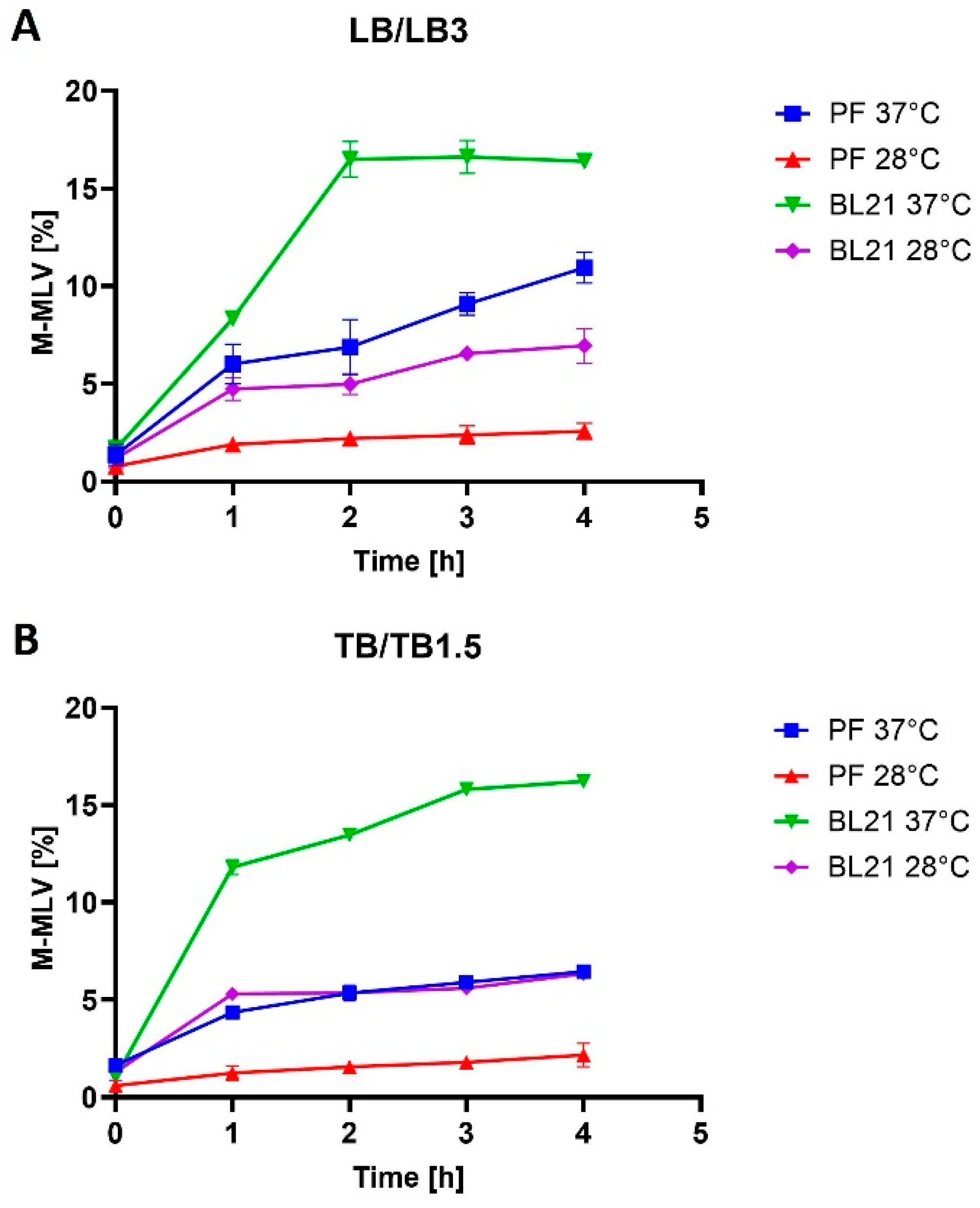
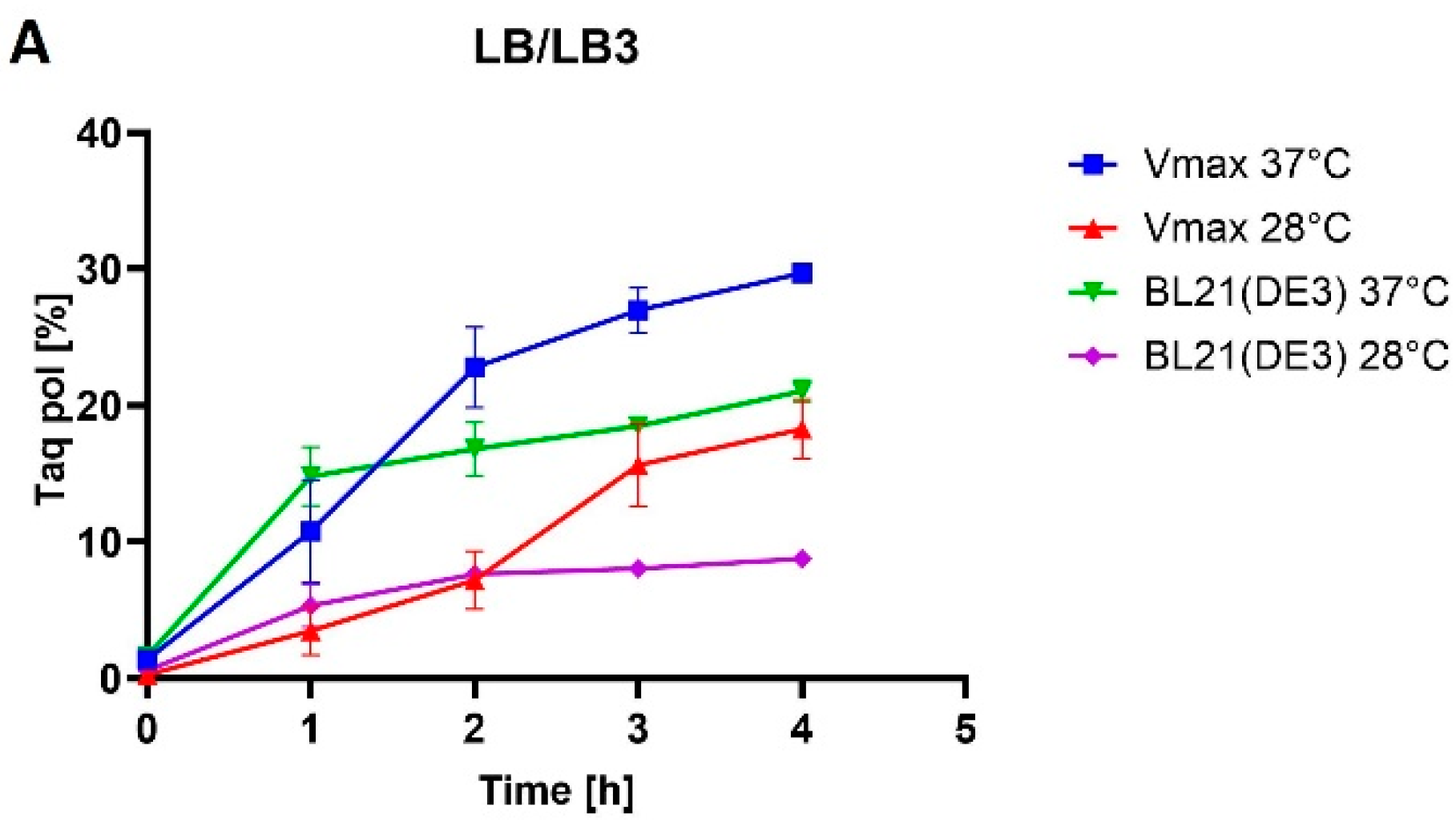
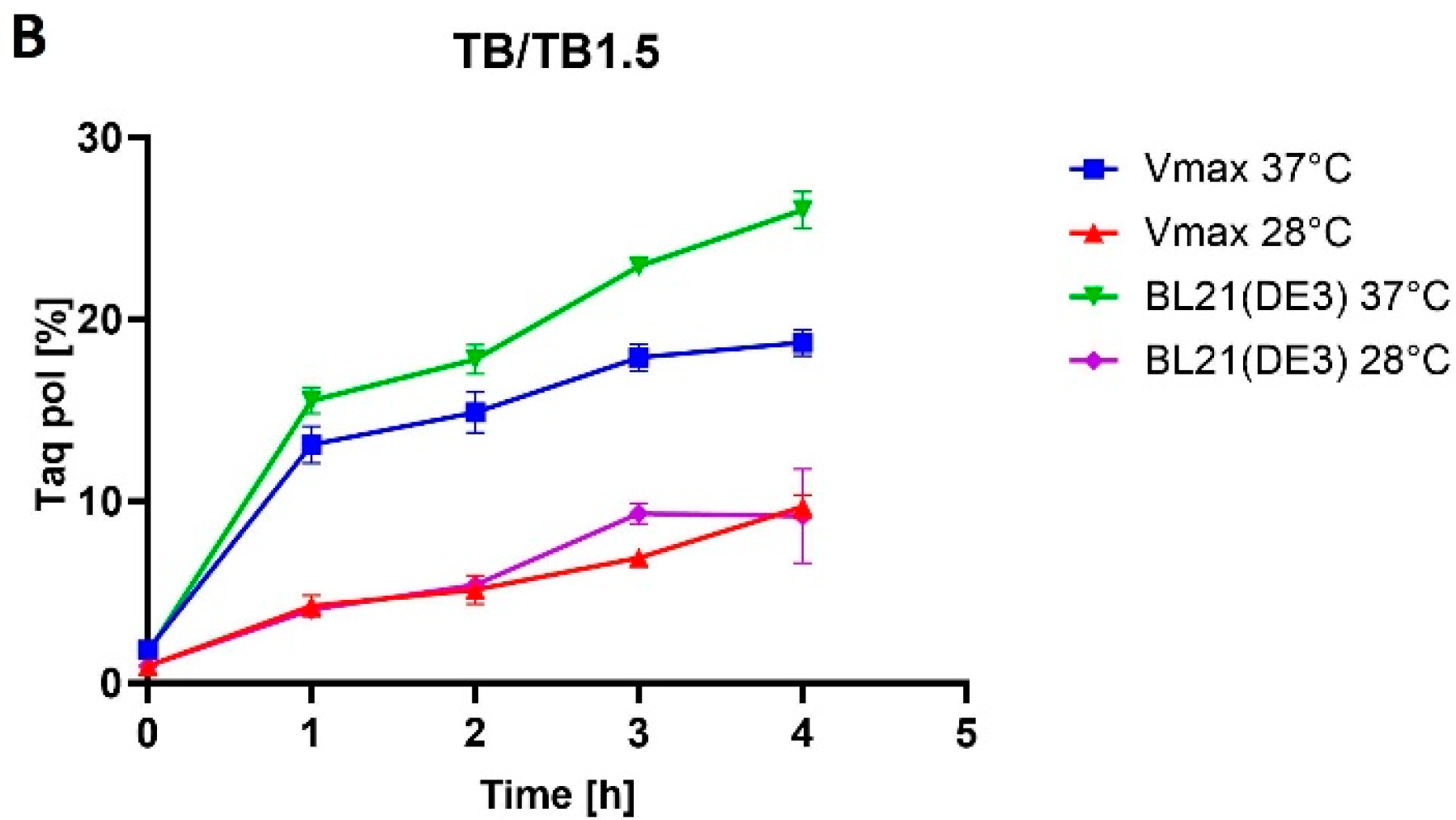
