Assessment of Microvascular Hemodynamic Adaptations in Finger Flexors of Climbers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Experimental Protocol
2.2.2. Data Collection
2.2.3. Vascular Occlusion Test
2.3. Data Processing and Variables
2.4. Statistical Analysis
3. Results
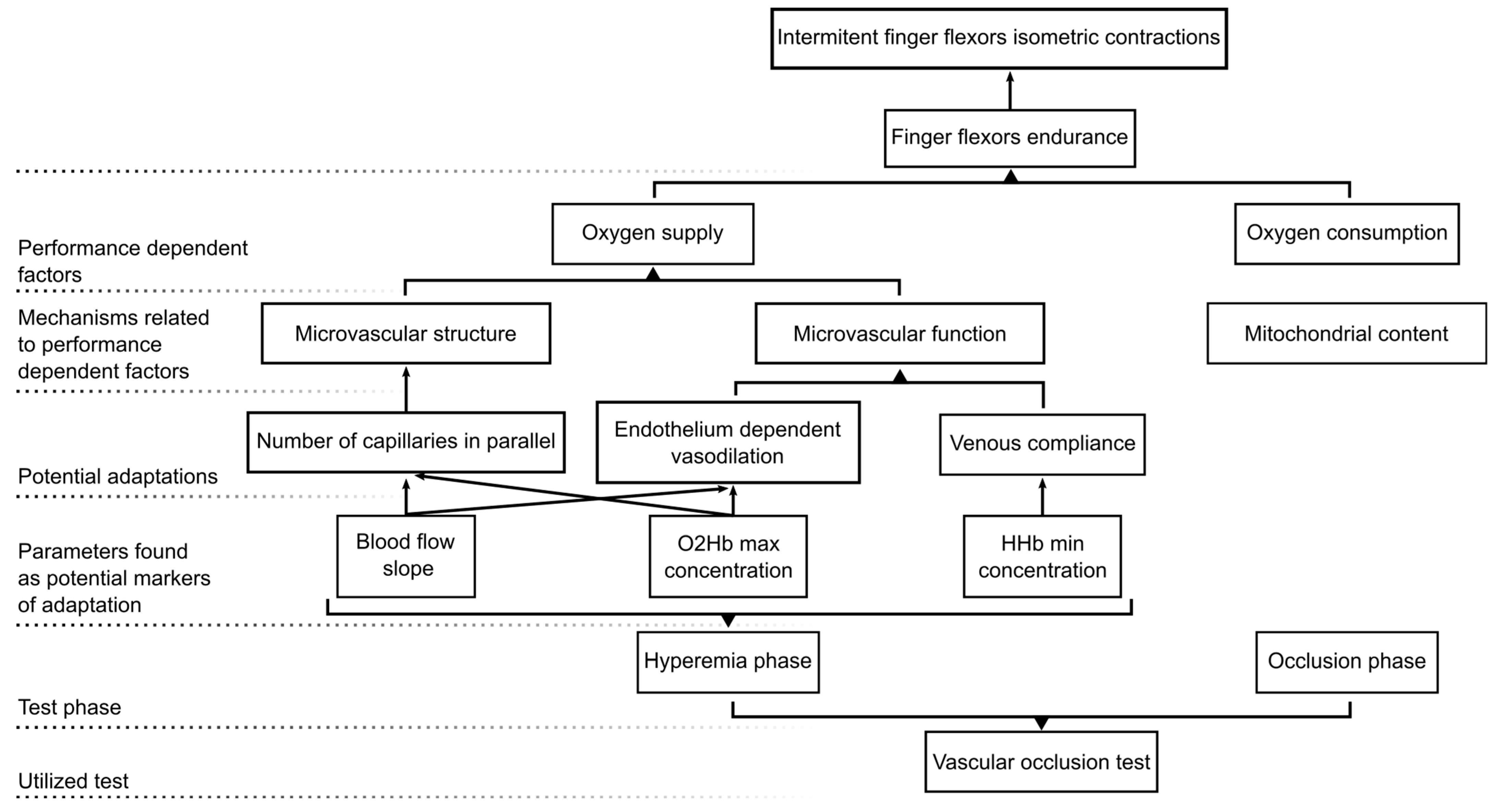
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giles, D.; Hartley, C.; Maslen, H.; Hadley, J.; Taylor, N.; Torr, O.; Chidley, J.; Randall, T.; Fryer, S. An All-out Test to Determine Finger Flexor Critical Force in Rock Climbers. Int. J. Sports Physiol. Perform. 2020, 16, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Philippe, M.; Wegst, D.; Müller, T.; Raschner, C.; Burtscher, M. Climbing-Specific Finger Flexor Performance and Forearm Muscle Oxygenation in Elite Male and Female Sport Climbers. Eur. J. Appl. Physiol. 2012, 112, 2839–2847. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Uris, B.; Arias, D.; Torrado, P.; Marina, M.; Busquets, A. Exploring Forearm Muscle Coordination and Training Applications of Various Grip Positions during Maximal Isometric Finger Dead-Hangs in Rock Climbers. PeerJ 2023, 11, e15464. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Quaresima, V. Near-Infrared Spectroscopy and Skeletal Muscle Oxidative Function in Vivo in Health and Disease: A Review from an Exercise Physiology Perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef] [PubMed]
- Sejersted, O.M.; Hargens, A.R.; Kardel, K.R.; Blom, P.; Jensen, O.; Hermansen, L. Intramuscular Fluid Pressure during Isometric Contraction of Human Skeletal Muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- McNeil, C.J.; Allen, M.D.; Olympico, E.; Shoemaker, J.K.; Rice, C.L. Blood Flow and Muscle Oxygenation during Low, Moderate, and Maximal Sustained Isometric Contractions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R475–R481. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Hopman, M.T.E.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H.J. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.; Stoner, L.; Dickson, T.G.; Draper, S.B.; McCluskey, M.J.; Hughes, J.D.; How, S.C.; Draper, N. Oxygen Recovery Kinetics in the Forearm Flexors of Multiple Ability Groups of Rock Climbers. J. Strength Cond. Res. 2015, 29, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Walther, G.; Diaz-Cañestro, C.; Pyke, K.E.; Padilla, J. Microvascular Dilator Function in Athletes: A Systematic Review and Meta-Analysis. Med. Sci. Sports Exerc. 2015, 47, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Tryon, L.D.; Vainshtein, A.; Memme, J.; Chen, C.; Pauly, M.; Crilly, M.J.; Carter, H. Exercise and the Regulation of Mitochondrial Turnover, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 135, ISBN 9780128039915. [Google Scholar]
