Prostate-Specific Membrane Antigen Radioligand Therapy in Non-Prostate Cancers: Where Do We Stand?
Abstract
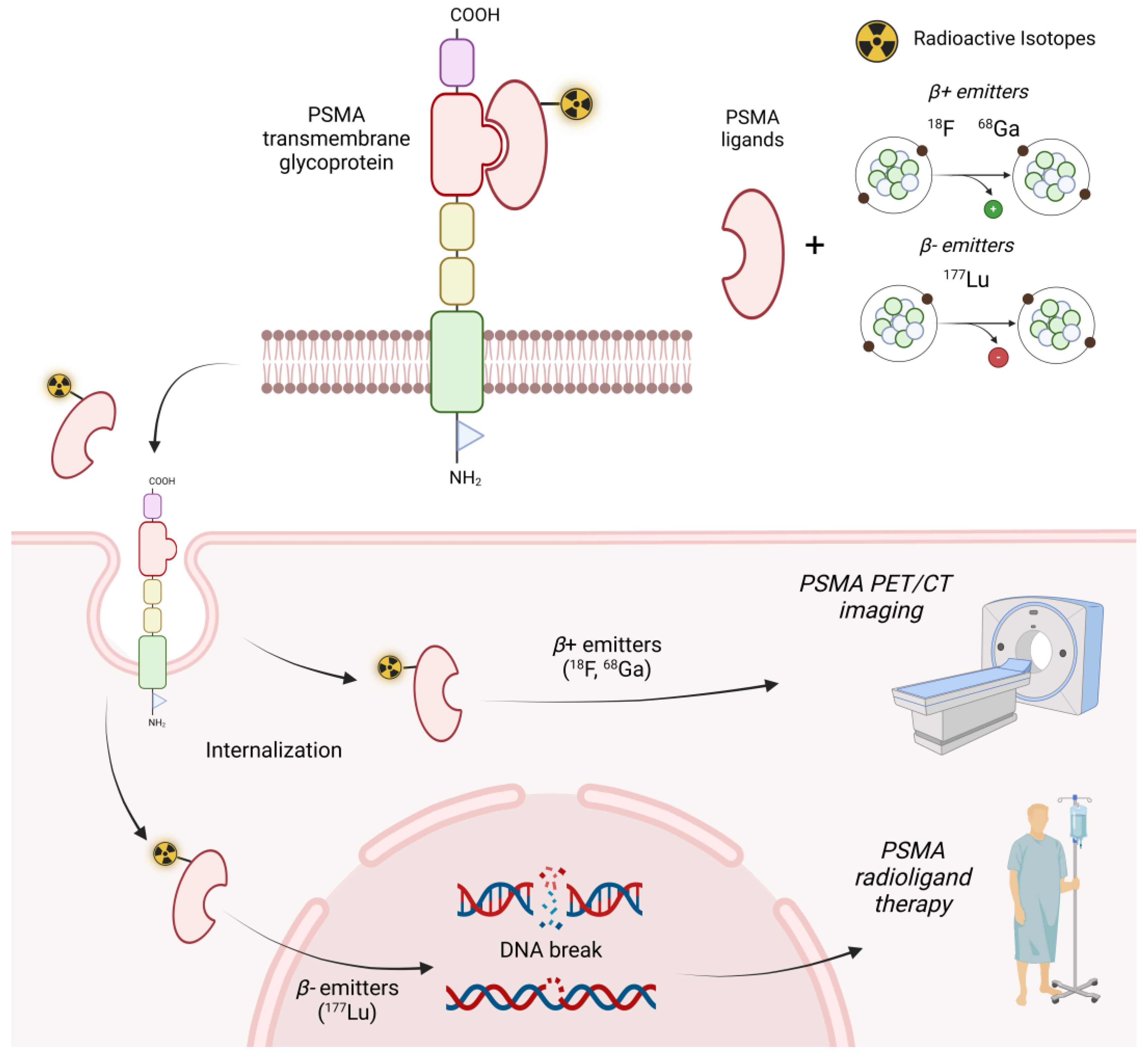
1. Introduction
2. Search Strategies
3. Results
3.1. Preclinical Studies
| First Author [Ref.] | Year | Type of Disease | Cell Lines/Mouse Model | Main Findings |
|---|---|---|---|---|
| Heesch A [27] | 2023 | Breast cancer | Endothelial cell line (HUVEC), benign breast epithelial cell line (MCF-10A), PCa cell line (LNCaP), and TNBC cell lines (MDA-MB-231, MDA-MB-468, BT-20, Hs578T, SUM149PT, SUM1315MO2, HCC1937) | PSMA expression was detected in 91% of the investigated TNBC cell lines. Hypoxic conditions significantly increased the uptake of [177Lu]Lu-PSMA in MDA-MB-231 (0.4% vs. 3.4%) and MCF-10A (0.3% vs. 3.0%). [177Lu]Lu-PSMA-induced apoptosis rates were highest in BT-20- and MDA-MB-231-associated endothelial cells. |
| Morgenroth A [28] | 2019 | Breast cancer | - Human breast cancer cell lines (MDA-MB 231 and MCF-7); endothelial cells (HUVEC). - Subcutaneous xenograft. | [177Lu]Lu-PSMA-617 impaired the vitality and angiogenic potential of cells. In vivo, PSMA accumulated specifically in triple-negative breast cancer xenografts. |
| Heesch A [29] | 2024 | Breast cancer | - Human breast cancer cell lines MDA-MB-231. - Orthotopic xenograft. | The tumour volume 30 days after therapy was significantly smaller for the single-dose (p < 0.001) and fractionated dose (p < 0.001) groups compared with the control. In the tumour tissue, both therapy groups showed a higher amount of apoptotic cells compared with the control group |
| Lu Q [30] | 2023 | HCC | - Human hepatocellular cancer cells HepG2. - Subcutaneous xenograft. | Tumour growth was significantly suppressed in the 37 MBq [177Lu]Lu-PSMA-617, 18.5 MBq [177Lu]Lu-PSMA-617, and 7.4 MBq [177Lu]Lu-EB-PSMA-617 groups compared with the saline group. Median survival was 40, 44, 43, and 30 days, respectively. No healthy organ toxicity was observed. |
3.2. Clinical Studies
| First Author [Ref.] | Year | Type of Disease | Patients | 177Lu-PSMA RLT | Main Findings |
|---|---|---|---|---|---|
| Civan C [31] | 2023 | Salivary gland tumours | 5 | One cycle in three patients and RLT completed in two patients; 6.8 ± 1.4 GBq; time interval 6 weeks | PSMA RLT was well tolerated and stabilized disease in one patient. However, frequent discontinuation after one PSMA RLT cycle and low tumor absorbed doses were shown. |
| Klein Nulent TJW [32] | 2021 | Metastatic salivary gland tumours | 6 | One to four cycles; 6.0–7.4 GBq; interval time 6–8 weeks | When tumour targeting was sufficient, palliative PSMA RLT of advanced/metastasized salivary gland cancer may cause a significant relief of tumour-associated discomfort and may induce disease control in one-third of the cases. |