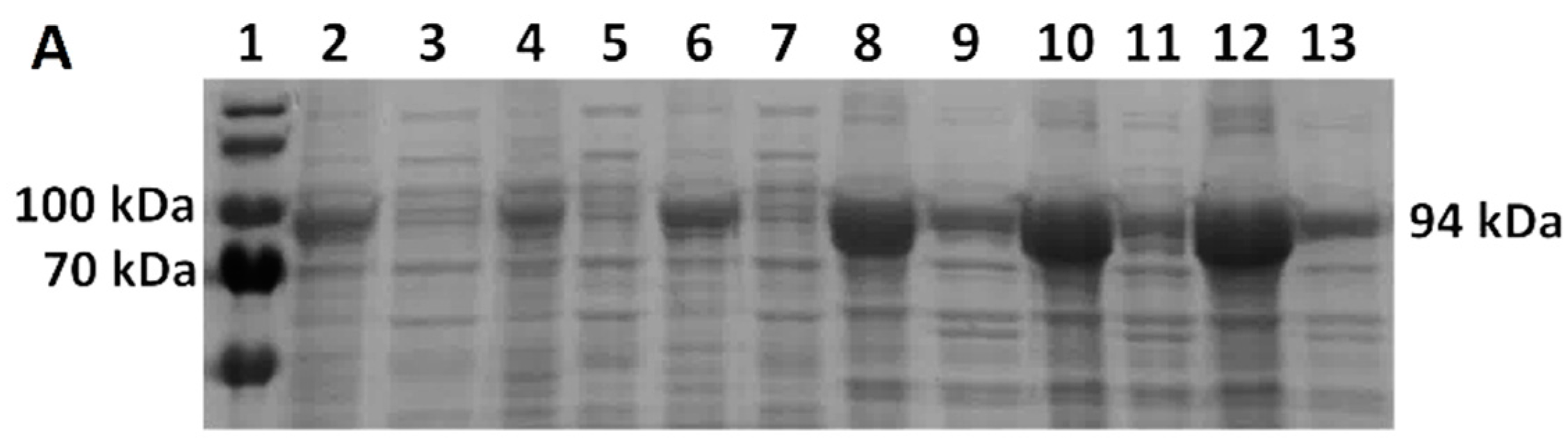
E. coli BL21(DE3) cells increased from 21% of TCPs in LB medium to 26% of TCPs in TB medium at 37 °C. M-MLV reverse transcriptase production was comparable in both media at 37 °C and accounted for approximately 16% of TCPs.