- Padilla, J.; Simmons, G.H.; Bender, S.B.; Arce-Esquivel, A.A.; Whyte, J.J.; Laughlin, M.H. Vascular Effects of Exercise: Endothelial Adaptations beyond Active Muscle Beds. Physiology 2011, 26, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Niezen, C.K.; Massari, D.; Vos, J.J.; Scheeren, T.W.L. The Use of a Vascular Occlusion Test Combined with Near-Infrared Spectroscopy in Perioperative Care: A Systematic Review. J. Clin. Monit. Comput. 2022, 36, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, R.; Nelson, M.D. Reactive Hyperemia: A Review of Methods, Mechanisms, and Considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R605–R618. [Google Scholar] [CrossRef] [PubMed]
- Kranen, S.H.; Oliveira, R.S.; Bond, B.; Williams, C.A.; Barker, A.R. The Utility of the Reperfusion Rate of Tissue Oxygen Saturation as a Measure of Vascular Endothelial Function in Adolescents: Reliability, Validity and Sensitivity. Front. Physiol. 2023, 14, 1163474. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.V.; Volino-Souza, M.; Leitão, R.; Pinheiro, V.; Conte-Júnior, C.A.; Alvares, T.S. Suitability of the Muscle O2 Resaturation Parameters Most Used for Assessing Reactive Hyperemia: A near-Infrared Spectroscopy Study. J. Vasc. Bras. 2021, 20, e20200143. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, Y.; Nyberg, M.; Jensen, L.G.; Mortensen, S.P. Vasodilator Interactions in Skeletal Muscle Blood Flow Regulation. J. Physiol. 2012, 590, 6297–6305. [Google Scholar] [CrossRef] [PubMed]
- Pyke, K.E.; Poitras, V.; Tschakovsky, M.E. Brachial Artery Flow-Mediated Dilation during Handgrip Exercise: Evidence for Endothelial Transduction of the Mean Shear Stimulus. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Casey, D.P. Regulation of Increased Blood Flow (Hyperemia) to Muscles During Exercise: A Hierarchy of Competing Physiological Needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [PubMed]
- Barstow, T.J. Understanding near Infrared Spectroscopy and Its Application to Skeletal Muscle Research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.; Stone, K.J.; Sveen, J.; Dickson, T.; España-Romero, V.; Giles, D.; Baláš, J.; Stoner, L.; Draper, N. Differences in Forearm Strength, Endurance, and Hemodynamic Kinetics between Male Boulderers and Lead Rock Climbers. Eur. J. Sport Sci. 2017, 17, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.; Giles, D.; Palomino, I.G.; de la O Puerta, A.; España-Romero, V. Hemodynamic and Cardiorespiratory Predictors of Sport Rock Climbing Performance. J. Strength Cond. Res. 2018, 32, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.; Stoner, L.; Stone, K.; Giles, D.; Sveen, J.; Garrido, I.; España-Romero, V. Forearm Muscle Oxidative Capacity Index Predicts Sport Rock-Climbing Performance. Eur. J. Appl. Physiol. 2016, 116, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Sanni, A.A.; McCully, K.K. Interpretation of Near-Infrared Spectroscopy (NIRS) Signals in Skeletal Muscle. J. Funct. Morphol. Kinesiol. 2019, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Durduran, T.; Choe, R.; Baker, W.B.; Yodh, A.G. Diffuse Optics for Tissue Monitoring and Tomography. Reports Prog. Phys. 2010, 73, 076701. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, V.; Farzam, P.; Anderson, P.; Farzam, P.Y.; Wiese, D.; Carp, S.A.; Ferrari, M.; Franceschini, M.A. Diffuse Correlation Spectroscopy and Frequency-Domain near-Infrared Spectroscopy for Measuring Microvascular Blood Flow in Dynamically Exercising Human Muscles. J. Appl. Physiol. 2019, 127, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Symons, T.B.; Durduran, T.; Yodh, A.G.; Yu, G. Effects of Muscle Fiber Motion on Diffuse Correlation Spectroscopy Blood Flow Measurements during Exercise. Biomed. Opt. Express 2010, 1, 500. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Li, T.; Yu, G. Clinical Applications of Near-Infrared Diffuse Correlation Spectroscopy and Tomography for Tissue Blood Flow Monitoring and Imaging. Physiol. Meas. 2017, 38, R1–R26. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Liu, Y.; Qian, L.; Zheng, Y.; Gao, J.; Cao, W.; Shang, Y. Portable Near-Infrared Technologies and Devices for Noninvasive Assessment of Tissue Hemodynamics. J. Healthc. Eng. 2019, 2019, 3750495. [Google Scholar] [CrossRef]
- Draper, N.; Giles, D.; Schöffl, V.; Konstantin Fuss, F.; Watts, P.; Wolf, P.; Baláš, J.; Espana-Romero, V.; Blunt Gonzalez, G.; Fryer, S.; et al. Comparative Grading Scales, Statistical Analyses, Climber Descriptors and Ability Grouping: International Rock Climbing Research Association Position Statement. Sport. Technol. 2015, 8, 88–94. [Google Scholar] [CrossRef]
- Giles, D.; España Romero, V.; Garrido, I.; De La O Puerta, A.; Stone, K.; Fryer, S. Differences in Oxygenation Kinetics between the Dominant and Nondominant Flexor Digitorum Profundus in Rock Climbers. Int. J. Sports Physiol. Perform. 2017, 12, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 0805802835. [Google Scholar]
