Could Dental Material Reuse Play a Significant Role in Preservation of Raw Materials, Water, Energy, and Costs? Microbiological Analysis of New versus Reused Healing Abutments: An In Vitro Study
Abstract
1. Introduction
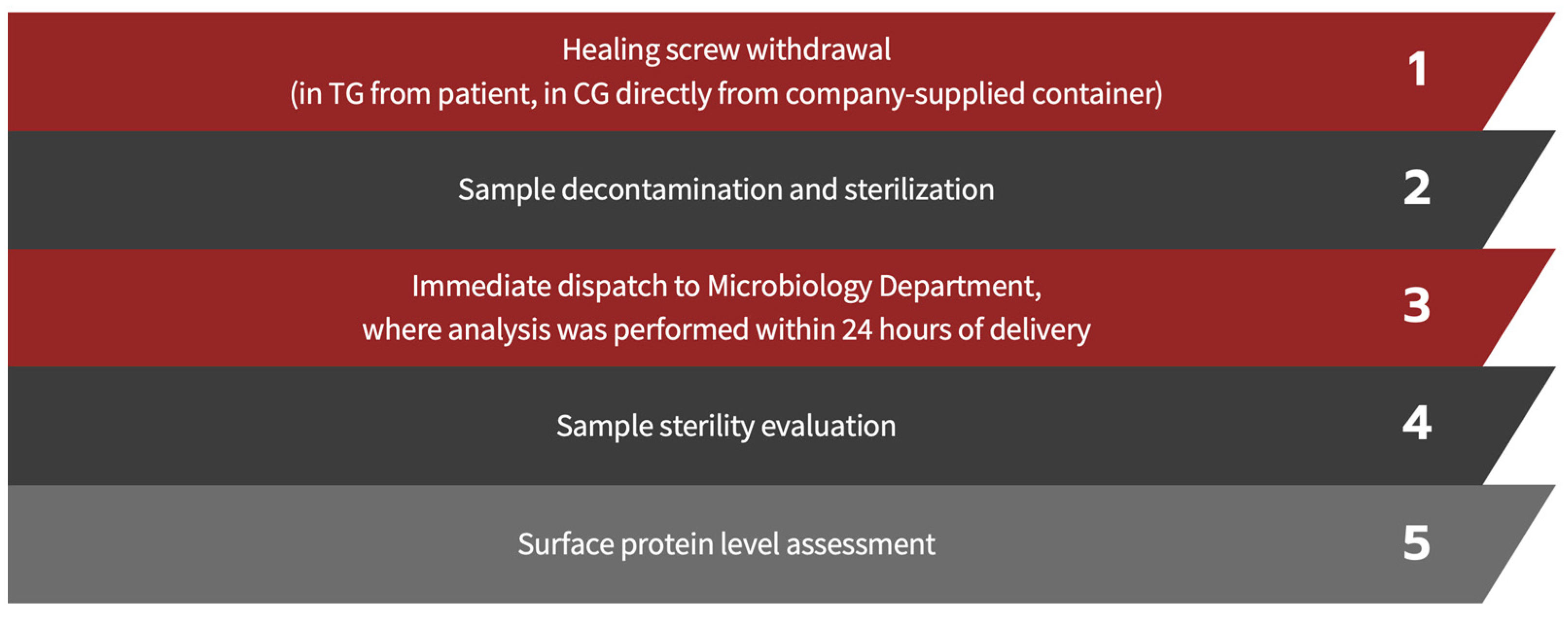
2. Materials and Methods
2.1. Sample Selection
- Inclusion criteria
- Age over 18 years;
- Systemic health according to American Society of Anesthesiologists guidelines (ASA I or II) [19];
- Non-smokers [20];
- Undergoing single implant placement in the mandibular or maxillary molar region;
- Screwed prostheses;
- Implant diameter of 3.8 mm;
- Not adequate for immediate loading and therefore undergoing deferred prosthetic loading;
- Healing screw inserted that corresponds to the characteristics listed in Table 1;
- Absence of periodontal disease with probing depth ≤ 3 mm, negativity for bleeding on probing and adherent gingiva ≥ 4 mm.
