Extracellular Overexpression of a Neutral Pullulanase in Bacillus subtilis through Multiple Copy Genome Integration and Atypical Secretion Pathway Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Chemicals
2.2. Construction of the Genome Integration Strains
2.3. Overexpression of the Encoding Genes of the Key Transporters
2.4. Fermentation of the Recombinant Strains
2.5. Pululanase Assay
2.6. Determination of Pullulanase Expression Using SDS-PAGE
2.7. Trehalose Production Process from Maltodextrin in Multi-Enzyme Catalyzed System
3. Results and Discussion
3.1. Multiple Copies Genome Integration of PulA3E in B. subtilis 0127
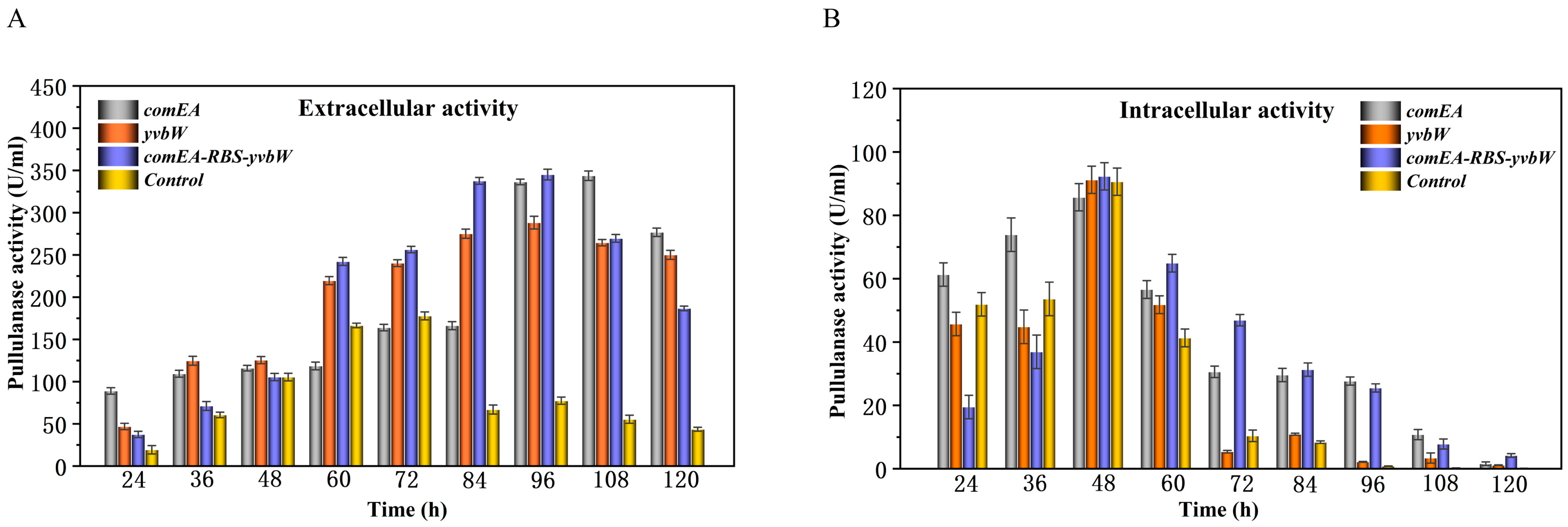
3.2. Improving Extracellular Expression of PulA3E by Overexpressing Specific Transporters
3.3. Fermentation of the Recombinant Strains in a 5 L Reactor
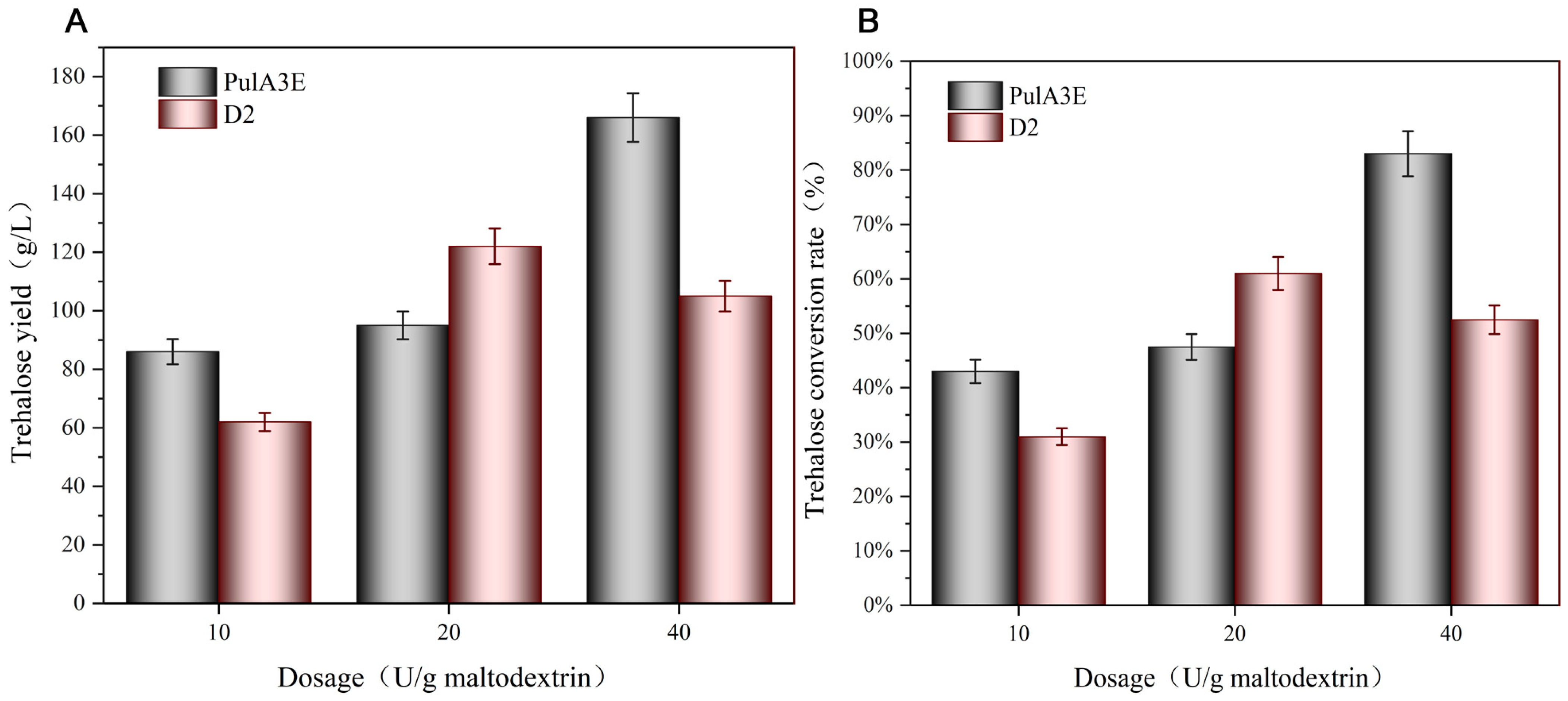
3.4. Enhanced Trehalose Production by Adding Neutral Pullulanase PulA3E
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, P.; Zhang, S.Y.; Luo, Z.G.; Zong, M.H.; Li, X.X.; Lou, W.Y. Biotechnology and bioengineering of pullulanase: State of the art and perspectives. World J. Microbiol. Biotechnol. 2021, 37, 43. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamoori, Z.Z.; Embaby, A.M.; Hussein, A.; Mahmoud, H.E. A molecular study on recombinant pullulanase type I from Metabacillus indicus. AMB Express 2023, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.H.; Zhang, X.; Zhu, C.; Wang, C.; Cheng, Y.; Tang, N. Structural, physicochemical and digestive properties of rice starch modified by preheating and pullulanase treatments. Carbohydr. Polym. 2023, 313, 120866. [Google Scholar] [CrossRef]
- Wang, X.; Nie, Y.; Xu, Y. Industrially produced pullulanases with thermostability: Discovery, engineering, and heterologous expression. Bioresour. Technol. 2019, 278, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhang, K.; Su, L.; Wu, J. Microbial starch debranching enzymes: Developments and applications. Biotechnol. Adv. 2021, 50, 107786. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Liu, X.; Zhang, P.; Zhang, H. Improved thermostability of type I pullulanase from Bacillus thermoliquefaciens by error-prone PCR. Enzyme Microb. Technol. 2023, 169, 110290. [Google Scholar] [CrossRef] [PubMed]