- Thompson, E.B.; Farrow, L.; Hunt, J.E.A.; Lewis, M.P.; Ferguson, R.A. Brachial Artery Characteristics and Micro-Vascular Filtration Capacity in Rock Climbers. Eur. J. Sport Sci. 2015, 15, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.F.; Oneglia, A.; Jaffery, M.; Manitowabi-Huebner, S.; Hueber, D.M.; Nelson, M.D. Kinetic Differences between Macro- And Microvascular Measures of Reactive Hyperemia. J. Appl. Physiol. 2020, 129, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.N.; George, M.A.; Proctor, D.N.; Murias, J.M. Differences in Vascular Function between Trained and Untrained Limbs Assessed by Near-Infrared Spectroscopy. Eur. J. Appl. Physiol. 2018, 118, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- McLay, K.M.; Gilbertson, J.E.; Pogliaghi, S.; Paterson, D.H.; Murias, J.M. Vascular Responsiveness Measured by Tissue Oxygen Saturation Reperfusion Slope Is Sensitive to Different Occlusion Durations and Training Status. Exp. Physiol. 2016, 101, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.L.; Frandsen, U.; Jensen, K.Y.; Prokhorova, T.A.; Dalgaard, L.B.; Bech, R.D.; Nygaard, T.; Suetta, C.; Aagaard, P. Skeletal Muscle Microvascular Changes in Response to Short-Term Blood Flow Restricted Training—Exercise-Induced Adaptations and Signs of Perivascular Stress. Front. Physiol. 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Holloway, T.M.; Morton, R.W.; Oikawa, S.Y.; McKellar, S.; Baker, S.K.; Phillips, S.M. Microvascular Adaptations to Resistance Training Are Independent of Load in Resistance-Trained Young Men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R267–R273. [Google Scholar] [CrossRef] [PubMed]
- Egginton, S. Invited Review: Activity-Induced Angiogenesis. Eur. J. Physiol. 2009, 457, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.; Stoner, L.; Lucero, A.; Witter, T.; Scarrott, C.; Dickson, T.; Cole, M.; Draper, N. Haemodynamic Kinetics and Intermittent Finger Flexor Performance in Rock Climbers. Int. J. Sports Med. 2014, 36, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Koutlas, A.; Smilios, I.; Kokkinou, E.M.; Myrkos, A.; Kounoupis, A.; Dipla, K.; Zafeiridis, A. NIRS-Derived Muscle-Deoxygenation and Microvascular Reactivity during Occlusion–Reperfusion at Rest Are Associated With Whole-Body Aerobic Fitness. Res. Q. Exerc. Sport 2023, 95, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, Y.; Nyberg, M. Cardiovascular Adaptations to Exercise Training. Compr. Physiol. 2016, 6, 1–32. [Google Scholar] [CrossRef]
