Optimizing Acute Coronary Syndrome Patient Treatment: Leveraging Gated Transformer Models for Precise Risk Prediction and Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Preprocessing
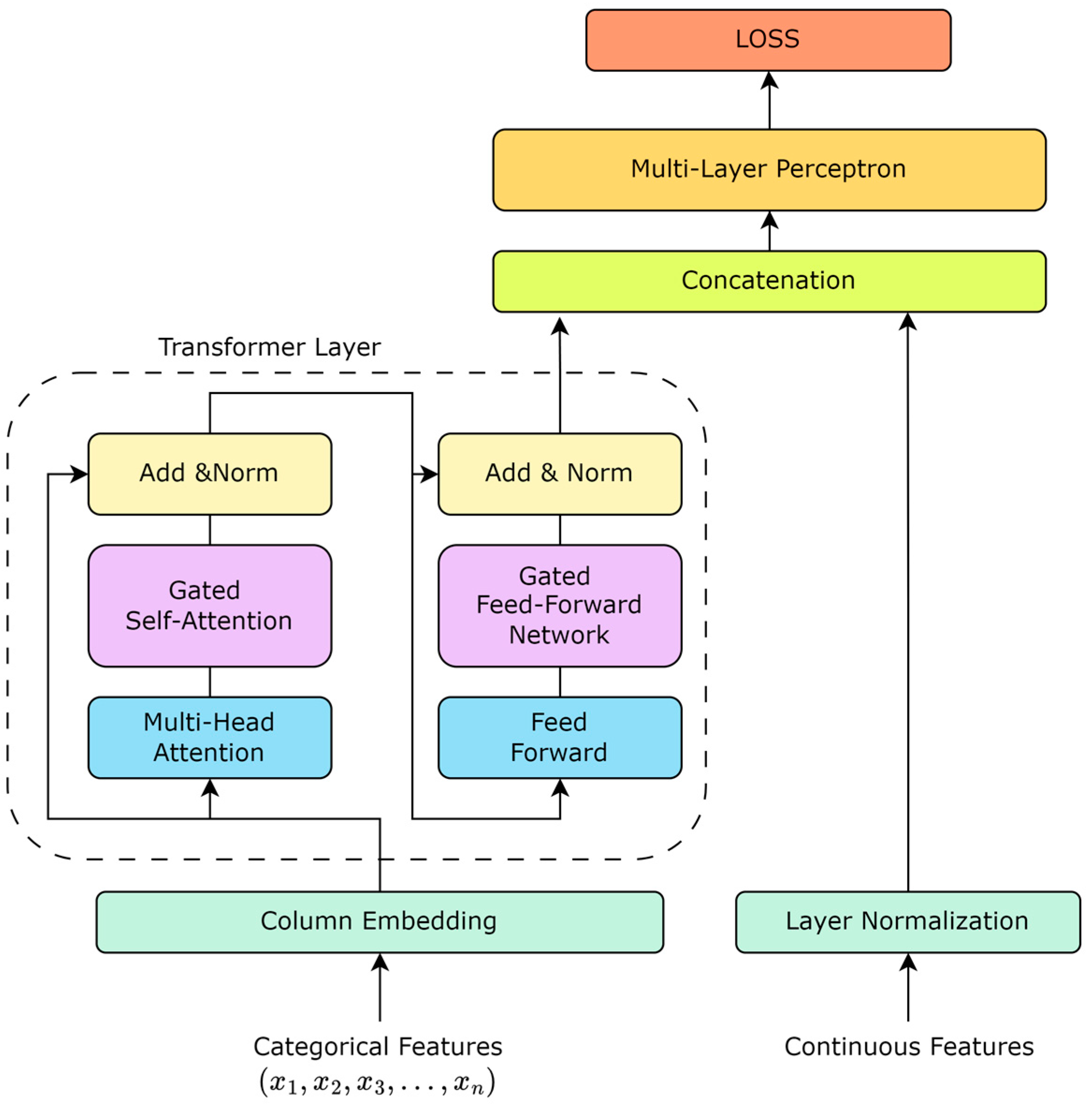
2.2. Table Data Modeling Based on Gated Controllers
| Algorithm 1: Gated Transformer Model for MACE Risk Prediction |
| 1: Initialize dataset D containing EHR data of ACS patients |
| 2: Preprocess D to extract features F for each patient |
| 3: Initialize the gated Transformer model M |
| 4: Define gate structure within M that adjusts information flow |
| 5: while model M has not converged do |
| 6: for each batch B from F do |
| 7: for each feature set f in B do |
| 8: Encode f using input embeddings to receive E |
| 9: for each Transformer layer L in M do |
| 10: Apply self-attention to E to receive attention outputs A |
| 11: Apply gate G to A: |
| 12: |
| 13: |
| 14: to the next layer or output layer |
| 15: end for |
| 16: end for |
| 17: Calculate predictions P for B using final outputs from M |
| 18: Compute loss L between P and actual outcomes of B |
| 19: Update weights of M, including gate parameters, to minimize L |
| 20: end for |
| 21: Validate M on a separate validation set and adjust if necessary |
| 22: end while |
| 23: |
| 24: do |
| 25: using M for p’s features |
| 26: indicating MACE risk |
| 27: end for |
| Ensure: High-accuracy MACE risk prediction incorporating dynamic gating |
2.2.1. Pre-Training Embedding
2.2.2. Transformer Layer
2.2.3. Column Embedding
2.2.4. Gated Self-Attention Mechanism
2.2.5. Gated Feed-Forward Network
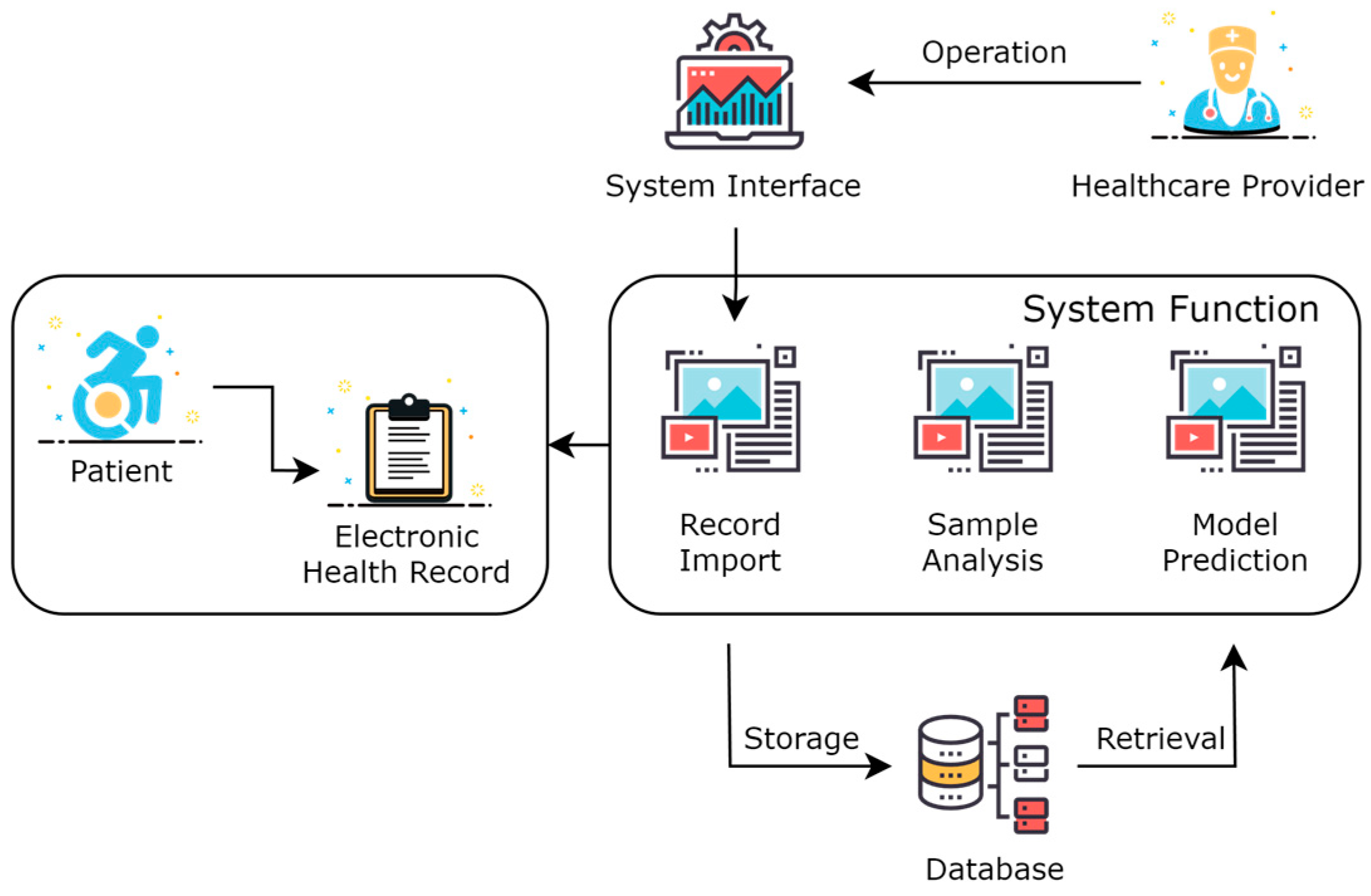
2.3. Design of Acute Coronary Syndrome Patient Management Platform
3. Results
3.1. Results of Data Processing
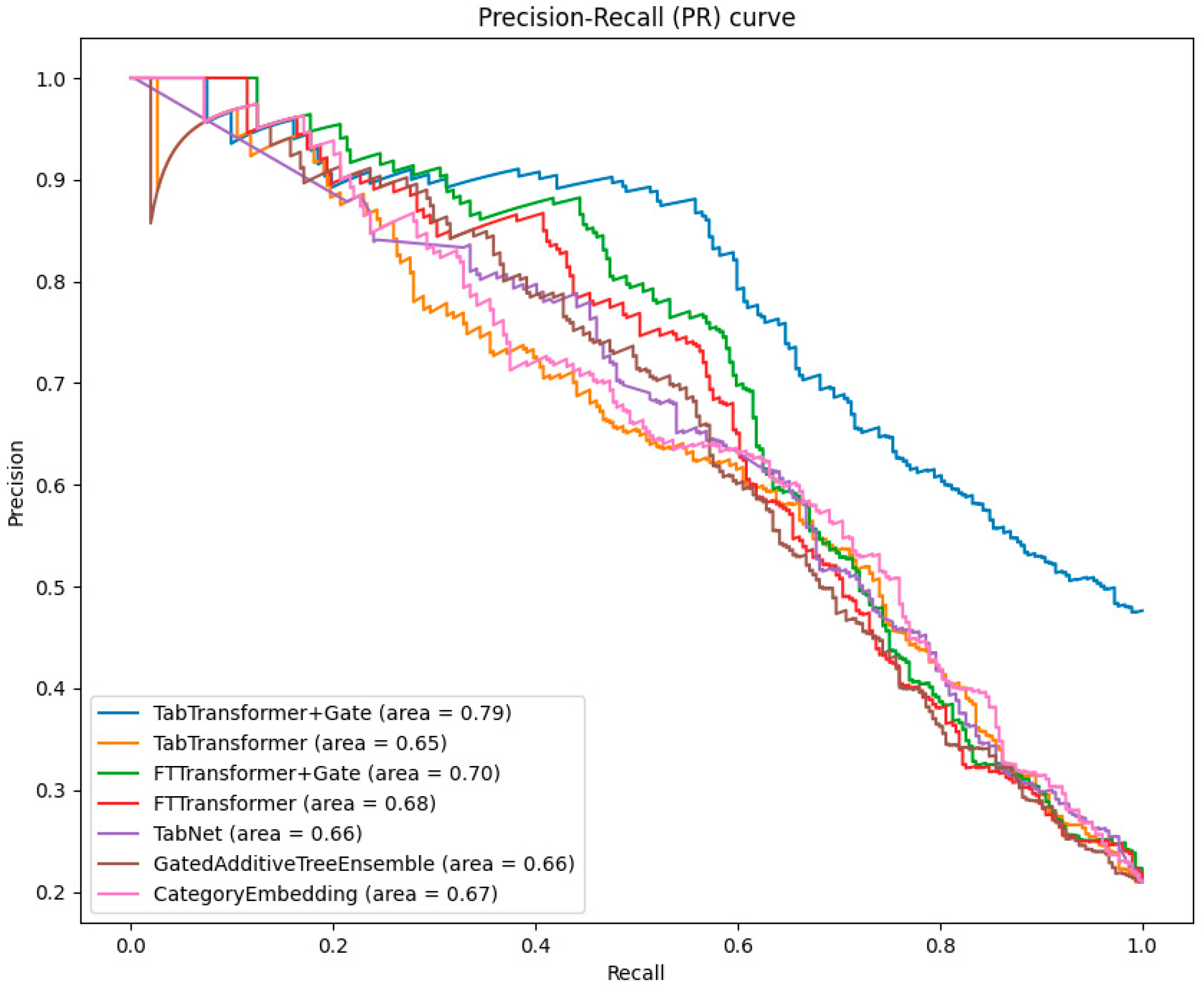
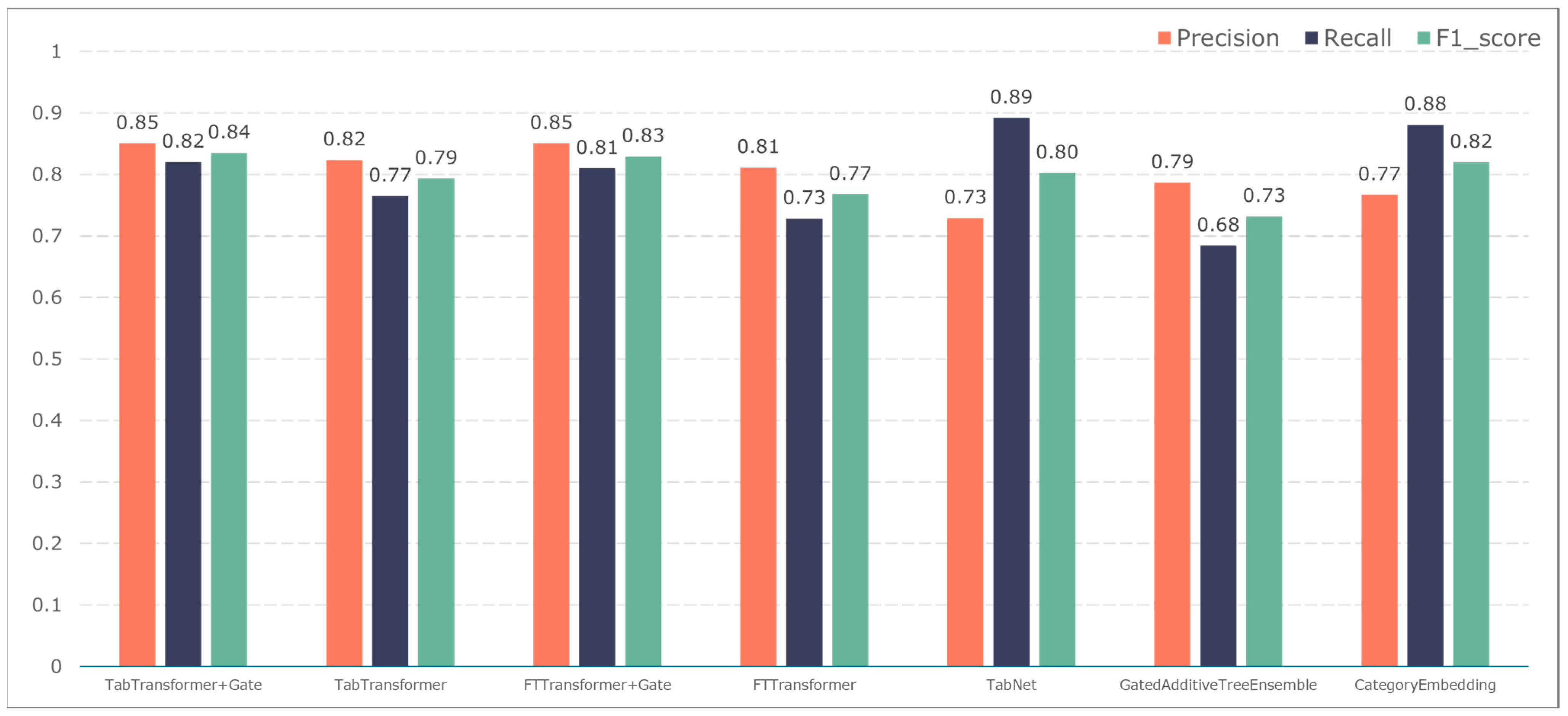
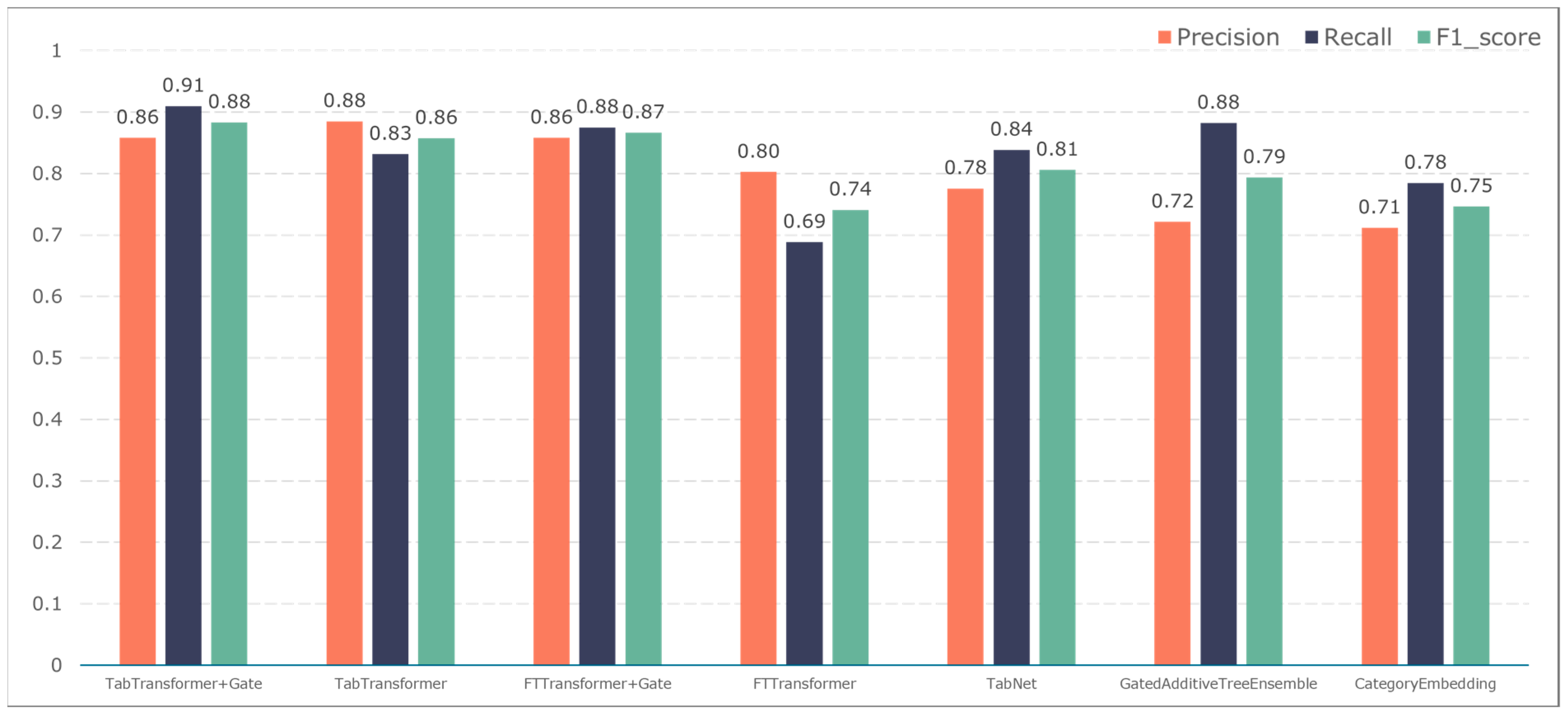
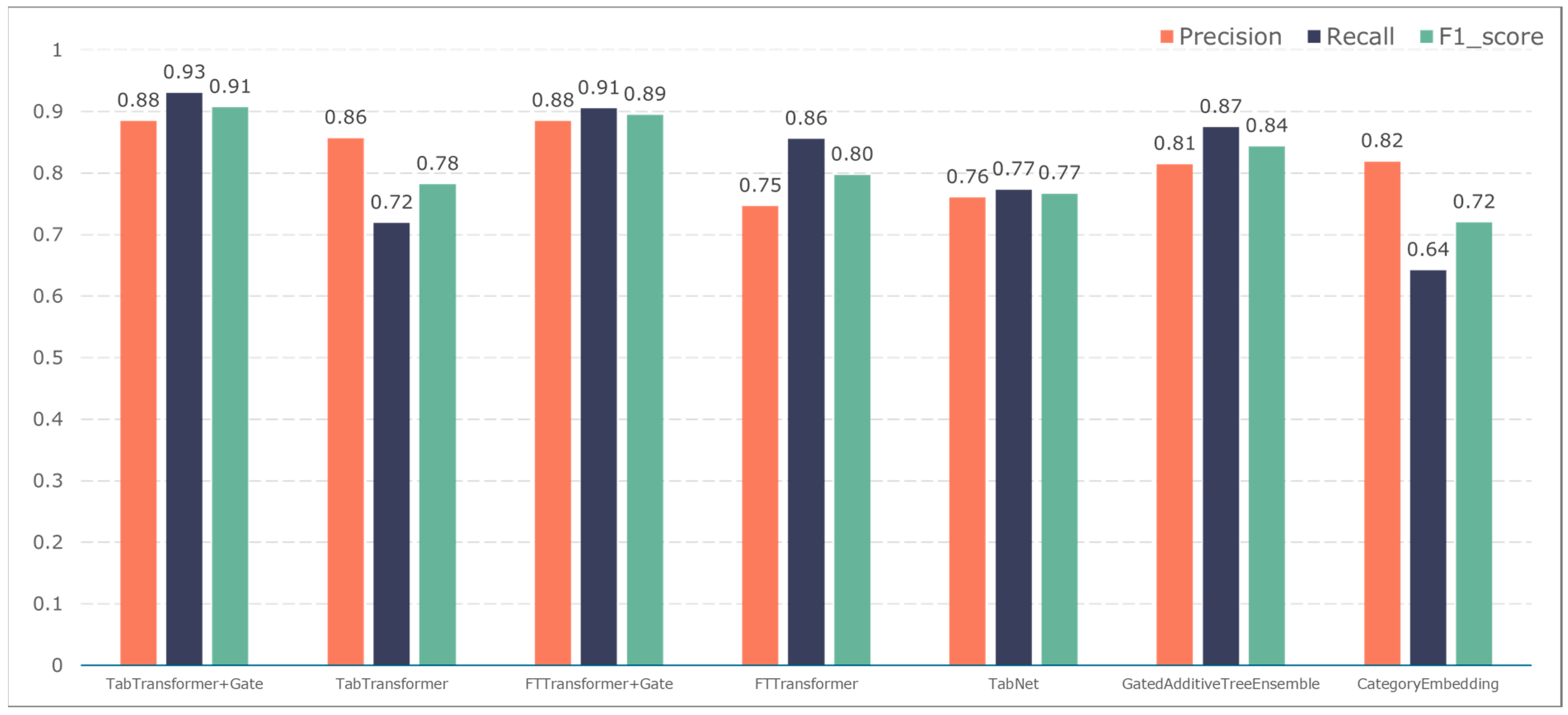
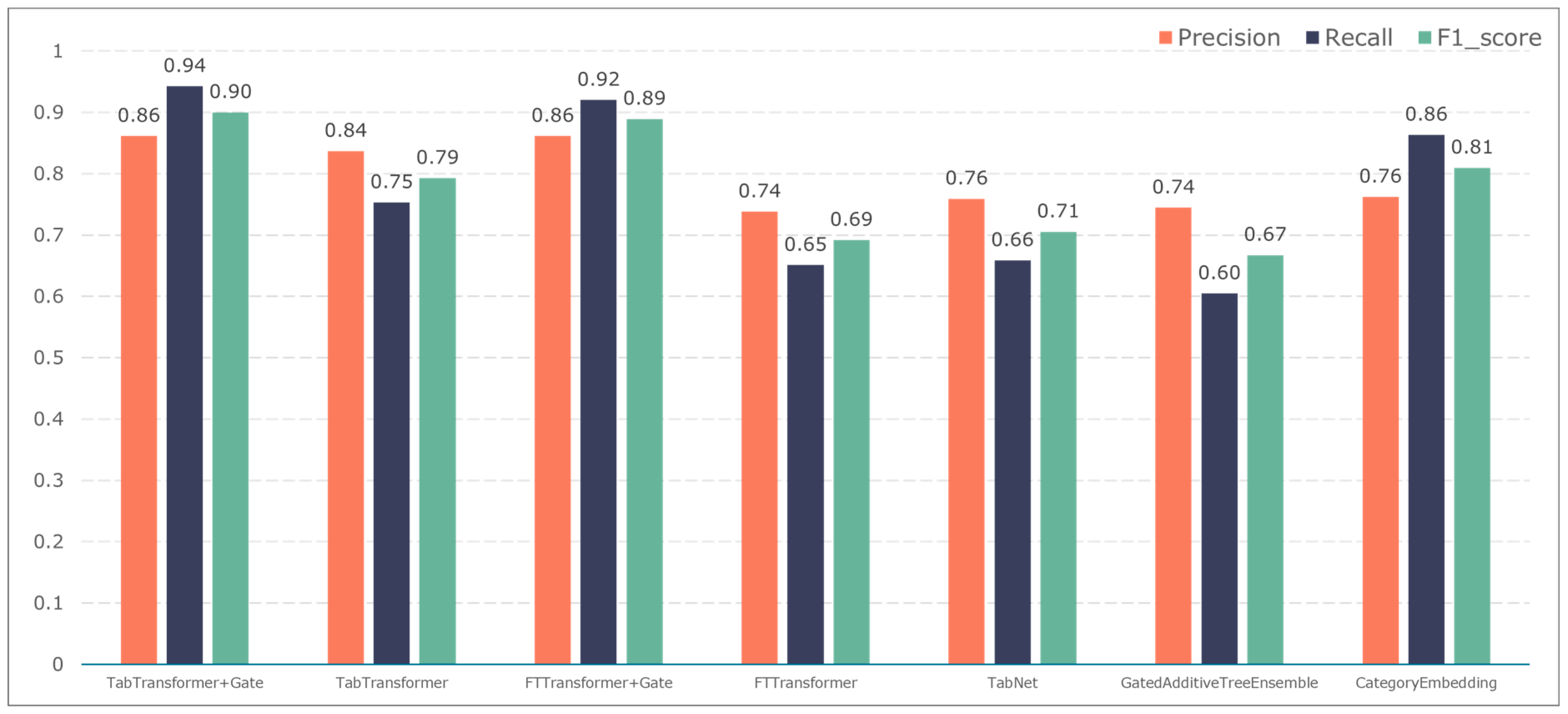
3.2. Comparison of Classification Results
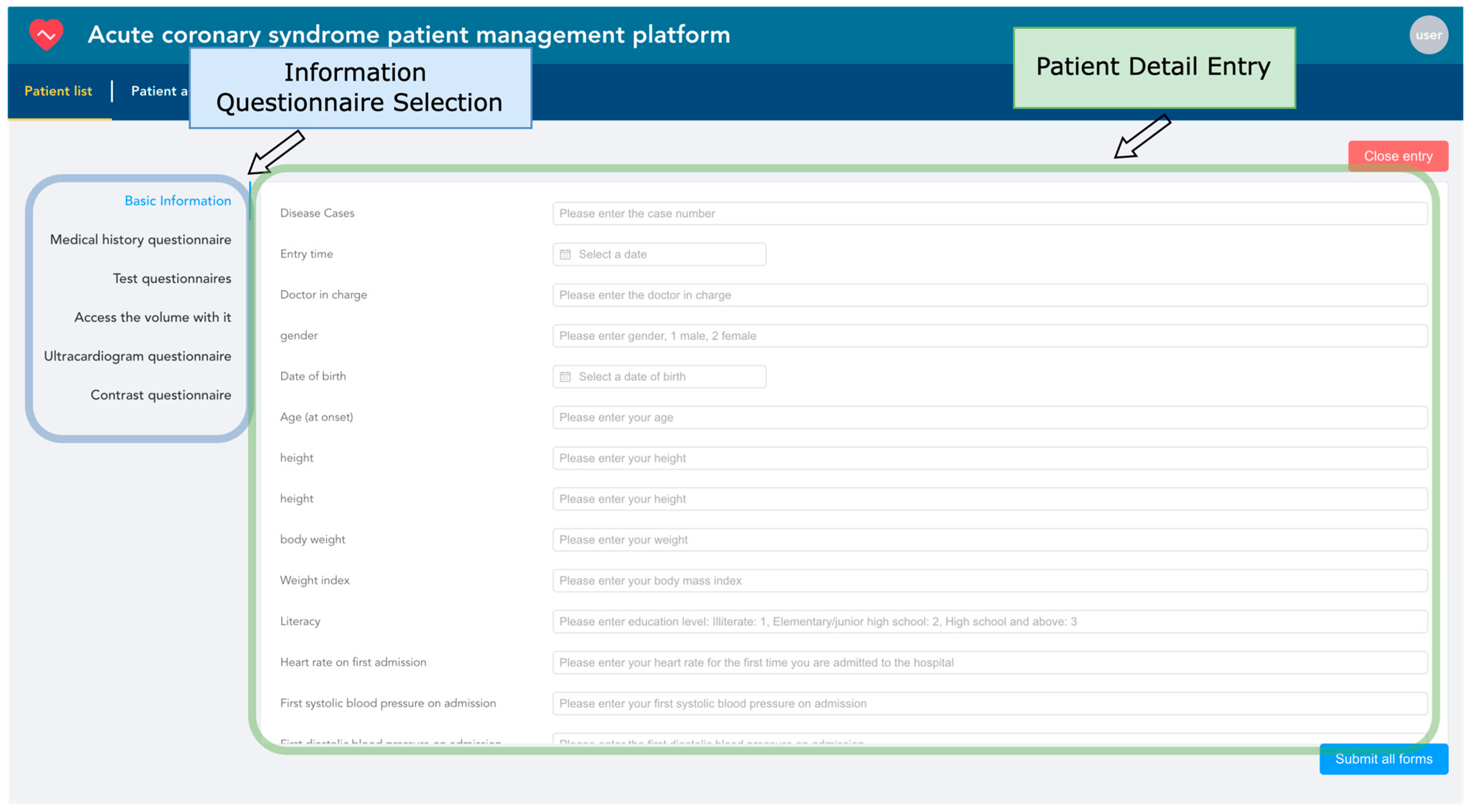
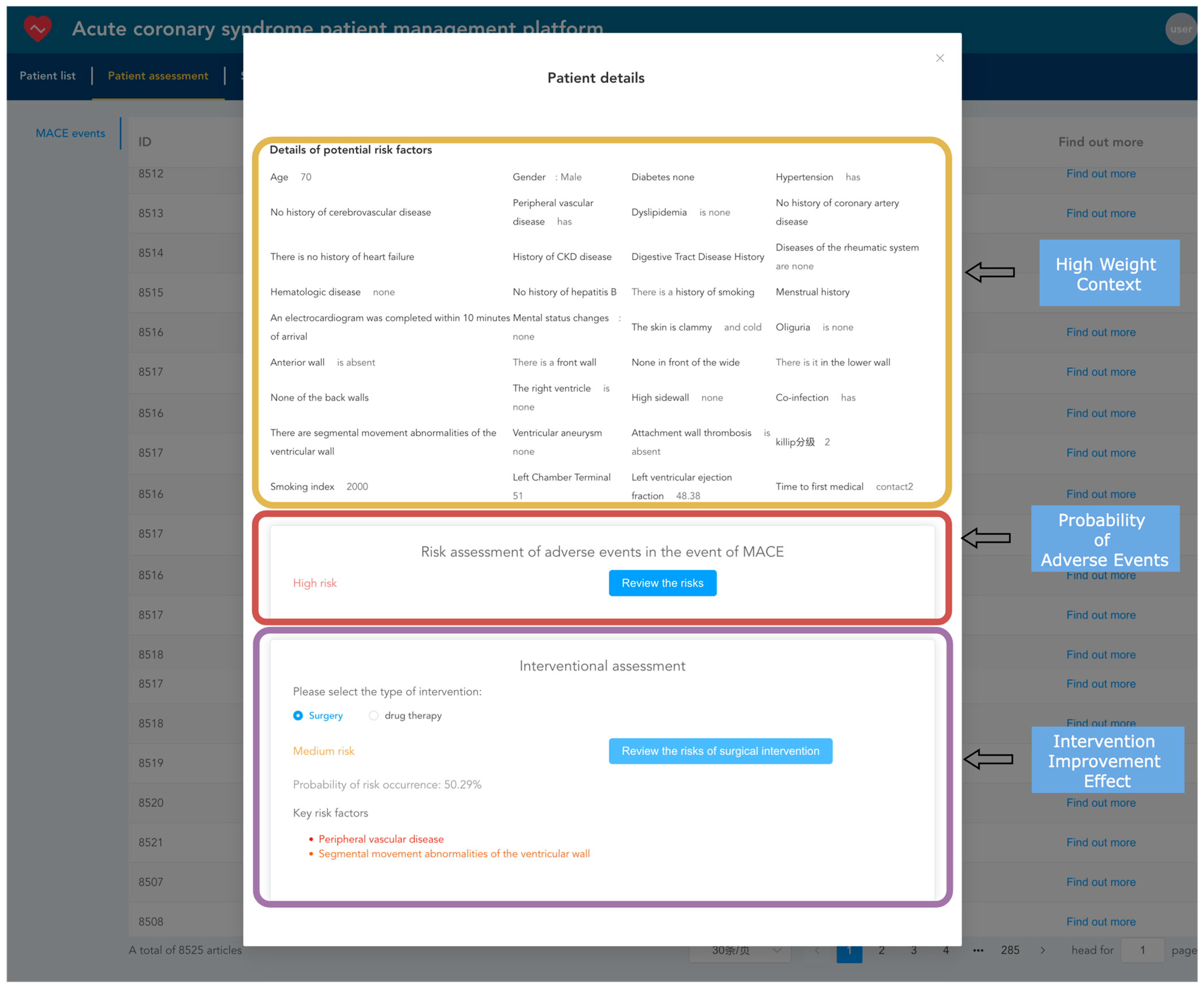
3.3. Implementation of the Design of Acute Coronary Syndrome Patient Management Platform
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable Name | Category | Type | Description |
|---|---|---|---|
| Gender | Basic information | Categorical | The biological sex of the patient. |
| Birth Date | Basic information | Numerical | The date of birth of the patient. |
| Age at Onset | Basic information | Numerical | The age at which the patient first experienced symptoms. |
| Body Mass Index (BMI) | Basic information | Numerical | The body mass index of the patient. |
| Initial Heart Rate | Basic information | Numerical | The patient’s heart rate upon first admission. |
| Initial Systolic BP | Basic information | Numerical | The patient’s systolic blood pressure upon first admission. |
| Initial Diastolic BP | Basic information | Numerical | The patient’s diastolic blood pressure upon first admission. |
| Initial Oxygenation | Basic information | Numerical | The patient’s oxygenation level upon first admission. |
| History of Diabetes | Medical history | Categorical | Whether the patient has a history of diabetes. |