| Has Simsek D [33] | 2019 | Adenoid cystic carcinoma of the parotid | 1 | One cycle, 7.5 GBq | The treatment was well tolerated with no side effects reported. Significant but not complete pain relief was expressed by the patient. |
| Wang G [34] | 2022 | Adenoid cystic carcinoma | 4 | Up to three cycles; 1.85 GBq; interval time 8–10 weeks | PSMA RLT based on [177Lu]Lu-EB-PSMA-617 may be a promising treatment for adenoid cystic carcinoma. |
| Graef J [35] | 2023 | High-grade glioma | 3 | Two cycles; median activity of 6.03 GBq (IQR 5.74–6.10) | In high-grade glioma, a minority of patients were eligible for PSMA-RLT, and the tumour dose was too low for a sufficient therapeutic effect. |
| Truckenmueller P [36] | 2022 | High-grade glioma | 3 | Two cycles; median activity of 6.03 GBq (5.74–6.10); time interval 9–11 weeks | Only a minor proportion of the patients were eligible for PSMA-RLT based on the TBRmax threshold. |
| Hirmas N [37] | 2021 | Hepatocellular carcinoma | 2 | One cycle; 5.9–6.2 GBq | PSMA-RLT was not effective since it did not yield a sufficient tumour radiation dose. |
| Wächter S [40] | 2021 | Anaplastic and poorly differentiated thyroid carcinoma | 1 | Two cycles, 6.3 GBq e 7.4 GBq, time interval 8 weeks | PSMA-targeted therapy could be used as an alternative option in selected patients if they showed progression after established therapeutic lines. |
| de Vries LH [41] | 2020 | Radioactive iodine-refractory differentiated thyroid cancer | 2 | Two cycles; 6 GBq, time interval 6 and 11 weeks | PSMA-RLT showed a modest, temporary response. |
| Digklia A [42] | 2022 | Uterine leiomyosarcoma | 1 | Two cycles (2 months apart) combined with 240 mg of nivolumab (every 2 weeks) | At 6 months post-treatment, a reduction in the tumor growth rate (TGR (%/month) from 36.46%/m to 11.25%/m was shown. |
| Jüptner [43] | 2019 | Vena cava leiomyosarcoma | 1 | One cycle, 6 GBq | Treatment was well tolerated. However, because of the week retention of the radiotracer, the therapy was discontinued, and no further treatment cycles were arranged. |
| Simsek [44] | 2021 | Testicular mixed germ cell tumour | 1 | One cycle, 7.5 GBq | Treatment was well tolerated without any adverse effects. However, the disease progressed. |
3.3. Ongoing Clinical Trials
| Type of Cancer | Centre/Sponsor | Patients | Study Phase | Trial ID (Reference) | Status |
|---|---|---|---|---|---|
| Adenoid cystic carcinoma | Peking Union Medical College Hospital, Beijing, China | 40 | Early Phase I | NCT04801264 | Unknown |
| Renal cell carcinoma | Peking Union Medical College Hospital, Beijing, China | 40 | Not Applicable | NCT05170555 | Unknown |
| Salivary gland cancer | Radboud University Medical Center, Nijmegen, Gelderland, Netherlands | 12 | Phase II | NCT04291300 | Completed |
| PSMA-positive tumours | Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori, Cesena, Italy | 100 | Phase II | NCT05867615 | Recruiting |
