An Investigation of Running Kinematics with Recovered Anterior Cruciate Ligament Reconstruction on a Treadmill and In-Field Using Inertial Measurement Units: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
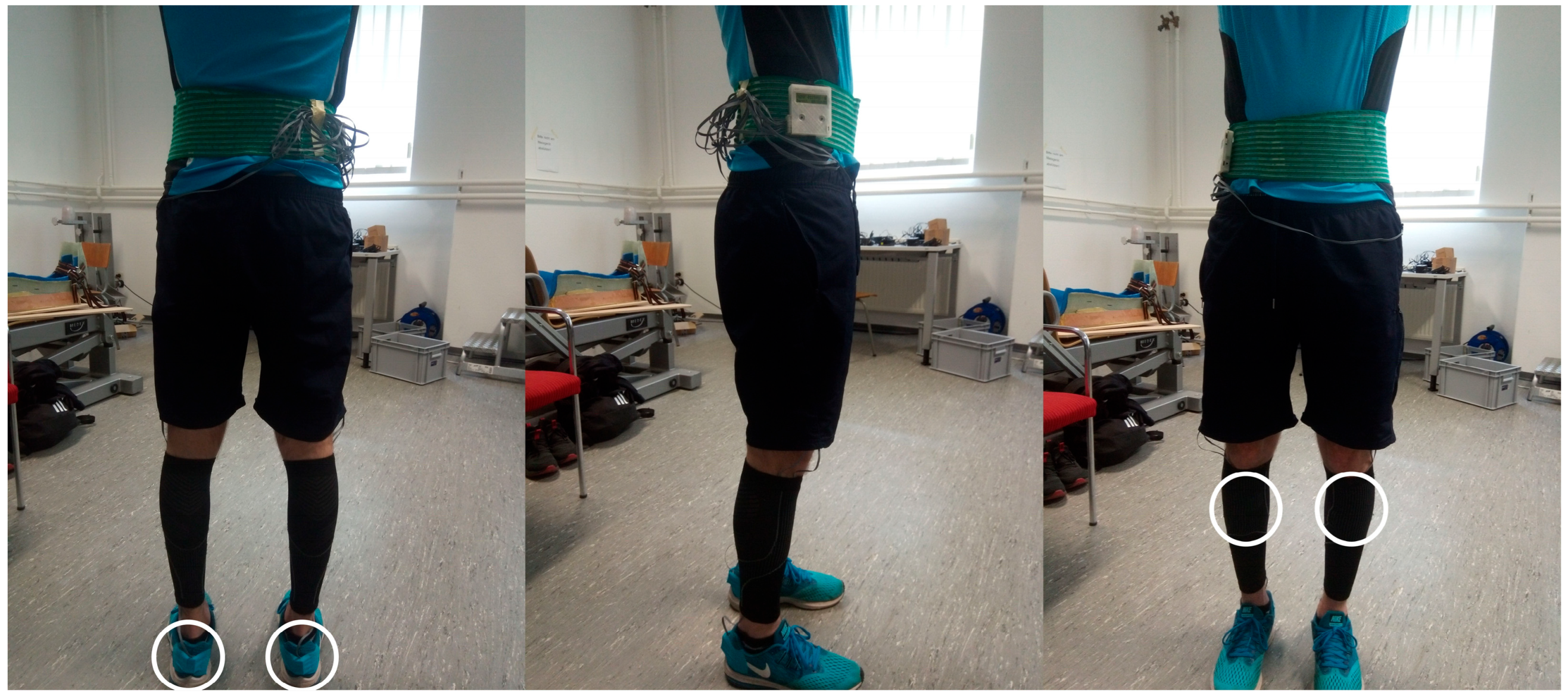
2.2. Testing
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Spatiotemporal Parameters
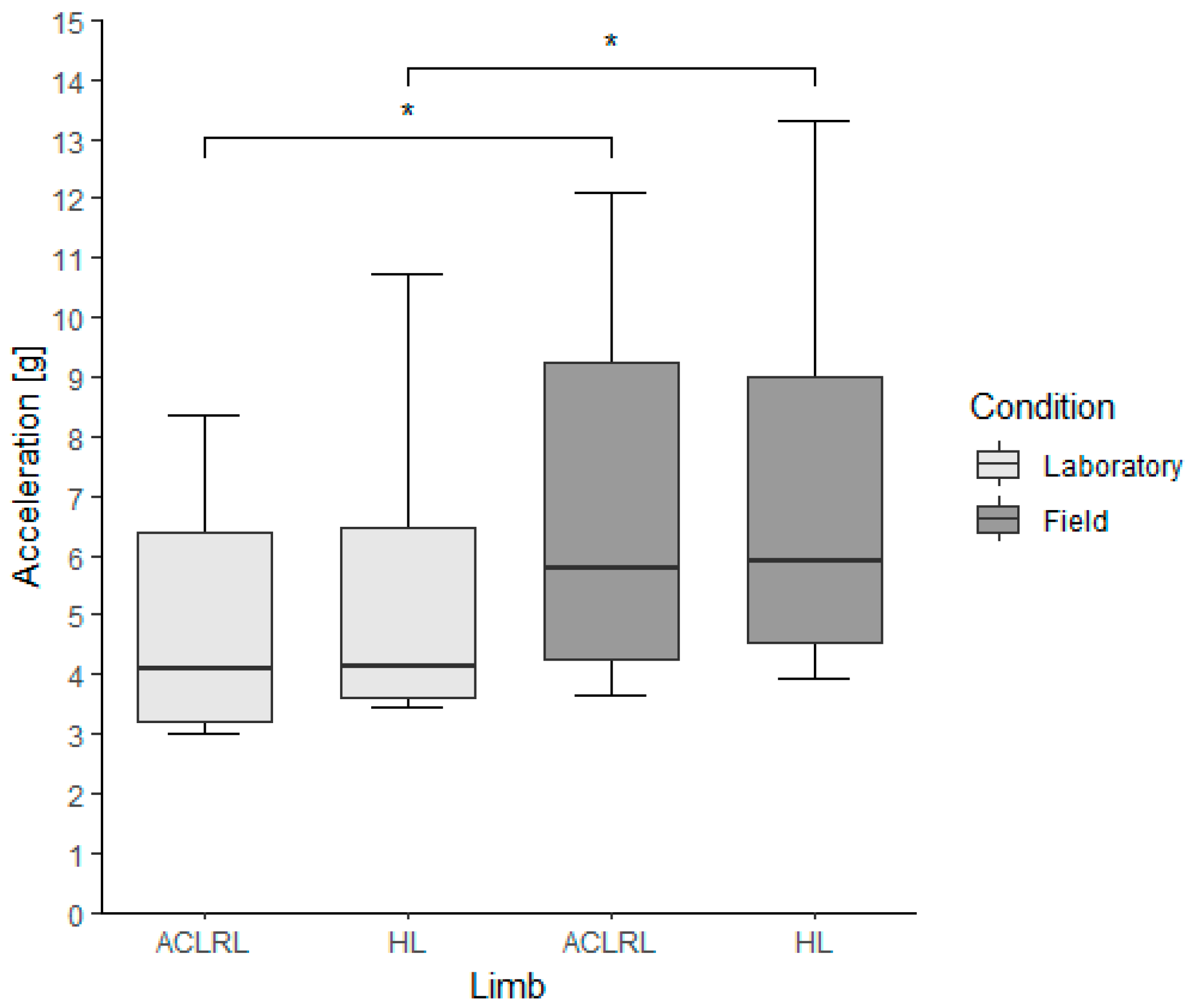
3.3. Peak Tibial Accelerations
3.4. Time after Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fulton, J.; Wright, K.; Kelly, M.; Zebrosky, B.; Zanis, M.; Drvol, C.; Butler, R. Injury Risk Is Altered by Previous Injury: A Systematic Review of the Literature and Presentation of Causative Neuromuscular Factors. Int. J. Sports Phys. Ther. 2014, 9, 583–595. [Google Scholar] [PubMed]
- Waldén, M.; Hägglund, M.; Ekstrand, J. High Risk of New Knee Injury in Elite Footballers with Previous Anterior Cruciate Ligament Injury. Br. J. Sports Med. 2006, 40, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, A.J.; Grandhi, R.K.; Schneider, D.K.; Stanfield, D.; Webster, K.E.; Myer, G.D. Risk of Secondary Injury in Younger Athletes after Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2016, 44, 1861–1876. [Google Scholar] [CrossRef] [PubMed]
- Hart, H.F.; Culvenor, A.G.; Collins, N.J.; Ackland, D.C.; Cowan, S.M.; Machotka, Z.; Crossley, K.M. Knee Kinematics and Joint Moments during Gait Following Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2016, 50, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Lepley, A.S.; Kuenze, C.M. Hip and Knee Kinematics and Kinetics during Landing Tasks after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. J. Athl. Train. 2018, 53, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Pairot-de-Fontenay, B.; Willy, R.W.; Elias, A.R.C.; Mizner, R.L.; Dubé, M.O.; Roy, J.S. Running Biomechanics in Individuals with Anterior Cruciate Ligament Reconstruction: A Systematic Review. Sports Med. 2019, 49, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Slater, L.V.; Hart, J.M.; Kelly, A.R.; Kuenze, C.M. Progressive Changes in Walking Kinematics and Kinetics after Anterior Cruciate Ligament Injury and Reconstruction: A Review and Meta-Analysis. J. Athl. Train. 2017, 52, 847–860. [Google Scholar] [CrossRef]
- van der Kruk, E.; Reijne, M.M. Accuracy of Human Motion Capture Systems for Sport Applications; State-of-the-Art Review. Eur. J. Sport Sci. 2018, 18, 806–819. [Google Scholar] [CrossRef]