| History of Hypertension | Medical history | Categorical | Whether the patient has a history of hypertension. |
| History of Stroke | Medical history | Categorical | Whether the patient has a history of cerebrovascular disease. |
| History of PVD | Medical history | Categorical | Whether the patient has a history of peripheral vascular disease. |
| History of Dyslipidemia | Medical history | Categorical | Whether the patient has a history of dyslipidemia. |
| History of CAD | Medical history | Categorical | Whether the patient has a history of coronary artery disease. |
| History of CABG | Medical history | Categorical | Whether the patient has a history of coronary artery bypass graft surgery. |
| History of Heart Failure | Medical history | Categorical | Whether the patient has a history of heart failure. |
| History of COPD | Medical history | Categorical | Whether the patient has a history of chronic obstructive pulmonary disease. |
| History of CKD | Medical history | Categorical | Whether the patient has a history of chronic kidney disease. |
| History of GI Ulcer | Medical history | Categorical | Whether the patient has a history of gastrointestinal ulcers. |
| Rheumatic Diseases | Medical history | Categorical | Whether the patient has rheumatic diseases. |
| Hematologic Diseases | Medical history | Categorical | Whether the patient has hematologic diseases. |
| History of Hepatitis B | Medical history | Categorical | Whether the patient has a history of hepatitis B. |
| Smoking History | Medical history | Categorical | Whether the patient has a history of smoking. |
| Smoking Index | Medical history | Numerical | The smoking index of the patient. |
| Menstrual History | Medical history | Categorical | The patient’s menstrual history. |
| Age at Menopause | Medical history | Numerical | The age at which the patient experienced menopause. |
| Alcohol Consumption History | Medical history | Categorical | Whether the patient has a history of alcohol consumption. |
| History of Chest Pain | Medical history | Categorical | Whether the patient has a history of chest pain. |
| Angina Episodes > 2 in 24 h Before Admission | Medical history | Categorical | Whether the patient experienced more than two episodes of angina in the 24 h before admission. |
| Time to First Medical Contact | Medical history | Numerical | The time from symptom onset to first medical contact. |
| Symptoms at Onset | Medical history | Categorical | The symptoms experienced by the patient at onset. |
| Chest Pain | Medical history | Categorical | Whether the patient experienced chest pain. |
| Radiating Pain | Medical history | Categorical | Whether the patient experienced radiating pain. |
| Nausea and Vomiting | Medical history | Categorical | Whether the patient experienced nausea and vomiting. |
| Profuse Sweating | Medical history | Categorical | Whether the patient experienced profuse sweating. |
| Shortness of Breath | Medical history | Categorical | Whether the patient experienced shortness of breath. |
| Loss of Consciousness | Medical history | Categorical | Whether the patient experienced loss of consciousness. |