V. natriegens is a marine bacterium, so it needs an increased salt concentration for optimal growth. Lee et al. [
4] used LB medium with the addition of 3% NaCl and standardized LB3 medium as a medium suitable for
V. natriegens because of its simplicity. Xu et al. [
7] employed a modified TB medium with the addition of 1.5% NaCl (designated here as TB1.5). We utilized these two media in
V. natriegens as complementary to the media in which we grew
E. coli. However, the use of the richer TB1.5 medium did not increase the production rate, as the Taq DNA polymerase production rate dropped from 30% to 19% of TCPs (LB3 versus TB1.5), and similarly for MMLV reverse transcriptase, from 11% to 6% of TCPs.
Protein misfolding is a significant challenge in the bacterial production of recombinant proteins [
40]. The lowering of the expression temperature is typically one of the first and easiest steps to increase the solubility of the produced protein [
41,
42]. The use of lower temperatures in production processes usually results in higher yields of soluble protein and improved biological activity of the protein [
43]. However, in all our cases, no obvious benefit arose from a reduction of expression temperature. Our results indicate a significant effect of temperature on the yield of the expression system. In the case of the production of mutant M-MLV reverse transcriptase in
E. coli BL21, a 2.5-fold decrease occurred in the percentage representation of the target protein at a lower temperature in both media. During the production at lower temperatures in
V. natriegens PF, a more significant reduction was detected in yield compared with production in
E. coli. In LB medium, the yield decreased by 4.4-fold, and in TB medium by 3-fold. Interestingly, for mutant Taq DNA polymerase production, as expected, a decrease was also found in production, but unlike in the case of mutant M-MLV reverse transcriptase production, the decrease was higher in
E. coli than in
V. natriegens. Regarding production in
V. natriegens Vmax™ Express, a smaller decrease was recorded in production at lower temperatures compared with production in
E. coli, in which protein production decreased in LB medium by 1.6-fold and in TB medium by 1.9-fold. For the production of mutant Taq DNA polymerase in
E. coli BL21(DE3), the production decrease was 2.2-fold in LB medium and 2.6-fold in TB medium. The decrease in production temperature had no impact on protein solubility in any of the cases. Given the low yields at lower temperatures, we assessed production at lower temperatures as being less favorable. The variance in our results might arise not only from the target protein but also from the utilization of distinct strains of the same organisms. Another factor to consider is that even single-point mutations can cause significant changes in protein solubility [
44].