| High-grade glioma | St. Olavs Hospital, Trondheim, Norway | 10 | Not Applicable | NCT05644080 | Recruiting |
| Soft tissue sarcoma | University of Lausanne Hospitals, Lausanne, Vaud, Switzerland | 20 | Phase I | NCT05420727 | Recruiting |
| Metastatic clear cell renal cancer | Centre Leon Berard, Lyon, France | 48 | Phase I/II | NCT06059014 | Recruiting |
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrmann, K.; Larson, S.M.; Weber, W.A. Theranostic Concepts: More Than Just a Fashion Trend-Introduction and Overview. J. Nucl. Med. 2017, 58 (Suppl. S2), 1S–2S. [Google Scholar] [CrossRef]
- Weber, W.A.; Barthel, H.; Bengel, F.; Eiber, M.; Herrmann, K.; Schäfers, M. What Is Theranostics? J. Nucl. Med. 2023, 64, 669–670. [Google Scholar] [CrossRef]
- Hertz, S.; Roberts, A. Radioactive iodine in the study of thyroid physiology; the use of radioactive iodine therapy in hyperthyroidism. J. Am. Med. Assoc. 1946, 131, 81–86. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. Radionuclide Therapy of Metastatic Prostate Cancer. Semin. Nucl. Med. 2019, 49, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.L., Jr.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Calais, J.; Ceci, F.; Cho, S.Y.; Fanti, S.; Giesel, F.L.; Goffin, K.; et al. PSMA PET/CT: Joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Broeck, T.V.D.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024; in press. [Google Scholar] [CrossRef]
- Alberts, I.L.; Seide, S.E.; Mingels, C.; Bohn, K.P.; Shi, K.; Zacho, H.D.; Rominger, A.; Afshar-Oromieh, A. Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: A systematic review and network meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2978–2989, Erratum in Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3014–3016. [Google Scholar] [CrossRef]
- Gühne, F.; Radke, S.; Winkens, T.; Kühnel, C.; Greiser, J.; Seifert, P.; Drescher, R.; Freesmeyer, M. Differences in Distribution and Detection Rate of the [68Ga]Ga-PSMA Ligands PSMA-617, -I&T and -11-Inter-Individual Comparison in Patients with Biochemical Relapse of Prostate Cancer. Pharmaceuticals 2021, 15, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berliner, C.; Tienken, M.; Frenzel, T.; Kobayashi, Y.; Helberg, A.; Kirchner, U.; Klutmann, S.; Beyersdorff, D.; Budäus, L.; Wester, H.J.; et al. Detection rate of PET/CT in patients with biochemical relapse of prostate cancer using [68Ga]PSMA I&T and comparison with published data of [68Ga]PSMA HBED-CC. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Maurer, T.; van derPoel, H.; Alongi, F.; Kunikowska, J.; Laudicella, R.; Fanti, S.; Hofman, M.S. [68Ga]Ga-PSMA Versus [18F]PSMA Positron Emission Tomography/Computed Tomography in the Staging of Primary and Recurrent Prostate Cancer. A Systematic Review of the Literature. Eur. Urol. Oncol. 