| Duration of Symptoms | Medical history | Numerical | The duration of the symptoms experienced by the patient. |
| ECG Completed within 10 Minutes of Arrival | Medical history | Categorical | Whether an ECG was completed within 10 min of the patient’s arrival. |
| High-Sensitivity CRP | Laboratory test | Numerical | The level of high-sensitivity C-reactive protein in the patient. |
| BNP | Laboratory test | Numerical | The level of brain natriuretic peptide in the patient. |
| cTnI | Laboratory test | Numerical | The level of cardiac troponin I in the patient. |
| PCT | Laboratory test | Numerical | The level of procalcitonin in the patient. |
| IL-6 | Laboratory test | Numerical | The level of interleukin-6 in the patient. |
| D-Dimer | Laboratory test | Numerical | The level of D-dimer in the patient. |
| pH | Laboratory test | Numerical | The pH level of the patient’s blood. |
| Albumin | Laboratory test | Numerical | The level of albumin in the patient. |
| ALT | Laboratory test | Numerical | The level of alanine aminotransferase in the patient. |
| WBC Count | Laboratory test | Numerical | The white blood cell count of the patient. |
| RDW | Laboratory test | Numerical | The red cell distribution width of the patient. |
| RBC Count | Laboratory test | Numerical | The red blood cell count of the patient. |
| Hematocrit (HCT) | Laboratory test | Numerical | The hematocrit level of the patient. |
| Creatinine (Cr) | Laboratory test | Numerical | The creatinine level of the patient. |
| Creatine Kinase (CK) | Laboratory test | Numerical | The creatine kinase level of the patient. |
| Potassium (K) | Laboratory test | Numerical | The potassium level of the patient. |
| Calcium (Ca) | Laboratory test | Numerical | The calcium level of the patient. |
| Total Cholesterol (TC) | Laboratory test | Numerical | The total cholesterol level of the patient. |
| LDL-C | Laboratory test | Numerical | The low-density lipoprotein cholesterol level of the patient. |
| HDL-C | Laboratory test | Numerical | The high-density lipoprotein cholesterol level of the patient. |
| Triglycerides (TG) | Laboratory test | Numerical | The triglyceride level of the patient. |
| Glucose | Laboratory test | Numerical | The glucose level of the patient. |
| Fibrinogen (FIB) | Laboratory test | Numerical | The fibrinogen level of the patient. |
| Hemoglobin (Hb) | Laboratory test | Numerical | The hemoglobin level of the patient. |
| Platelet Count (PLT) | Laboratory test | Numerical | The platelet count of the patient. |
| Blood Urea Nitrogen (BUN) | Laboratory test | Numerical | The blood urea nitrogen level of the patient. |
| Uric Acid (UA) | Laboratory test | Numerical | The uric acid level of the patient. |
| Triiodothyronine (T3) | Laboratory test | Numerical | The triiodothyronine level of the patient. |
| Thyroxine (T4) | Laboratory test | Numerical | The thyroxine level of the patient. |
| Free Thyroxine (FT4) | Laboratory test | Numerical | The free thyroxine level of the patient. |
| Quality of Life | Follow-up | Categorical | The patient’s perceived quality of life. |
| Regular Follow-up | Follow-up | Categorical | Whether the patient has regular follow-ups. |