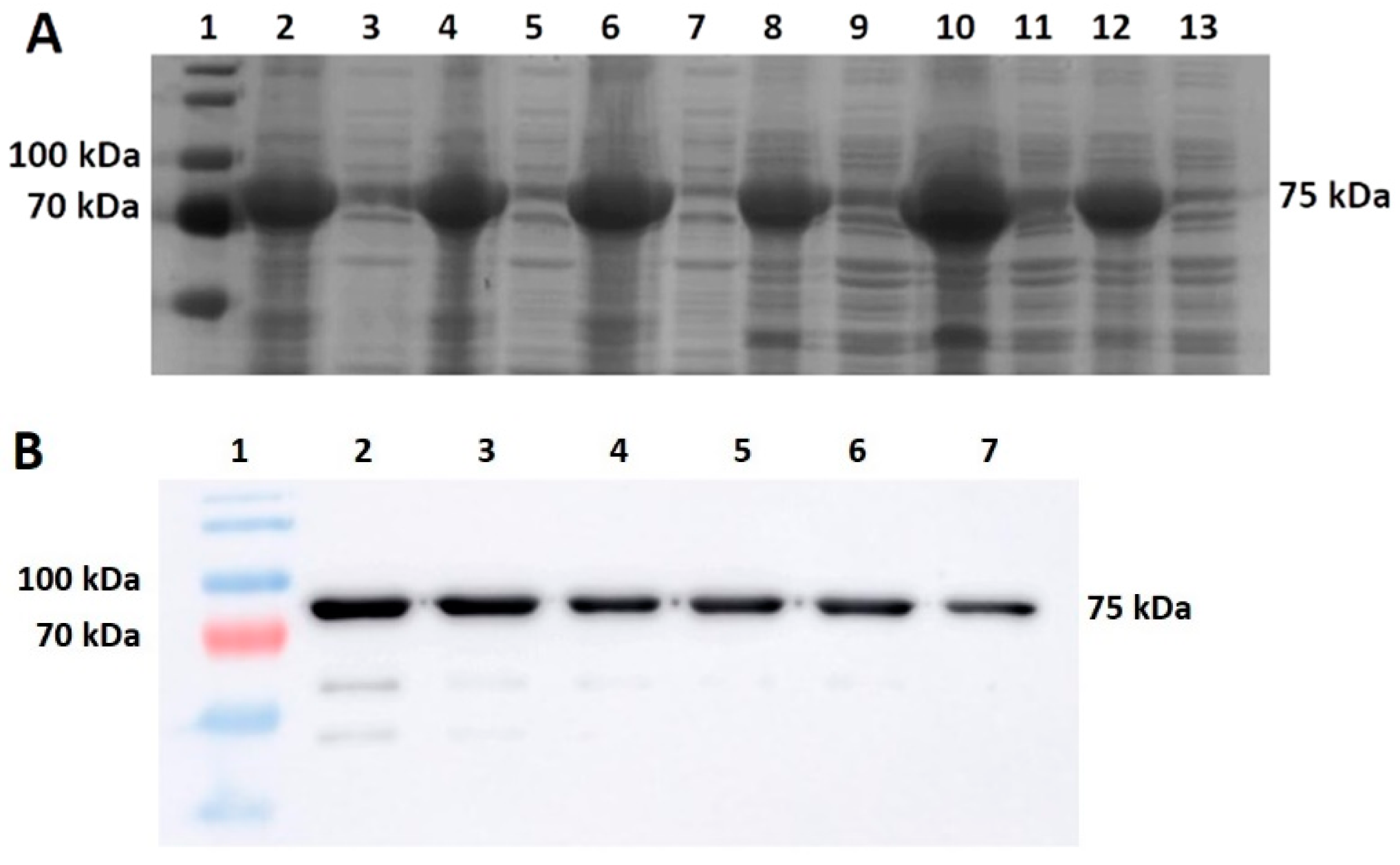
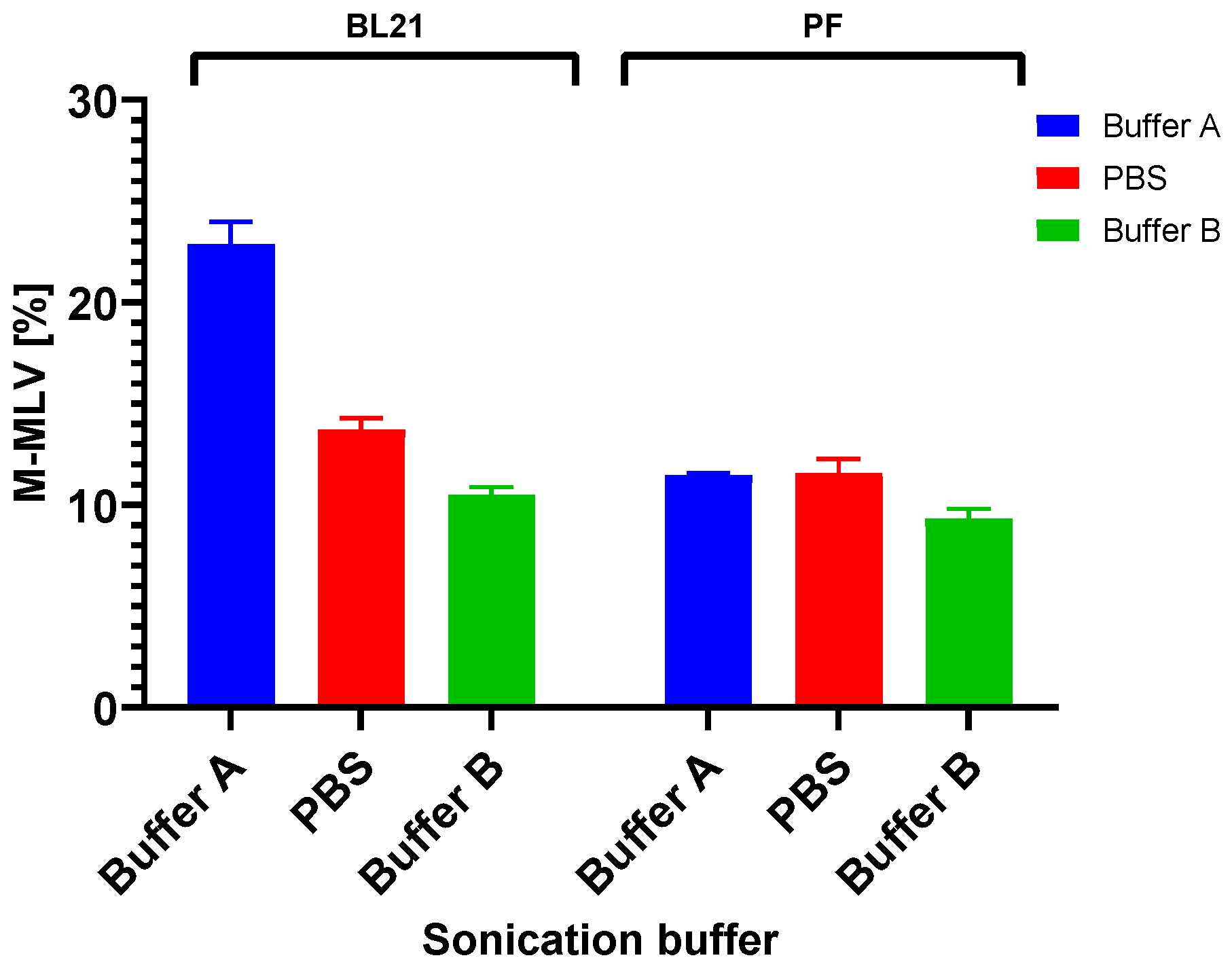
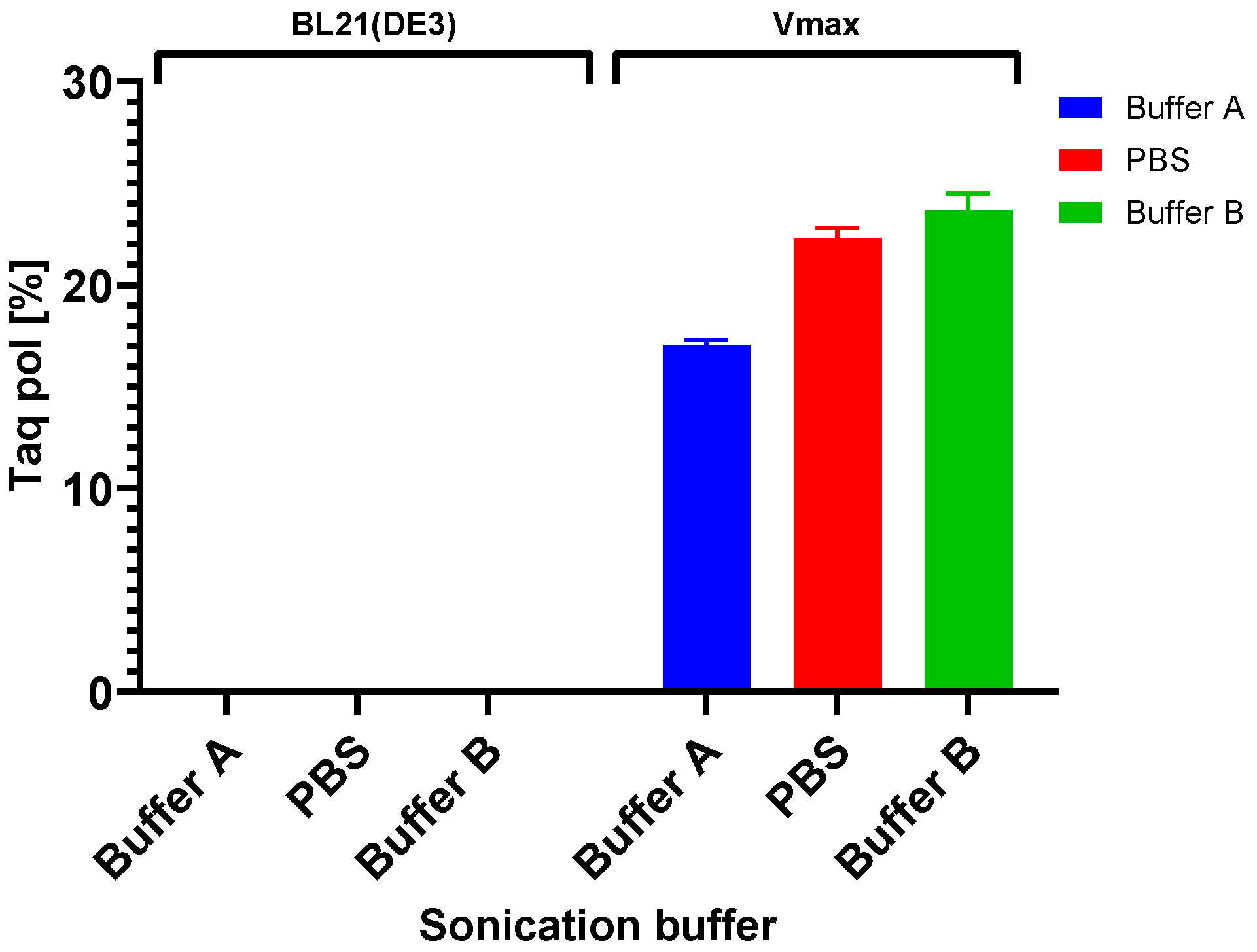
Additionally, since lowering the temperature of expression did not help to increase the solubility of the target protein in any of the cases in our study, we focused on increasing the solubility by using various sonication buffers. Importantly, when selecting a suitable sonication buffer, not only should the host organism be considered, but also the protein being produced whose properties, e.g., protein activity, might be affected. Our results show, in both cases, the importance of the determination of a suitable buffer. Regarding the evaluation of the solubility of M-MLV, our findings distinctly demonstrate a higher production of the protein in E. coli in the soluble fraction. Interestingly, in the case of production in E. coli, we noticed relatively large differences in the effect of the sonication buffer on the solubility of M-MLV in comparison to V. natriegens. Based on our findings, Buffer A is the most suitable option for M-MLV production in E. coli, yielding 1.6 times more soluble protein compared with that in PBS buffer and 2 times more than that in Buffer B. The highest solubility was attained in the PBS buffer, but