- Exclusion criteria
2.2. Decontamination and Sterilization Protocol
- Decontamination [27,28,29]; HAs are soaked for 15 min in decontamination baths containing 2 g of powdered disinfectant solution diluted in 1 liter of warm water. Composition details, chemical-physical characteristics, and properties of the disinfected agent are summarized in the following table (Table 2);
- Cleaning: washing in an ultrasonic tank filled with the same solution as described above for 15 min, effectively promoting the breakdown and elimination of potential contaminants from HA surfaces;
- Rinsing: take the material and rinse it thoroughly under flowing water;
- Drying: the material must be thoroughly dried before being bagged;
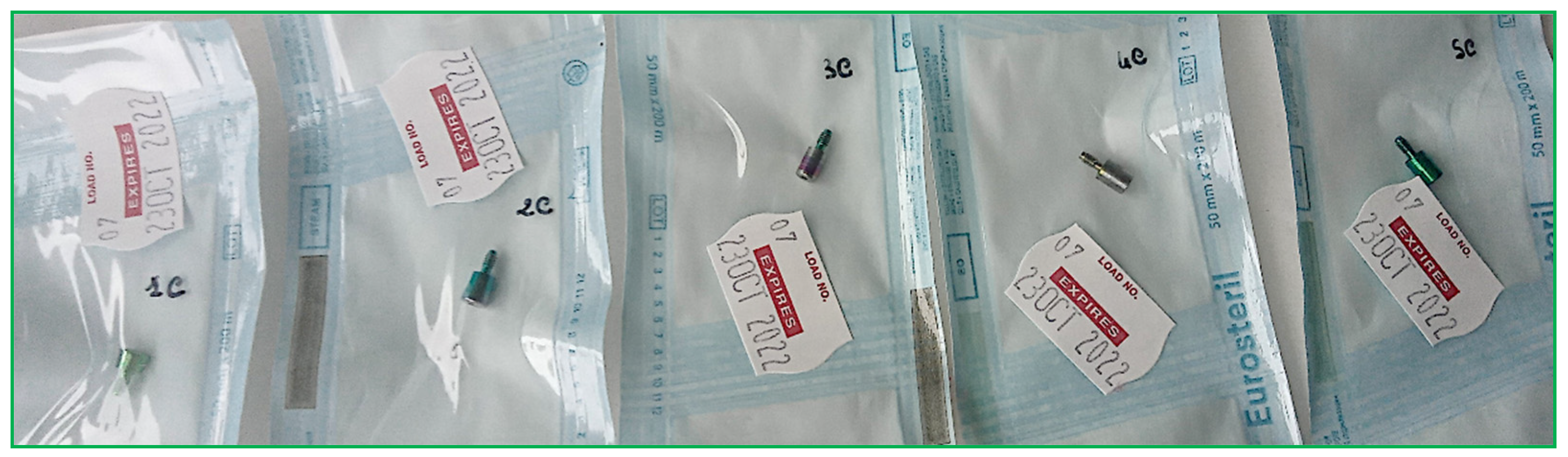
- Packaging: dried HAs were packaged in a sterile barrier system (SBS) consisting of paper and polypropylene. The packaging included traceability data for the sterilization cycle, such as the sterilization operator’s signature, the autoclave-indicated sterilization cycle number, packaging date, and expiration date (set at 30 days from packaging). The packages must feature an external chemical indicator of class 1 (UNI EN ISO 11140-1), which indicates whether the package has undergone processing (process indicator) [30];
- Storage (Figure 2).
2.3. Sample Sterility Evaluation
- Extraction of the HA from the sterilization pack using sterile forceps under a laminar flow hood;
- Incubation of the HA for 24 h at 37 °C;
- Assessment for the presence of turbidity in the culture broth by an operator; if absent, the component is considered sterile.