| Strict Smoking Cessation | Follow-up | Categorical | Whether the patient strictly abstains from smoking. |
| Blood Pressure Control | Follow-up | Categorical | Whether the patient’s blood pressure is controlled. |
| Blood Glucose Control | Follow-up | Categorical | Whether the patient’s blood glucose is controlled. |
| Deceased | Follow-up | Categorical | Whether the patient is deceased. |
| Cause of Death | Follow-up | Categorical | The cause of the patient’s death. |
| Recurrent MI | Follow-up | Categorical | Whether the patient has had a recurrent myocardial infarction. |
| Number of Recurrent MIs | Follow-up | Numerical | The number of recurrent myocardial infarctions the patient has had. |
| Arrhythmia | Follow-up | Categorical | Whether the patient has experienced arrhythmia. |
| Time of Arrhythmia Onset | Follow-up | Numerical | The time when the patient experienced arrhythmia. |
| Atrial Fibrillation | Follow-up | Categorical | Whether the patient has atrial fibrillation. |
| AV Block | Follow-up | Categorical | Whether the patient has atrioventricular block. |
| Bundle Branch Block | Follow-up | Categorical | Whether the patient has bundle branch block. |
| Ventricular Arrhythmia | Follow-up | Categorical | Whether the patient has ventricular arrhythmia. |
| Arrhythmia Treatment | Follow-up | Categorical | The treatment for the patient’s arrhythmia. |
| Heart Failure | Follow-up | Categorical | Whether the patient has heart failure. |
| Time of Heart Failure Onset | Follow-up | Numerical | The time when the patient experienced heart failure. |
| Stroke | Follow-up | Categorical | Whether the patient has experienced a stroke. |
| Time of Stroke Onset | Follow-up | Numerical | The time when the patient experienced a stroke. |
| Type of Stroke | Follow-up | Categorical | The type of stroke the patient experienced. |
| Bleeding | Follow-up | Categorical | Whether the patient has experienced bleeding. |
| Time of Bleeding Onset | Follow-up | Numerical | The time when the patient experienced bleeding. |
| Bleeding Classification | Follow-up | Categorical | The classification of the patient’s bleeding. |
| Current Oral Medications | Follow-up | Categorical | The current oral medications the patient is taking. |
| Ascending Aorta Diameter | ECHO | Numerical | The diameter of the patient’s ascending aorta. |
| Aortic Root Diameter | ECHO | Numerical | The diameter of the patient’s aortic root. |
| Right Ventricular Outflow Tract | ECHO | Numerical | The measurement of the right ventricular outflow tract. |
| Right Ventricular Anteroposterior Diameter | ECHO | Numerical | The anteroposterior diameter of the right ventricle. |
| Aortic Diameter | ECHO | Numerical | The diameter of the patient’s aorta. |
| Left Ventricular End-Diastolic Diameter | ECHO | Numerical | The end-diastolic diameter of the left ventricle. |
| Left Ventricular End-Systolic Diameter | ECHO | Numerical | The end-systolic diameter of the left ventricle. |
| Left Atrial Anteroposterior Diameter | ECHO | Numerical | The anteroposterior diameter of the left atrium. |
| Interventricular Septal End-Diastolic Thickness | ECHO | Numerical | The end-diastolic thickness of the interventricular septum. |