- Semwal, J.; Meera, M.S. Modification of sorghum starch as a function of pullulanase hydrolysis and infrared treatment. Food Chem. 2023, 416, 135815. [Google Scholar] [CrossRef]
- Sun, X.; Yang, J.; Fu, X.; Zhao, X.; Zhen, J.; Song, H.; Xu, J.; Zheng, H.; Bai, W. Trehalose Production Using Three Extracellular Enzymes Produced via One-Step Fermentation of an Engineered Bacillus subtilis Strain. Bioengineering 2023, 10, 977. [Google Scholar] [CrossRef]
- Naik, B.; Kumar, V.; Goyal, S.K.; Dutt Tripathi, A.; Mishra, S.; Joakim Saris, P.E.; Kumar, A.; Rizwanuddin, S.; Kumar, V.; Rustagi, S. Pullulanase: Unleashing the power of enzyme with a promising future in the food industry. Front. Bioeng. Biotechnol. 2023, 11, 1139611. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, S.; Liang, X.; Han, P.; Liu, Y. Characterization of a novel detergent-resistant type I pullulanase from Bacillus megaterium Y103 and its application in laundry detergent. Prep. Biochem. Biotechnol. 2023, 53, 683–689. [Google Scholar] [CrossRef]
- Hassan, N.A.; Darwesh, O.M.; Smuda, S.S.; Altemimi, A.B.; Hu, A.J.; Cacciola, F.; Haoujar, I.; Abedelmaksoud, T.G. Recent Trends in the Preparation of Nano-Starch Particles. Molecules 2022, 27, 5497. [Google Scholar] [CrossRef]
- Su, L.; Yao, K.; Wu, J. Improved Activity of Sulfolobus acidocaldarius Maltooligosyltrehalose Synthase through Directed Evolution. J. Agric. Food Chem. 2020, 68, 4456–4463. [Google Scholar] [CrossRef]
- Su, L.; Wu, S.; Feng, J.; Wu, J. High-efficiency expression of Sulfolobus acidocaldarius maltooligosyl trehalose trehalohydrolase in Escherichia coli through host strain and induction strategy optimization. Bioprocess. Biosyst. Eng. 2019, 42, 345–354. [Google Scholar] [CrossRef]
- Fu, L.L.; Xu, Z.R.; Li, W.F.; Shuai, J.B.; Lu, P.; Hu, C.X. Protein secretion pathways in Bacillus subtilis: Implication for optimization of heterologous protein secretion. Biotechnol. Adv. 2007, 25, 1–12. [Google Scholar] [CrossRef]
- Olaniyi, O.O.; Damilare, A.O.; Lawal, O.T.; Igbe, F.O. Properties of a neutral, thermally stable and surfactant-tolerant pullulanase from worker termite gut-dwelling Bacillus safensis as potential for industrial applications. Heliyon 2022, 8, e10617. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qu, J.; Zou, W.; Shen, W.; Chen, X. An overview and future prospects of recombinant protein production in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2021, 105, 6607–6626. [Google Scholar] [CrossRef] [PubMed]