2022, 5, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.; Sengul, S.S.; Cetin, B.; Avcı, M.; Yagci, S.; Ozkoç, I.; Barikan, D.E.; Yildiz, M. The role of Ga68 PSMA PET/CT imaging in Lu177 PSMA treatment planning in metastatic castration-resistant prostate cancer. Ann. Nucl. Med. 2022, 36, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.O.; Bruchertseifer, F.; Vorster, M.; Morgenstern, A.; Sathekge, M.M. Prostate-specific membrane antigen-targeted endoradiotherapy in metastatic prostate cancer. Curr. Opin. Urol. 2020, 30, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fallah, J.; Agrawal, S.; Gittleman, H.; Fiero, M.H.; Subramaniam, S.; John, C.; Chen, W.; Ricks, T.K.; Niu, G.; Fotenos, A.; et al. FDA Approval Summary: Lutetium Lu 177 Vipivotide Tetraxetan for Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2023, 29, 1651–1657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M., Jr.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar] [PubMed]
- Chang, S.S.; O’Keefe, D.S.; Bacich, D.J.; Reuter, V.E.; Heston, W.D.; Gaudin, P.B. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 1999, 5, 2674–2681. [Google Scholar] [PubMed]
- Conway, R.E.; Rojas, C.; Alt, J.; Nováková, Z.; Richardson, S.M.; Rodrick, T.C.; Fuentes, J.L.; Richardson, N.H.; Attalla, J.; Stewart, S.; et al. Prostate-specific membrane antigen (PSMA)-mediated laminin proteolysis generates a pro-angiogenic peptide. Angiogenesis 2016, 19, 487–500. [Google Scholar] [CrossRef]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D.W. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Krohn, T.; Heinzel, A.; Mottaghy, F.M.; Behrendt, F.F. First evidence of PSMA expression in differentiated thyroid cancer using [68Ga]PSMA-HBED-CC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1622–1623. [Google Scholar] [CrossRef] [PubMed]
- Udovicich, C.; Callahan, J.; Bressel, M.; Ong, W.L.; Perera, M.; Tran, B.; Azad, A.; Haran, S.; Moon, D.; Chander, S.; et al. Impact of Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography in the Management of Oligometastatic Renal Cell Carcinoma. Eur. Urol. Open Sci. 2022, 44, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Dall’Armellina, S.; Pizzuto, D.A.; Perotti, G.; Zagaria, L.; Lanni, V.; Treglia, G.; Racca, M.; Annunziata, S. PSMA Radioligand Uptake as a Biomarker of Neoangiogenesis in Solid. Tumours: Diagnostic or Theragnostic Factor? Cancers 2022, 14, 4039. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, C.; Sathekge, M.; de Spiegeleer, B.; De Jonghe, P.J.; Debruyne, P.R.; Borms, M.; Beels, L.; Maes, A. PSMA expression on neovasculature of solid tumors. Histol. Histopathol. 2020, 35, 919–927. [Google Scholar] [PubMed]