in comparison with the lowest solubility achieved in Buffer B, this value was only 1.3 times higher. Interestingly, we obtained nearly opposite results when assessing the solubility of Taq pol. We detected only a negligible amount of soluble protein detected by Western blot when produced in E. coli. On the other hand, we were able to obtain 23% of TCPs when using Buffer B, which is 1.3 times higher than the lowest amount of protein obtained when using Buffer A. We did not observe a significant difference in the amount of soluble protein when using Buffer B compared with PBS.
We were only able to produce negligible amounts of soluble Taq polymerase in
E. coli intracellularly.
V. natriegens intracellular production resulted in increased solubility when we used diverse buffers. Extracellular protein production can be an excellent strategy for producing recombinant proteins that are insoluble when produced intracellularly. Other advantages include the simplification of downstream processes, cost-effectiveness, and time-saving. Overexpression of D,D-carboxypeptidases causes the non-selective leakage of proteins into the media [
15]. Interestingly, extracellular production of Taq polymerase without co-expression after 24 h was 1.13 times higher compared with extracellular production with co-expression. The extracellular protein production without co-expression was probably caused by the induction of a prophage naturally present in
V. natriegens.
V. natriegens contains two prophage regions (VNP1 and VNP2) that can exhibit spontaneous activation under standard cultivation conditions [
30]. The higher amounts of protein produced extracellularly without co-expression might be caused by the overall better production without co-expression, as it imposes less burden on the cell. These results suggest that extracellular production might be advantageous for the production of mutant Taq DNA polymerase; however, activity verification will be necessary. On the contrary, for extracellular M-MLV production, this strategy did not prove to be successful. This may be attributable to such production involving the passive transport of proteins across the membrane without any secretion signals or to low production overall. Nevertheless, extracellular production might be suitable for proteins such as enzymes, as it allows the shortening and simplification of downstream processes, eliminating the need for harsh cell homogenization and similar steps that might affect the activity of the produced enzyme.