- Sharif Bidabadi, S.; Murray, I.; Lee, G.Y.F. Validation of Foot Pitch Angle Estimation Using Inertial Measurement Unit against Marker-Based Optical 3D Motion Capture System. Biomed. Eng. Lett. 2018, 8, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Higginson, B.K. Methods of Running Gait Analysis. Curr. Sports Med. Rep. 2009, 8, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Pueo, B.; Jimenez-Olmedo, J.M. Application of Motion Capture Technology for Sport Performance Analysis. Retos 2017, 32, 241–247. [Google Scholar] [CrossRef]
- Van Hooren, B.; Fuller, J.T.; Buckley, J.D.; Miller, J.R.; Sewell, K.; Rao, G.; Barton, C.; Bishop, C.; Willy, R.W. Is Motorized Treadmill Running Biomechanically Comparable to Overground Running? A Systematic Review and Meta-Analysis of Cross-Over Studies. Sports Med. 2020, 50, 785–813. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.; Outerleys, J.; Jamison, S.S.; Tenforde, A.S.; Ruder, M.; Davis, I.S. Comparison of Tibial Shock during Treadmill and Real-World Running. Med. Sci. Sports Exerc. 2020, 52, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Milner, C.E.; Hawkins, J.L.; Aubol, K.G. Tibial Acceleration during Running Is Higher in Field Testing than Indoor Testing. Med. Sci. Sports Exerc. 2020, 52, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Colino, E.; Garcia-Unanue, J.; Gallardo, L.; Foster, C.; Lucia, A.; Felipe, J.L. Mechanical Properties of Treadmill Surfaces and Their Effects on Endurance Running. Int. J. Sports Physiol. Perform. 2020, 15, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.C.; Gust, G.; Bazett-Jones, D.M. Tibial Acceleration and Shock Attenuation While Running over Different Surfaces in a Trail Environment. J. Sci. Med. Sport. 2021, 24, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Napier, C.; Fridman, L.; Blazey, P.; Tran, N.; Michie, T.V.; Schneeberg, A. Differences in Peak Impact Accelerations among Foot Strike Patterns in Recreational Runners. Front. Sports Act. Living 2022, 4, 802019. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Hayano, T.; Jamison, S.T.; Outerleys, J.; Davis, I.S. Tibial Acceleration Measured from Wearable Sensors Is Associated with Loading Rates in Injured Runners. PM R 2020, 12, 679–684. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, J.A.; Pérez-Soriano, P.; Llana Belloch, S.; Lucas-Cuevas, Á.G.; Sánchez-Zuriaga, D. Effects of Treadmill Running and Fatigue on Impact Acceleration in Distance Running. Sports Biomech. 2014, 13, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Havens, K.L.; Cohen, S.C.; Pratt, K.A.; Sigward, S.M. Accelerations from Wearable Accelerometers Reflect Knee Loading during Running after Anterior Cruciate Ligament Reconstruction. Clin. Biomech. 