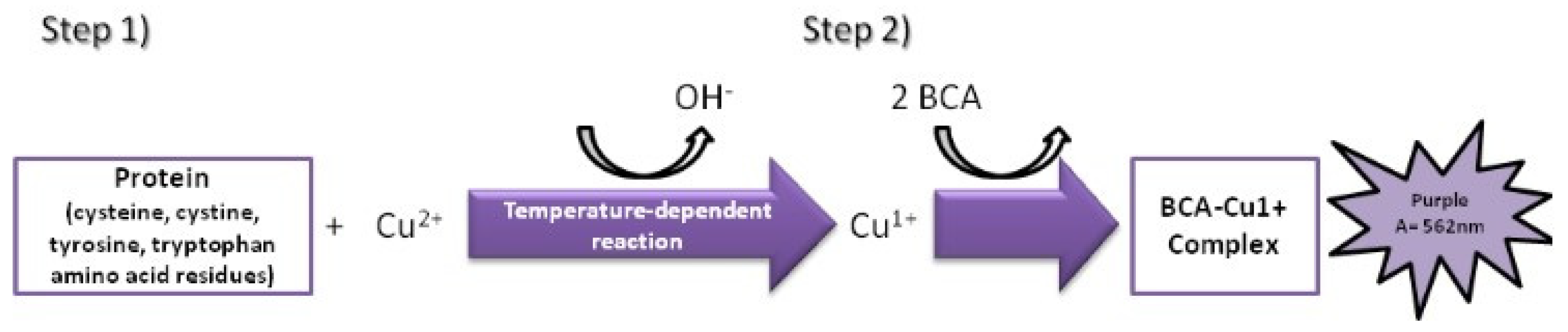
2.4. Assessment of Surface Protein Levels
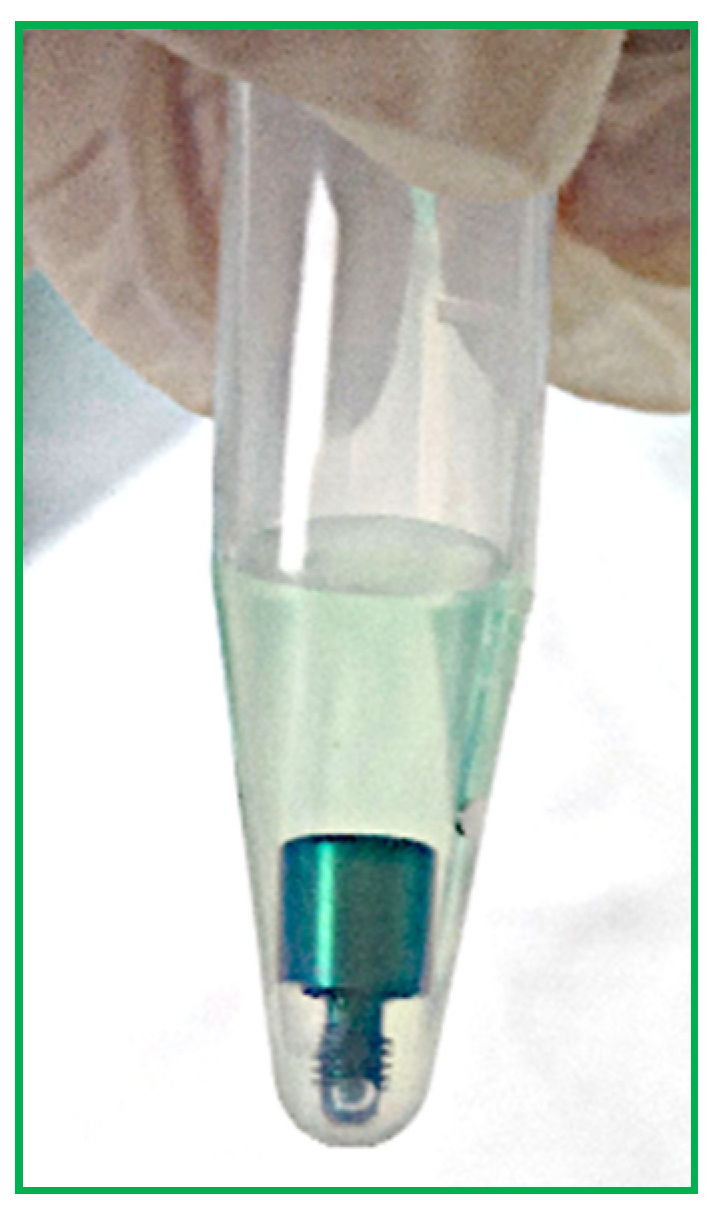
- Each HA was removed from the bags using tweezers and deposited in sterile 1.5 milliliter (mL) tubes.
- Then, 500 µL of BCA solution was added to each test tube to completely cover each screw (Figure 4).