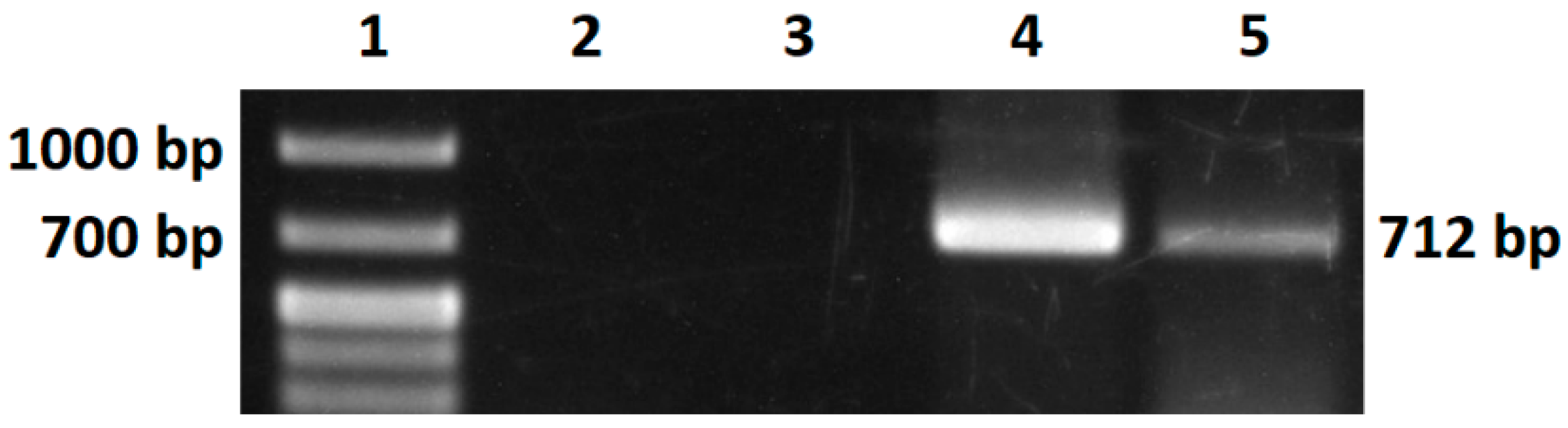
M-MLV reverse transcriptase activity was confirmed in both expression systems, so we were able to produce an active enzyme. The activity was determined by reverse transcription followed by cDNA amplification. In our case, RNA for GFP protein was used, a similar procedure for qualitative assay having been chosen by Nuryana et al. [
45] when they used RNA of SARS-CoV-2 as a template. Subsequently, they also determined the specific activity of the enzyme; for the quantitative assay, the reverse transcriptase activity was measured using an EnzChek
TM RT assay kit. We will focus on the quantification of synthesized cDNA by using qPCR and other quantification methods in future work. We have not been able to obtain an active Taq DNA polymerase as yet. The inactivity of the enzyme might be caused by the protein precipitation method or by the degradation of the protein itself. Chen et al. have found that ammonium sulfate precipitation leads to the loss of Taq Polymerase I activity by 30–40% [
46]. We could probably increase the activity of mutant Taq DNA polymerase by ethanol precipitation, as originally suggested by Chen et al. [
46]. The huge advantage of ethanol precipitation is that it does not require dialysis, which saves time, reduces costs, and prevents the loss of enzyme activity. Another aspect to take into consideration is the storage time. According to Chen et al., the Taq Pol Ι activity is lost after 2 days of storage at −20 °C [
46]. However, we analyzed the activity of mutant Taq DNA polymerase immediately after the purification step. Nevertheless, we propose that, in our case, the loss of enzymatic activity is probably caused by the combination of the single-point mutations that were introduced into the sequence of the enzyme. According to Mildvan et al. [
47], when two mutations have opposing structural effects, they can negatively impact the enzyme’s ability to bind to its substrate. If the negative impact is significant enough, it can lead to the inactivity of the enzyme. We will now focus on the gradual mutation of the enzyme and on monitoring the impact of individual mutations on the enzyme activity.