2018, 58, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Sheerin, K.R.; Reid, D.; Besier, T.F. The Measurement of Tibial Acceleration in Runners—A Review of the Factors That Can Affect Tibial Acceleration during Running and Evidence-Based Guidelines for Its Use. Gait Posture 2019, 67, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Lafortune, M.A. Three-Dimensional Acceleration of the Tibia during Walking and Running. J. Biomech. 1991, 24, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.; Outerleys, J.; Davis, I.S. Relationships between Tibial Acceleration and Ground Reaction Force Measures in the Medial-Lateral and Anterior-Posterior Planes. J. Biomech. 2021, 117, 110250. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, S.; Kiesewetter, P.; Milani, T.L.; Mitschke, C. The ‘Ride’ Feeling during Running under Field Conditions—Objectified with a Single Inertial Measurement Unit. Sensors 2021, 21, 5010. [Google Scholar] [CrossRef]
- Kiesewetter, P.; Bräuer, S.; Haase, R.; Nitzsche, N.; Mitschke, C.; Milani, T.L. Do Carbon-Plated Running Shoes with Different Characteristics Influence Physiological and Biomechanical Variables during a 10 km Treadmill Run? Appl. Sci. 2022, 12, 7949. [Google Scholar] [CrossRef]
- Maiwald, C.; Dannemann, A.; Gaudel, J.; Oriwol, D. A Simple Method to Detect Stride Intervals in Continuous Acceleration and Gyroscope Data Recorded during Treadmill Running. Footwear Sci. 2015, 7, 143–144. [Google Scholar] [CrossRef]
- Mitschke, C.; Heß, T.; Milani, T.L. Which Method Detects Foot Strike in Rearfoot and Forefoot Runners Accurately When Using an Inertial Measurement Unit? Appl. Sci. 2017, 7, 959. [Google Scholar] [CrossRef]
- Strohrmann, C.; Harms, H.; Kappeler-Setz, C.; Tröster, G. Monitoring Kinematic Changes with Fatigue in Running Using Body-Worn Sensors. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Knurr, K.A.; Kliethermes, S.A.; Stiffler-Joachim, M.R.; Cobian, D.G.; Baer, G.S.; Heiderscheit, B.C. Running Biomechanics Before Injury and 1 Year after Anterior Cruciate Ligament Reconstruction in Division I Collegiate Athletes. Am. J. Sports Med. 2021, 49, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Noehren, B.; Abraham, A.; Curry, M.; Johnson, D.; Ireland, M.L. Evaluation of Proximal Joint Kinematics and Muscle Strength Following ACL Reconstruction Surgery in Female Athletes. J. Orthop. Res. 2014, 32, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Pamukoff, D.N.; Montgomery, M.M.; Choe, K.H.; Moffit, T.J.; Garcia, S.A.; Vakula, M.N. Bilateral Alterations in Running Mechanics and Quadriceps Function Following Unilateral Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2018, 48, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Milandri, G.; Posthumus, M.; Small, T.J.; Bothma, A.; van der Merwe, W.; Kassanjee, R.; Sivarasu, S. Kinematic and Kinetic Gait Deviations in Males Long after Anterior Cruciate Ligament Reconstruction. Clin. Biomech. 