- Samples and blanks, i.e., sterile tubes without HAs and containing only BCS for the purpose of visual comparison, were subject to a 2 h incubation at 37 °C (Figure 5).
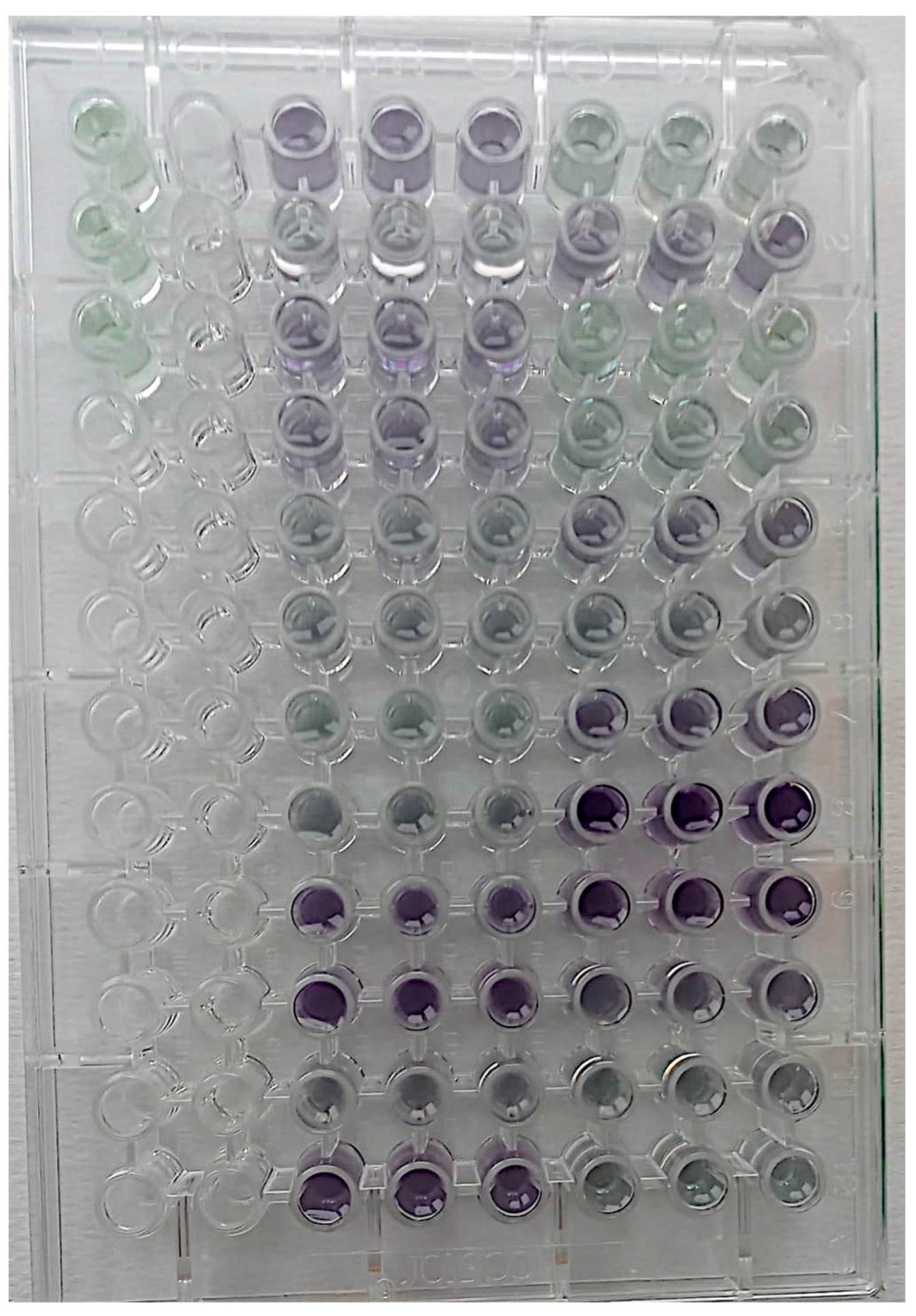
- Aliquots of 150 µL were added to 96-well plates in triplicate, and absorbance was read using a plate reader at 562 nm (Figure 6).