| Right Atrial Vertical Diameter | ECHO | Numerical | The vertical diameter of the right atrium. |
| Right Atrial Horizontal Diameter | ECHO | Numerical | The horizontal diameter of the right atrium. |
| Left Ventricular Ejection Fraction (LVEF) | ECHO | Numerical | The ejection fraction of the left ventricle. |
| Left Ventricular Shortening Fraction | ECHO | Numerical | The shortening fraction of the left ventricle. |
| Aortic Valve Regurgitation | ECHO | Categorical | Whether the patient has aortic valve regurgitation. |
| Aortic Valve Calcification | ECHO | Categorical | Whether the patient has aortic valve calcification. |
| Ventricular Wall Thinning | ECHO | Categorical | Whether the patient has thinning of the ventricular wall. |
| Location of Wall Thinning: Anterior Septum | ECHO | Categorical | Whether the wall thinning is located at the anterior septum. |
| Location of Wall Thinning: Septum | ECHO | Categorical | Whether the wall thinning is located at the septum. |
| Location of Wall Thinning: Anterior Wall | ECHO | Categorical | Whether the wall thinning is located at the anterior wall. |
| Location of Wall Thinning: Lateral Wall | ECHO | Categorical | Whether the wall thinning is located at the lateral wall. |
| Admission Heart Rate (HR1) | Angiography | Numerical | The patient’s heart rate upon admission. |
| Admission Systolic BP (SP1) | Angiography | Numerical | The patient’s systolic blood pressure upon admission. |
| Admission Diastolic BP (DP1) | Angiography | Numerical | The patient’s diastolic blood pressure upon admission. |
| Initial Oxygen Saturation (Spo21) | Angiography | Numerical | The patient’s oxygen saturation level upon first measurement. |
| Use of Vasopressors During Surgery | Angiography | Categorical | Whether vasopressors were used during surgery. |
| Intraoperative Complications | Angiography | Categorical | The complications that occurred during surgery. |
| Surgical Procedure | Angiography | Categorical | Whether the patient underwent surgery. |
| Intraoperative Contrast Agent Dosage | Angiography | Numerical | The dosage of contrast agent used during surgery. |
| Intraoperative Heparin Dosage | Angiography | Numerical | The dosage of heparin used during surgery. |
| Coronary Dominance | Angiography | Categorical | The dominance pattern of the coronary arteries. |
| Culprit Vessel Characteristics | Angiography | Categorical | The characteristics of the culprit vessel. |
| TIMI Flow Grade Pre- and Post-Intervention in Culprit Vessel | Angiography | Categorical | The TIMI flow grade in the culprit vessel before and after intervention. |
| Coronary Lesion Location and Stenosis Degree | Angiography | Categorical | The location and degree of stenosis of the coronary lesion. |
| Number and Type of Stents Implanted | Angiography | Numerical | The number and types of stents implanted. |
| Use of Thrombus Aspiration During Surgery | Angiography | Categorical | Whether thrombus aspiration was used during surgery. |
| Planned Second PCI | Angiography | Categorical | Whether a second PCI is planned. |
| Medication Adherence at Discharge | Angiography | Categorical | The adherence to prescribed medications at discharge. |