- Thomas, K.N.; Akerman, A.P.; Gibbons, T.D.; Campbell, H.A.; Cotter, J.D.; van Rij, A.M. The Athlete’s Vein: Venous Adaptations in the Lower Limbs of Endurance Athletes. Am. J. Physiol. Endocrinol. Metab. 2023, 325, H66–H76. [Google Scholar] [CrossRef] [PubMed]
- Oue, A.; Saito, M.; Iimura, Y. Effect of Short-Term Endurance Training on Venous Compliance in the Calf and Forearm Differs between Continuous and Interval Exercise in Humans. Physiol. Rep. 2019, 7, e14211. [Google Scholar] [CrossRef] [PubMed]
- Muthalib, M.; Millet, G.Y.; Quaresima, V.; Nosaka, K. Reliability of Near-Infrared Spectroscopy for Measuring Biceps Brachii Oxygenation during Sustained and Repeated Isometric Contractions. J. Biomed. Opt. 2010, 15, 017008. [Google Scholar] [CrossRef] [PubMed]
- Hamaoka, T.; McCully, K.K.; Niwayama, M.; Chance, B. The Use of Muscle Near-Infrared Spectroscopy in Sport, Health and Medical Sciences: Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 4591–4604. [Google Scholar] [CrossRef] [PubMed]
- Rasica, L.; Inglis, E.C.; Iannetta, D.; Soares, R.N.; Murias, J.M. Fitness Level- and Sex-Related Differences in Macrovascular and Microvascular Responses during Reactive Hyperemia. Med. Sci. Sports Exerc. 2022, 54, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.; Stoner, L.; Scarrott, C.; Lucero, A.; Witter, T.; Love, R.; Dickson, T.; Draper, N. Forearm Oxygenation and Blood Flow Kinetics during a Sustained Contraction in Multiple Ability Groups of Rock Climbers. J. Sports Sci. 2015, 33, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.E.; Erickson, M.L.; Brizendine, J.T.; Young, H.J.; McCully, K.K. Noninvasive Evaluation of Skeletal Muscle Mitochondrial Capacity with Near-Infrared Spectroscopy: Correcting for Blood Volume Changes. J. Appl. Physiol. 2012, 113, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.E.; Southern, W.M.; Reynolds, M.A.; McCully, K.K. A Cross-Validation of near-Infrared Spectroscopy Measurements of Skeletal Muscle Oxidative Capacity with Phosphorus Magnetic Resonance Spectroscopy. J. Appl. Physiol. 2013, 115, 1757–1766. [Google Scholar] [CrossRef] [PubMed]


| Climbers | Non-Climbers | |
|---|---|---|
| N | 17 | 15 |
| Age (years) | 28.28 ± 6.0 | 25.83 ± 3.8 |
| Biological sex | 8 male/7 female | 8 male/7 female |
| Height (cm) | 170.5 ± 9.3 | 168.9 ± 8.4 |
| Weight (Kg) | 64.1 ± 9.6 | 65.8 ± 7.4 |
| Arm span (cm) | 174.8 ± 10.3 | 170.2 ± 10.3 |
| Forearm perimeter | 27.2 ± 2.4 | 25.7 ± 1.8 |
| Forearm skinfold (mm) | 1.7 ± 0.5 | 2.7 ± 1.3 |
| Climbing experience (years) | 8.6 ± 7.7 | - |