2017, 49, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Pamukoff, D.N.; Montgomery, M.M.; Choe, K.H.; Moffit, T.J.; Vakula, M.N. Effect of Whole-Body Vibration on Sagittal Plane Running Mechanics in Individuals with Anterior Cruciate Ligament Reconstruction: A Randomized Crossover Trial. Arch. Phys. Med. Rehabil. 2018, 99, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Edwards, W.B.; Derrick, T.R.; Hamill, J. Musculoskeletal Attenuation of Impact Shock in Response to Knee Angle Manipulation. J. Appl. Biomech. 2012, 28, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Karanikas, K.; Arampatzis, A.; Brüggemann, G.P. Motor Task and Muscle Strength Followed Different Adaptation Patterns after Anterior Cruciate Ligament Reconstruction Reliability. Eur. J. Phys. Rehabil. Med. 2008, 45, 37–45. [Google Scholar]
- Roewer, B.D.; Di Stasi, S.L.; Snyder-Mackler, L. Quadriceps Strength and Weight Acceptance Strategies Continue to Improve Two Years after Anterior Cruciate Ligament Reconstruction. J. Biomech. 2011, 44, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Feller, J.A.; Wittwer, J.E. Longitudinal Changes in Knee Joint Biomechanics during Level Walking Following Anterior Cruciate Ligament Reconstruction Surgery. Gait Posture 2012, 36, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Gilgen-Ammann, R.; Taube, W.; Wyss, T. Gait Asymmetry during 400-to 1000-m High-Intensity Track Running in Relation to Injury History. Int. J. Sports Physiol. Perform. 2017, 12, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Morin, J.B.; Samozino, P.; Zameziati, K.; Belli, A. Effects of Altered Stride Frequency and Contact Time on Leg-Spring Behavior in Human Running. J. Biomech. 2007, 40, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Whittle, M.W. Generation and Attenuation of Transient Impulsive Forces beneath the Foot: A Review. Gait Posture 1999, 10, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Chia, L.; De Oliveira Silva, D.; Whalan, M.; McKay, M.J.; Sullivan, J.; Fuller, C.W.; Pappas, E. Non-Contact Anterior Cruciate Ligament Injury Epidemiology in Team-Ball Sports: A Systematic Review with Meta-Analysis by Sex, Age, Sport, Participation Level, and Exposure Type. Sports Med. 2022, 52, 2447–2467. [Google Scholar] [CrossRef] [PubMed]