- Miceli, A.; Liberini, V.; Pepe, G.; Dondi, F.; Vento, A.; Jonghi Lavarini, L.; Celesti, G.; Gazzilli, M.; Serani, F.; Guglielmo, P.; et al. Prostate-Specific Membrane Antigen Positron Emission Tomography Oncological Applications beyond Prostate Cancer in Comparison to Other Radiopharmaceuticals. Diagnostics 2024, 14, 1002. [Google Scholar] [CrossRef] [PubMed]
- Heesch, A.; Ortmanns, L.; Maurer, J.; Stickeler, E.; Sahnoun, S.E.M.; Mottaghy, F.M.; Morgenroth, A. The Potential of PSMA as a Vascular Target in TNBC. Cells 2023, 12, 551. [Google Scholar] [CrossRef]
- Morgenroth, A.; Tinkir, E.; Vogg, A.T.J.; Sankaranarayanan, R.A.; Baazaoui, F.; Mottaghy, F.M. Targeting of prostate-specific membrane antigen for radio-ligand therapy of triple-negative breast cancer. Breast Cancer Res. 2019, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Heesch, A.; Florea, A.; Maurer, J.; Habib, P.; Werth, L.S.; Hansen, T.; Stickeler, E.; Sahnoun, S.E.M.; Mottaghy, F.M.; Morgenroth, A. The prostate-specific membrane antigen holds potential as a vascular target for endogenous radiotherapy with [177Lu]Lu-PSMA-I&T for triple-negative breast cancer. Breast Cancer Res. 2024, 26, 30. [Google Scholar] [CrossRef]
- Lu, Q.; Long, Y.; Gai, Y.; Liu, Q.; Jiang, D.; Lan, X. [177Lu]Lu-PSMA-617 theranostic probe for hepatocellular carcinoma imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2342–2352. [Google Scholar] [CrossRef]
- Civan, C.; Kasper, S.; Berliner, C.; Fragoso-Costa, P.; Grünwald, V.; Pogorzelski, M.; Schaarschmidt, B.M.; Lang, S.; Kersting, D.; Nader, M.; et al. PSMA-Directed Imaging and Therapy of Salivary Gland Tumors: A Single-Center Retrospective Study. J. Nucl. Med. 2023, 64, 372–378. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; van Es, R.J.J.; Willems, S.M.; Braat, A.J.A.T.; Devriese, L.A.; de Bree, R.; de Keizer, B. First experiences with 177Lu-PSMA-617 therapy for recurrent or metastatic salivary gland cancer. EJNMMI Res. 2021, 11, 126. [Google Scholar] [CrossRef]
- Has Simsek, D.; Kuyumcu, S.; Agaoglu, F.Y.; Unal, S.N. Radionuclide Therapy With 177Lu-PSMA in a Case of Metastatic Adenoid Cystic Carcinoma of the Parotid. Clin. Nucl. Med. 2019, 44, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, M.; Zang, J.; Jiang, Y.; Chen, X.; Zhu, Z.; Chen, X. A pilot study of 68 Ga-PSMA-617 PET/CT imaging and 177Lu-EB-PSMA-617 radioligand therapy in patients with adenoid cystic carcinoma. EJNMMI Res. 2022, 12, 52. [Google Scholar] [CrossRef]
- Graef, J.; Bluemel, S.; Brenner, W.; Amthauer, H.; Truckenmueller, P.; Kaul, D.; Vajkoczy, P.; Onken, J.S.; Furth, C. [177Lu]Lu-PSMA Therapy as an Individual Treatment Approach for Patients with High-Grade Glioma: Dosimetry Results and Critical Statement. J. Nucl. Med. 2023, 64, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Truckenmueller, P.; Graef, J.; Scheel, M.; Vajkoczy, P.; Capper, D.; Kaul, D.; Furth, C.; Amthauer, H.; Brenner, W.; Onken, J.S. [68Ga]Ga-PSMA PET/MRI, histological PSMA expression and preliminary experience with [177Lu]Lu-PSMA therapy in relapsing high-grade glioma. Front. Oncol. 2022, 12, 980058. [Google Scholar] [CrossRef]