| Climbing/training session per week | 8.0 ± 0.9 | |
| IRCRA grading scale | 20.4 ± 3.2 | - |
| Climbers | Non-Climbers | |
|---|---|---|
| BA-O2Hb (µM) | 43.6 ± 10.7 | 39.1 ± 11.7 |
| BA-HHb (µM) | 24.9 ± 4 | 19.1 ± 4.1 |
| BA-TSI (%) | 63.3 ± 3.9 | 66.4 ± 4.6 |
| BA-BFI (cm2/s) | 1.70 × 10−9 ± 9.12 × 10−10 | 1.40 × 10−9 ± 4.25 × 10−10 |
| OC-∆O2Hbslope (µM/s) | −0.1 ± 0.1 | −0.1 ± 0.1 |
| OC-∆HHbslope (µM/s) | 0.1 ± 0 | 0.1 ± 0 |
| OC-∆TSIslope (%/s) | −0.1 ± 0 | −0.1 ± 0.1 |
| OC-∆O2Hbmin (µM) | −6.5 ± 6.4 | −8.6 ± 5.4 |
| OC-∆HHbmax (µM) | 14.9 ± 4.1 | 15 ± 6 |
| OC-∆TSImin (%) | −15.4 ± 8.5 | −20.3 ± 7.8 |
| OC-rBFmin (%) | 12.3 ± 7.1 | 12 ± 4.1 |
| OC-∆O2Hbtmin (s) | −22.2 ± 43.6 | −3.9 ± 7.8 |
| OC-∆HHbtmax (s) | −3.5 ± 12.8 | −2.8 ± 8.8 |
| OC-∆TSItmin (s) | −5.7 ± 13.9 | −2.3 ± 4.5 |
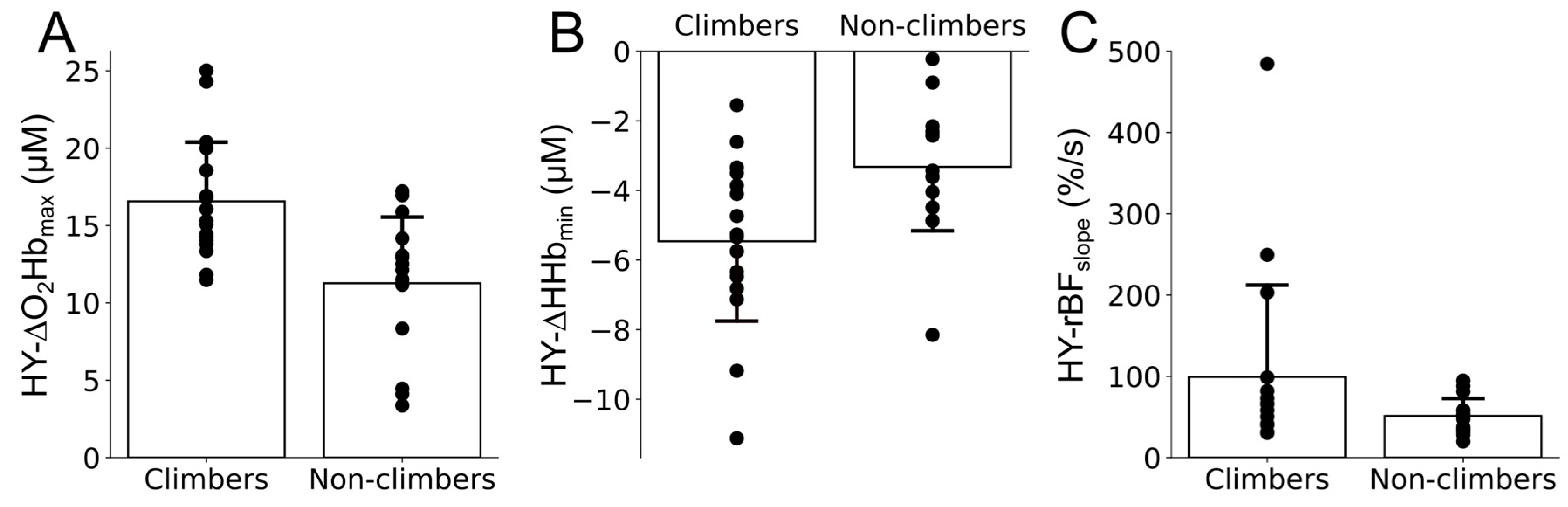
| HY-∆O2Hbmax (µM) | 16.6 ± 3.9 | 11.3 ± 4.4 |
| HY-∆Hhbmin (µM) | −5.5 ± 2.4 | −3.3 ± 1.9 |
| HY-∆TSImax (%) | 11.3 ± 4.2 | 9 ± 5.4 |
| HY-rBFmax (%) | 873.7 ± 440.3 | 712.4 ± 151 |
| HY-∆O2Hbtmax (s) | 14.4 ± 4.2 | 16.3 ± 4.6 |
| HY-∆HHbtmin (s) | 32 ± 7.8 | 33.9 ± 9.1 |
| HY-∆TSItmax (s) | 22.1 ± 6.1 | 24.5 ± 5.5 |
| HY-rBFtmax (s) | 13 ± 6.5 | 15.6 ± 5.9 |
| HY-∆O2Hbslope (µM/s) | 1.7 ± 0.6 | 1.6 ± 0.7 |
| HY-∆HHbslope (µM/s) | −1.4 ± 0.5 | −1.3 ± 0.6 |
| HY-∆TSI slope (%/s) | 2 ± 0.6 | 2.2 ± 1 |
| HY-rBFslope (%/s) | 100.8 ± 117.9 | 52.2 ± 22.7 |
| HY-∆O2HbHTR (s) | 4.8 ± 1.8 | 5.3 ± 1.9 |
| HY-∆HHbHTR (s) | 7.1 ± 3.1 | 7.6 ± 2.7 |
| HY-∆TSIHTR (s) | 5.9 ± 2.4 | 6.5 ± 2.1 |
| HY-rBFHTR (s) | 5.4 ± 4.3 | 4 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-Uris, B.; Busquets, A.; Beslija, F.; Durduran, T. Assessment of Microvascular Hemodynamic Adaptations in Finger Flexors of Climbers. Bioengineering 2024, 11, 401. https://doi.org/10.3390/bioengineering11040401
Ferrer-Uris B, Busquets A, Beslija F, Durduran T. Assessment of Microvascular Hemodynamic Adaptations in Finger Flexors of Climbers. Bioengineering. 2024; 11(4):401. https://doi.org/10.3390/bioengineering11040401
Chicago/Turabian StyleFerrer-Uris, Blai, Albert Busquets, Faruk Beslija, and Turgut Durduran. 2024. "Assessment of Microvascular Hemodynamic Adaptations in Finger Flexors of Climbers" Bioengineering 11, no. 4: 401. https://doi.org/10.3390/bioengineering11040401
APA StyleFerrer-Uris, B., Busquets, A., Beslija, F., & Durduran, T. (2024). Assessment of Microvascular Hemodynamic Adaptations in Finger Flexors of Climbers. Bioengineering, 11(4), 401. https://doi.org/10.3390/bioengineering11040401