| Use of Temporary Pacemaker, ECMO, IABP During Surgery | Angiography | Categorical | Whether a temporary pacemaker, ECMO, or IABP was used during surgery. |
| Management of Intraoperative Complications | Angiography | Categorical | The management strategies for complications during surgery. |
| Use of Intravascular Imaging During Stent Placement | Angiography | Categorical | Whether intravascular imaging was used during stent placement. |
References
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non–ST-Elevation Acute Coronary Syndromes A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart disease and stroke statistics—2022 up-date: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Emergency Physician Branch of the Chinese Medical Doctor Association; Emergency Medicine Expert Committee of the Center for Capacity Building and Continuing Education of the National Health Commission; Emergency and First Aid Branch of the China Association for the Promotion of International Healthcare Exchange. Guidelines for Rapid Diagnosis and Treatment of Acute Coronary Syndrome (2019). Chin. J. Emerg. Med. 2019, 29, 421–428. [Google Scholar]
- Zeng, W.; Sheng, H. Interpretation of key points of “China Cardiovascular Health and Disease Report 2022”. Chin. J. Cardiovasc. 2023, 28, 297–312. [Google Scholar]
- Antman, E.M.; McCabe, C.H.; Gurfinkel, E.P.; Turpie, A.G.; Bernink, P.J.; Salein, D.; Bayes De Luna, A.; Fox, K.; Lablanche, J.M.; Radley, D.; et al. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) 11B trial. Circulation 1999, 100, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Antman, E.M.; Cohen, M.; Bernink, P.J.; McCabe, C.H.; Horacek, T.; Papuchis, G.; Mautner, B.; Cor-balan, R.; Radley, D.; Braunwald, E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000, 284, 835–842. [Google Scholar] [CrossRef] [PubMed]
- GRACE Investigators. Rationale and design of the GRACE (Global Registry of Acute Coronary Events) Project: A multinational registry of patients hospitalized with acute coronary syndromes. Am. Heart J. 2001, 141, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van De Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Subherwal, S.; Bach, R.G.; Chen, A.Y.; Gage, B.F.; Rao, S.V.; Newby, L.K.; Wang, T.Y.; Gibler, W.B.; Ohman, E.M.; Roe, M.T.; et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: The CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009, 119, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhavnani, S.P. Digital health: Opportunities and challenges to develop the next-generation technology-enabled models of cardiovascular care. Methodist DeBakey Cardiovasc. J. 2020, 16, 296. [Google Scholar] [CrossRef]
- Shah, P.; Kendall, F.; Khozin, S.; Goosen, R.; Hu, J.; Laramie, J.; Ringel, M.; Schork, N. Artificial intelligence and machine learning in clinical development: A translational perspective. NPJ Digit. Med. 2019, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Quer, G.; Arnaout, R.; Henne, M.; Arnaout, R. Machine learning and the future of cardiovascular care: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 77, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Roy, J.; Stewart, W.F. Prediction modeling using EHR data: Challenges, strategies, and a comparison of machine learning approaches. Med. Care. 2010, 48 (Suppl. S6), S106–S113. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.; Guttag, J. Unsupervised Similarity-Based Risk Stratification for Cardiovascular Events Using Long-Term Time-Series Data. J. Mach. Learn. Res. 2011, 12, 999–1024. [Google Scholar]