- Hirmas, N.; Leyh, C.; Sraieb, M.; Barbato, F.; Schaarschmidt, B.M.; Umutlu, L.; Nader, M.; Wedemeyer, H.; Ferdinandus, J.; Rischpler, C.; et al. 68Ga-PSMA-11 PET/CT Improves Tumor Detection and Impacts Management in Patients with Hepatocellular Carcinoma. J. Nucl. Med. 2021, 62, 1235–1241. [Google Scholar] [CrossRef]
- Santhanam, P.; Russell, J.; Rooper, L.M.; Ladenson, P.W.; Pomper, M.G.; Rowe, S.P. The prostate-specific membrane antigen (PSMA)-targeted radiotracer 18F-DCFPyL detects tumor neovasculature in metastatic, advanced, radioiodine-refractory, differentiated thyroid cancer. Med. Oncol. 2020, 37, 98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pitalua-Cortes, Q.; García-Perez, F.O.; Vargas-Ahumada, J.; Gonzalez-Rueda, S.; Gomez-Argumosa, E.; Ignacio-Alvarez, E.; Soldevilla-Gallardo, I.; Torres-Agredo, L. Head-to-Head Comparison of 68Ga-PSMA-11 and 131I in the Follow-Up of Well-Differentiated Metastatic Thyroid Cancer: A New Potential Theragnostic Agent. Front. Endocrinol. 2021, 12, 794759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wächter, S.; Di Fazio, P.; Maurer, E.; Manoharan, J.; Keber, C.; Pfestroff, A.; Librizzi, D.; Bartsch, D.K.; Luster, M.; Eilsberger, F. Prostate-Specific Membrane Antigen in Anaplastic and Poorly Differentiated Thyroid Cancer-A New Diagnostic and Therapeutic Target? Cancers 2021, 13, 5688. [Google Scholar] [CrossRef]
- de Vries, L.H.; Lodewijk, L.; Braat, A.J.A.T.; Krijger, G.C.; Valk, G.D.; Lam, M.G.E.H.; Borel Rinkes, I.H.M.; Vriens, M.R.; de Keizer, B. 68Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with 177Lu-PSMA-617. EJNMMI Res. 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Digklia, A.; Boughdad, S.; Homicsko, K.; Dromain, C.; Trimech, M.; Dolcan, A.; Peters, S.; Prior, J.; Schaefer, N. First communication on the efficacy of combined 177Lutetium-PSMA with immunotherapy outside prostate cancer. J. Immunother. Cancer 2022, 10, e005383. [Google Scholar] [CrossRef] [PubMed]
- Jüptner, M.; Marx, M.; Zuhayra, M.; Lützen, U. Experimental 177Lu-PSMA-617 radioligand therapy in a patient with extended metastasized leiomyosarcoma. Nukl. Nucl. Med. 2019, 58, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Has Simsek, D.; Civan, C.; Ekenel, M.; Kuyumcu, S.; Sanli, Y. 177Lu-PSMA Therapy for Metastatic Testicular Mixed Germ Cell Tumor. Clin. Nucl. Med. 2021, 46, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29 (Suppl. S16), 15–18. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, A.M.; Alamoudi, A.J.; Abdel-Naim, A.B.; Neamatallah, T.A.; Ashour, O.M. Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies—A review. J. Adv. Res. 2017, 8, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Lauri, C.; Chiurchioni, L.; Russo, V.M.; Zannini, L.; Signore, A. PSMA Expression in Solid Tumors beyond the Prostate Gland: Ready for Theranostic Applications? J. Clin. Med. 2022, 11, 6590. [Google Scholar] [CrossRef] [PubMed]