- Renstrom, P.; Ljungqvist, A.; Arendt, E.; Beynnon, B.; Fukubayashi, T.; Garrett, W.; Georgoulis, T.; Hewett, T.E.; Johnson, R.; Krosshaug, T.; et al. Non-Contact ACL Injuries in Female Athletes: An International Olympic Committee Current Concepts Statement. Br. J. Sports Med. 2008, 42, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Chambon, N.; Delattre, N.; Guéguen, N.; Berton, E.; Rao, G. Shoe drop has opposite influence on running pattern when running overground or on a treadmill. Eur. J. Appl. Physiol. 2015, 115, 911–918. [Google Scholar] [CrossRef] [PubMed]


| Laboratory | Field | |||
|---|---|---|---|---|
| Variable (MED (IQR)) | ||||
| Group | ACLRL | HL | ACLRL | HL |
| LL | RL | LL | RL | |
| GCT (s) | ||||
| ACLR | 0.254 (0.038) | 0.258 (0.033) | 0.253 (0.034) | 0.257 (0.034) |
| Controls | 0.259 (0.021) | 0.257 (0.022) | 0.262 (0.018) | 0.267 (0.026) |
| vPTA (g) | ||||
| ACLR | 6.09 (0.72) | 5.81 (2.46) | 8.89 (2.11) * | 8.61 (5.06) * |
| Controls | 5.10 (1.64) | 5.42 (1.63) | 6.28 (0.68) * | 6.49 (1.61) * |
| mlPTA (g) | ||||
| ACLR | 4.08 (3.16) | 4.15 (2.86) | 5.76 (4.97) * | 5.91 (4.46) * |
| Controls | 3.71 (0.85) | 3.62 (1.04) | 4.93 (1.43) * | 4.73 (2.09) |
| apPTA (g) | ||||
| ACLR | 2.77 (0.46) | 3.33 (0.54) | 3.72 (1.73) * | 4.10 (1.31) * |
| Controls | 3.33 (1.27) | 3.16 (0.89) | 4.16 (1.57) * | 3.79 (1.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, M.; Kiesewetter, P.; Milani, T.L.; Mitschke, C. An Investigation of Running Kinematics with Recovered Anterior Cruciate Ligament Reconstruction on a Treadmill and In-Field Using Inertial Measurement Units: A Preliminary Study. Bioengineering 2024, 11, 404. https://doi.org/10.3390/bioengineering11040404
Hill M, Kiesewetter P, Milani TL, Mitschke C. An Investigation of Running Kinematics with Recovered Anterior Cruciate Ligament Reconstruction on a Treadmill and In-Field Using Inertial Measurement Units: A Preliminary Study. Bioengineering. 2024; 11(4):404. https://doi.org/10.3390/bioengineering11040404
Chicago/Turabian StyleHill, Matteo, Pierre Kiesewetter, Thomas L. Milani, and Christian Mitschke. 2024. "An Investigation of Running Kinematics with Recovered Anterior Cruciate Ligament Reconstruction on a Treadmill and In-Field Using Inertial Measurement Units: A Preliminary Study" Bioengineering 11, no. 4: 404. https://doi.org/10.3390/bioengineering11040404
APA StyleHill, M., Kiesewetter, P., Milani, T. L., & Mitschke, C. (2024). An Investigation of Running Kinematics with Recovered Anterior Cruciate Ligament Reconstruction on a Treadmill and In-Field Using Inertial Measurement Units: A Preliminary Study. Bioengineering, 11(4), 404. https://doi.org/10.3390/bioengineering11040404