- Churpek, M.M.; Yuen, T.C.; Park, S.Y.; Gibbons, R.; Edelson, D.P. Using electronic health record data to develop and validate a prediction model for adverse outcomes in the wards. Crit Care Med. 2014, 42, 841–848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, W.; Huang, Z.; Ji, L.; Duan, H. A genetic fuzzy system for unstable angina risk assessment. BMC Med. Inform. Decis. Mak. 2014, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Verma, L.; Srivastava, S.; Negi, P.C. A hybrid data mining model to predict coronary artery disease cases using non-invasive clinical data. J. Med. Syst. 2016, 40, 178. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Zhang, H.; Wang, Z.; Aydar, M.; Kitai, T. Artificial intelligence in precision cardiovascular medicine. J. Am. Coll. Cardiol. 2017, 69, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Muthukumarasamy, S.; Tamilarasan, A.K.; Ayeelyan, J.; Adimoolam, M. Machine learning in healthcare diagnosis. Blockchain Mach. Learn. E-Healthc. Syst. 2020, 13, 343–366. [Google Scholar]
- Popa-Fotea, N.M.; Calmac, L.; Micheu, M.M.; Cosmin, M.; Scarlatescu, A.; Zamfir, D.; Itu, L.M.; Tache, I.A.; Stoian, D.; Hatfaludi, C.A.; et al. A cloud-based platform for clinical decision support in acute coronary syndrome patients: Study methodology. Kardiol. Pol. Pol. Heart J. 2022, 80, 604–607. [Google Scholar] [CrossRef]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial intelligence in cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Khetan, A.; Cvitkovic, M.; Karnin, Z. Tabtransformer: Tabular data modeling using con-textual embeddings. arXiv 2020, arXiv:2012.06678. [Google Scholar]
- Han, J.; Pei, J.; Tong, H. Data Mining: Concepts and Techniques; Morgan kaufmann: Burlington, MA, USA, 2022. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J.H.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: New York, NY, USA, 2009. [Google Scholar]
- Tsay, R.S. Analysis of Financial Time Series; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Little, R.J.A.; Rubin, D.B. Statistical Analysis with Missing Data; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Barnett, V.; Lewis, T. Outliers in Statistical Data; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Arik, S.Ö.; Pfister, T. Tabnet: Attentive interpretable tabular learning. Proc. AAAI Conf. Artif. Intell. 2021, 35, 6679–6687. [Google Scholar] [CrossRef]
- Joseph, M.; Raj, H. Gate: Gated additive tree ensemble for tabular classification and regression. arXiv 2022, arXiv:2207.08548. [Google Scholar]
- Gorishniy, Y.; Rubachev, I.; Khrulkov, V.; Babenko, A. Revisiting deep learning models for tabular data. Adv. Neural Inf. Process. Syst. 2021, 34, 18932–18943. [Google Scholar]