- Stülke, J.; Grüppen, A.; Bramkamp, M.; Pelzer, S. Bacillus subtilis, a Swiss Army Knife in Science and Biotechnology. J. Bacteriol. 2023, 205, e0010223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, L.; Wu, J. Enhancing extracellular pullulanase production in Bacillus subtilis through dltB disruption and signal peptide optimization. Appl. Biochem. Biotechnol. 2022, 194, 1206–1220. [Google Scholar] [CrossRef]
- Ejaz, S.; Khan, H.; Sarwar, N.; Aqeel, S.M.; Al-Adeeb, A.; Liu, S. A Review on Recent Advancement in Expression Strategies Used in Bacillus subtilis. Protein Pept. Lett. 2022, 29, 733–743. [Google Scholar] [CrossRef]
- Xu, Y.; Xuan, X.; Gao, R.; Xie, G. Increased Expression Levels of Thermophilic Serine Protease TTHA0724 through Signal Peptide Screening in Bacillus subtilis and Applications of the Enzyme. Int. J. Mol. Sci. 2023, 24, 5950. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, X.; Zhao, X.; Xu, J.; Liu, Y.; Zheng, H.; Bai, W.; Song, H. Overexpression of a thermostable α-amylase through genome integration in Bacillus subtilis. Fermentation 2023, 9, 139. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Dong, Y.; Qin, G.; Zhao, X.; Shen, Y. Enhanced extracellular beta-mannanase production by overexpressing PrsA lipoprotein in Bacillus subtilis and optimizing culture conditions. J. Basic. Microbiol. 2022, 62, 815–823. [Google Scholar] [CrossRef]
- Liu, P.; Guo, J.; Miao, L.; Liu, H. Enhancing the secretion of a feruloyl esterase in Bacillus subtilis by signal peptide screening and rational design. Protein Expr. Purif. 2022, 200, 106165. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, Y.; Cheng, Y.; Hu, R.; Fang, Y.; Lyu, M.; Wang, S.; Lu, Z. Optimal Secretory Expression of Acetaldehyde Dehydrogenase from Issatchenkia terricola in Bacillus subtilis through a Combined Strategy. Molecules 2022, 27, 747. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Wang, B.; Pan, L. Efficient production of extracellular pullulanase in Bacillus subtilis ATCC6051 using the host strain construction and promoter optimization expression system. Microb. Cell Fact. 2018, 17, 163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. A genetic toolkit for efficient production of secretory protein in Bacillus subtilis. Bioresour. Technol. 