- Kleiburg, F.; Heijmen, L.; Gelderblom, H.; Kielbasa, S.M.; Bovée, J.V.; De Geus-Oei, L.F. Prostate-specific membrane antigen (PSMA) as a potential target for molecular imaging and treatment in bone and soft tissue sarcomas. Br. J. Radiol. 2023, 96, 20220886. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Halder, S.; Herwald, S.; Ghijsen, M.; Shafi, G.; Uttarwar, M.; Rosen, E.; Franc, B.; Kishore, S. Frequent Amplification and Overexpression of PSMA in Basallike Breast Cancer from Analysis of The Cancer Genome Atlas. J. Nucl. Med. 2024, 65, 1004–1006. [Google Scholar] [CrossRef]
- Bakht, M.K.; Yamada, Y.; Ku, S.Y.; Venkadakrishnan, V.B.; Korsen, J.A.; Kalidindi, T.M.; Mizuno, K.; Ahn, S.H.; Seo, J.-H.; Garcia, M.M.; et al. Landscape of prostate-specific membrane antigen heterogeneity and regulation in AR-positive and AR-negative metastatic prostate cancer. Nat. Cancer 2023, 4, 699–715. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Mokoala, K.; Reed, J.; Maseremule, L.; Ndlovu, H.; Hlongwa, K.; Maes, A.; et al. 225Ac-PSMA-617 radioligand therapy of de novo metastatic hormone-sensitive prostate carcinoma (mHSPC): Preliminary clinical findings. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dondi, F.; Miceli, A.; Rovera, G.; Feudo, V.; Battisti, C.; Rondini, M.; Marongiu, A.; Mura, A.; Camedda, R.; De Feo, M.S.; et al. Prostate-Specific Membrane Antigen Radioligand Therapy in Non-Prostate Cancers: Where Do We Stand? Bioengineering 2024, 11, 714. https://doi.org/10.3390/bioengineering11070714
Dondi F, Miceli A, Rovera G, Feudo V, Battisti C, Rondini M, Marongiu A, Mura A, Camedda R, De Feo MS, et al. Prostate-Specific Membrane Antigen Radioligand Therapy in Non-Prostate Cancers: Where Do We Stand? Bioengineering. 2024; 11(7):714. https://doi.org/10.3390/bioengineering11070714
Chicago/Turabian StyleDondi, Francesco, Alberto Miceli, Guido Rovera, Vanessa Feudo, Claudia Battisti, Maria Rondini, Andrea Marongiu, Antonio Mura, Riccardo Camedda, Maria Silvia De Feo, and et al. 2024. "Prostate-Specific Membrane Antigen Radioligand Therapy in Non-Prostate Cancers: Where Do We Stand?" Bioengineering 11, no. 7: 714. https://doi.org/10.3390/bioengineering11070714
APA StyleDondi, F., Miceli, A., Rovera, G., Feudo, V., Battisti, C., Rondini, M., Marongiu, A., Mura, A., Camedda, R., De Feo, M. S., Conte, M., Gorica, J., Ferrari, C., Nappi, A. G., & Santo, G. (2024). Prostate-Specific Membrane Antigen Radioligand Therapy in Non-Prostate Cancers: Where Do We Stand? Bioengineering, 11(7), 714. https://doi.org/10.3390/bioengineering11070714