- Türker, R.; Zhang, L.; Koutraki, M.; Sack, H. Knowledge-based short text categorization using entity and category embedding. In Proceedings of the Semantic Web: 16th International Conference, ESWC 2019, Portorož, Slovenia, 2–6 June 2019; Proceedings 16. Springer International Publishing: Cham, Switzerland; pp. 346–362. [Google Scholar]
- Zhang, X.; Wang, X.; Xu, L.; Liu, J.; Ren, P.; Wu, H. The predictive value of machine learning for mortality risk in patients with acute coronary syndromes: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 451. [Google Scholar] [CrossRef] [PubMed]
- Kasim, S.; Malek, S.; Song, C.; Ahmad, W.A.W.; Fong, A.; Ibrahim, K.S.; Safiruz, M.S.; Aziz, F.; Hiew, J.H.; Ibrahim, N. In-hospital mortality risk stratification of Asian ACS patients with artificial intelligence algorithm. PLoS ONE 2022, 17, e0278944. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Oren, E.; Chen, K.; Dai, A.M.; Hajaj, N.; Hardt, M.; Liu, P.J.; Liu, X.; Marcus, J.; Sun, M.; et al. Scalable and accurate deep learning with electronic health records. NPJ Digit. Med. 2018, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Angraal, S.; Krumholz, H.M.; Schulz, W.L. Blockchain technology: Applications in health care. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003800. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Beam, A.L.; Kohane, I.S. Big data and machine learning in health care. JAMA 2018, 319, 1317–1318. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Emanuel, E.J. Predicting the future—Big data, machine learning, and clinical medicine. N. Engl. J. Med. 2016, 375, 1216. [Google Scholar] [CrossRef]




| Data Types | Quantity | Proportion |
|---|---|---|
| Basic information | 8 | 6% |
| Medical history | 31 | 23.1% |
| Laboratory test results | 30 | 22.3% |
| Follow-up | 25 | 18.6% |
| ECHO | 20 | 15% |
| Angiography | 20 | 15% |
| Parameter | Value |
|---|---|
| Learning Rate | 5 × 10−6 |
| Input embedding dimension | 128 |
| Batch size | 256 |
| Epoch | 200 |
| Dropout | 0.1 |
| Attention heads | 8 |
| Attention blocks | 6 |
| Attention Dropout | 0.1 |
| AddNorm Dropout | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Y.; Jin, Z.; Ma, W.; Ma, Y.; Deng, N.; Fan, Z.; Wei, S. Optimizing Acute Coronary Syndrome Patient Treatment: Leveraging Gated Transformer Models for Precise Risk Prediction and Management. Bioengineering 2024, 11, 551. https://doi.org/10.3390/bioengineering11060551
Mei Y, Jin Z, Ma W, Ma Y, Deng N, Fan Z, Wei S. Optimizing Acute Coronary Syndrome Patient Treatment: Leveraging Gated Transformer Models for Precise Risk Prediction and Management. Bioengineering. 2024; 11(6):551. https://doi.org/10.3390/bioengineering11060551
Chicago/Turabian StyleMei, Yingxue, Zicai Jin, Weiguo Ma, Yingjun Ma, Ning Deng, Zhiyuan Fan, and Shujun Wei. 2024. "Optimizing Acute Coronary Syndrome Patient Treatment: Leveraging Gated Transformer Models for Precise Risk Prediction and Management" Bioengineering 11, no. 6: 551. https://doi.org/10.3390/bioengineering11060551
APA StyleMei, Y., Jin, Z., Ma, W., Ma, Y., Deng, N., Fan, Z., & Wei, S. (2024). Optimizing Acute Coronary Syndrome Patient Treatment: Leveraging Gated Transformer Models for Precise Risk Prediction and Management. Bioengineering, 11(6), 551. https://doi.org/10.3390/bioengineering11060551