2022, 363, 127885. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhu, X.; Nie, T.; Lu, F.; Bie, X.; Lu, Y.; Trouth, F.; Lu, Z. Enhanced expression of pullulanase in Bacillus subtilis by new strong promoters mined from transcriptome data, both alone and in combination. Front. Microbiol. 2018, 9, 2635. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Zheng, H.; Zhao, X.; Fu, X.; Yang, S.; Xu, J.; Song, H.; Ma, Y. Regulate the hydrophobic motif to enhance the non-classical secretory expression of pullulanase PulA in Bacillus subtilis. Int. J. Biol. Macromol. 2021, 193, 238–246. [Google Scholar] [CrossRef]
- Zhang, X.Z.; You, C.; Zhang, Y.H. Transformation of Bacillus subtilis. Methods Mol. Biol. 2014, 1151, 95–101. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Fu, X.; Zhou, D.; Teng, J.; Yang, J.; Zhen, J.; Zhao, X.; Liu, Y.; Zheng, H.; Bai, W. Extracellular Overexpression of a Neutral Pullulanase in Bacillus subtilis through Multiple Copy Genome Integration and Atypical Secretion Pathway Enhancement. Bioengineering 2024, 11, 661. https://doi.org/10.3390/bioengineering11070661
Dong W, Fu X, Zhou D, Teng J, Yang J, Zhen J, Zhao X, Liu Y, Zheng H, Bai W. Extracellular Overexpression of a Neutral Pullulanase in Bacillus subtilis through Multiple Copy Genome Integration and Atypical Secretion Pathway Enhancement. Bioengineering. 2024; 11(7):661. https://doi.org/10.3390/bioengineering11070661
Chicago/Turabian StyleDong, Wenkang, Xiaoping Fu, Dasen Zhou, Jia Teng, Jun Yang, Jie Zhen, Xingya Zhao, Yihan Liu, Hongchen Zheng, and Wenqin Bai. 2024. "Extracellular Overexpression of a Neutral Pullulanase in Bacillus subtilis through Multiple Copy Genome Integration and Atypical Secretion Pathway Enhancement" Bioengineering 11, no. 7: 661. https://doi.org/10.3390/bioengineering11070661
APA StyleDong, W., Fu, X., Zhou, D., Teng, J., Yang, J., Zhen, J., Zhao, X., Liu, Y., Zheng, H., & Bai, W. (2024). Extracellular Overexpression of a Neutral Pullulanase in Bacillus subtilis through Multiple Copy Genome Integration and Atypical Secretion Pathway Enhancement. Bioengineering, 11(7), 661. https://doi.org/10.3390/bioengineering11070661