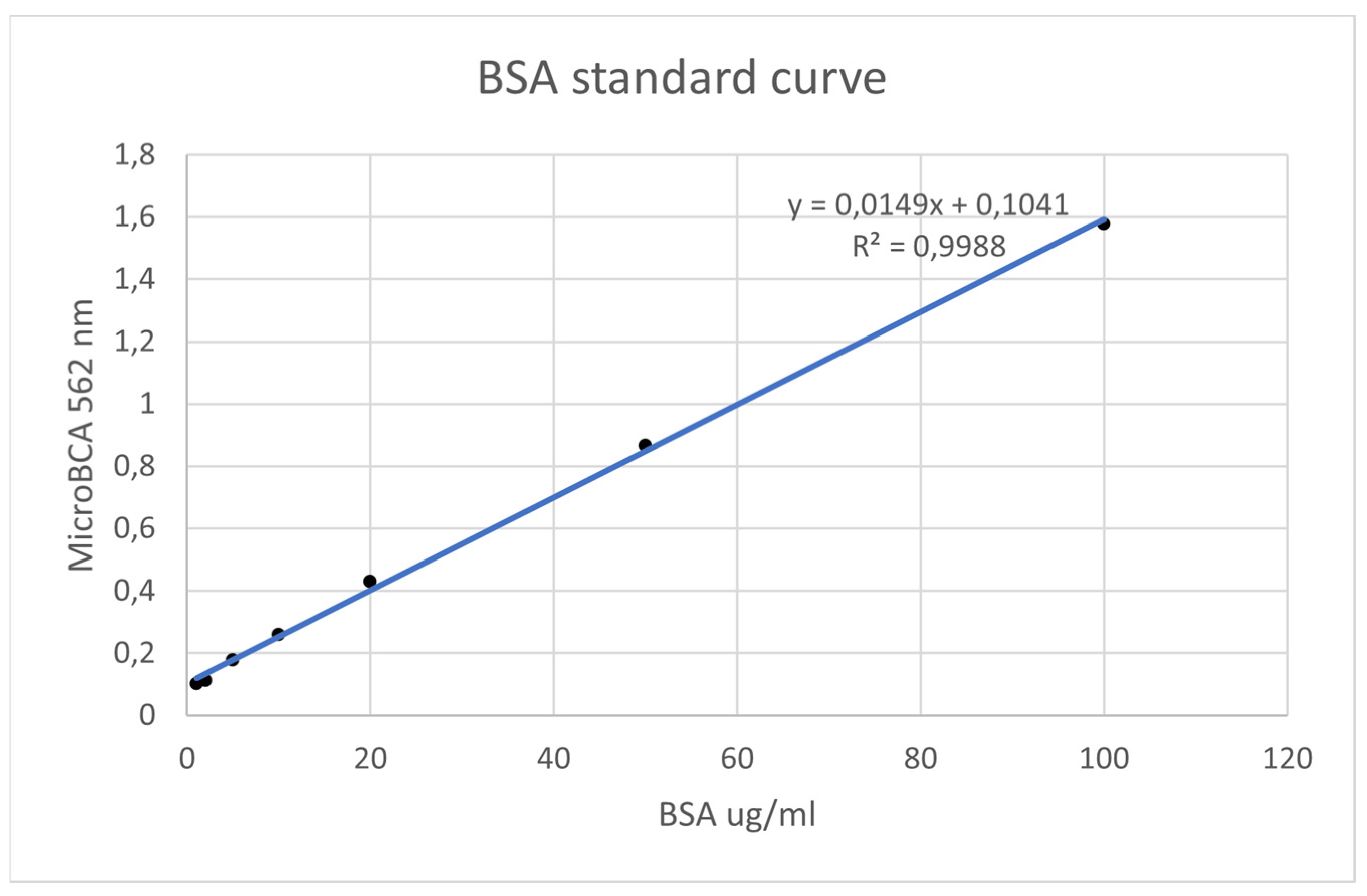
- Using known amounts of a reference protein (BSA, bovine serum albumin), a standard curve was created to trace the specific amount (ug/mL) of protein in each sample. Using the equation for the straight line, the amount of protein in ug/mL for each sample was obtained by substituting Y for the OD = 562 nm values obtained [37] (Figure 7).
2.5. Statistical Analysis
3. Results
3.1. Sample Sterility Assessment
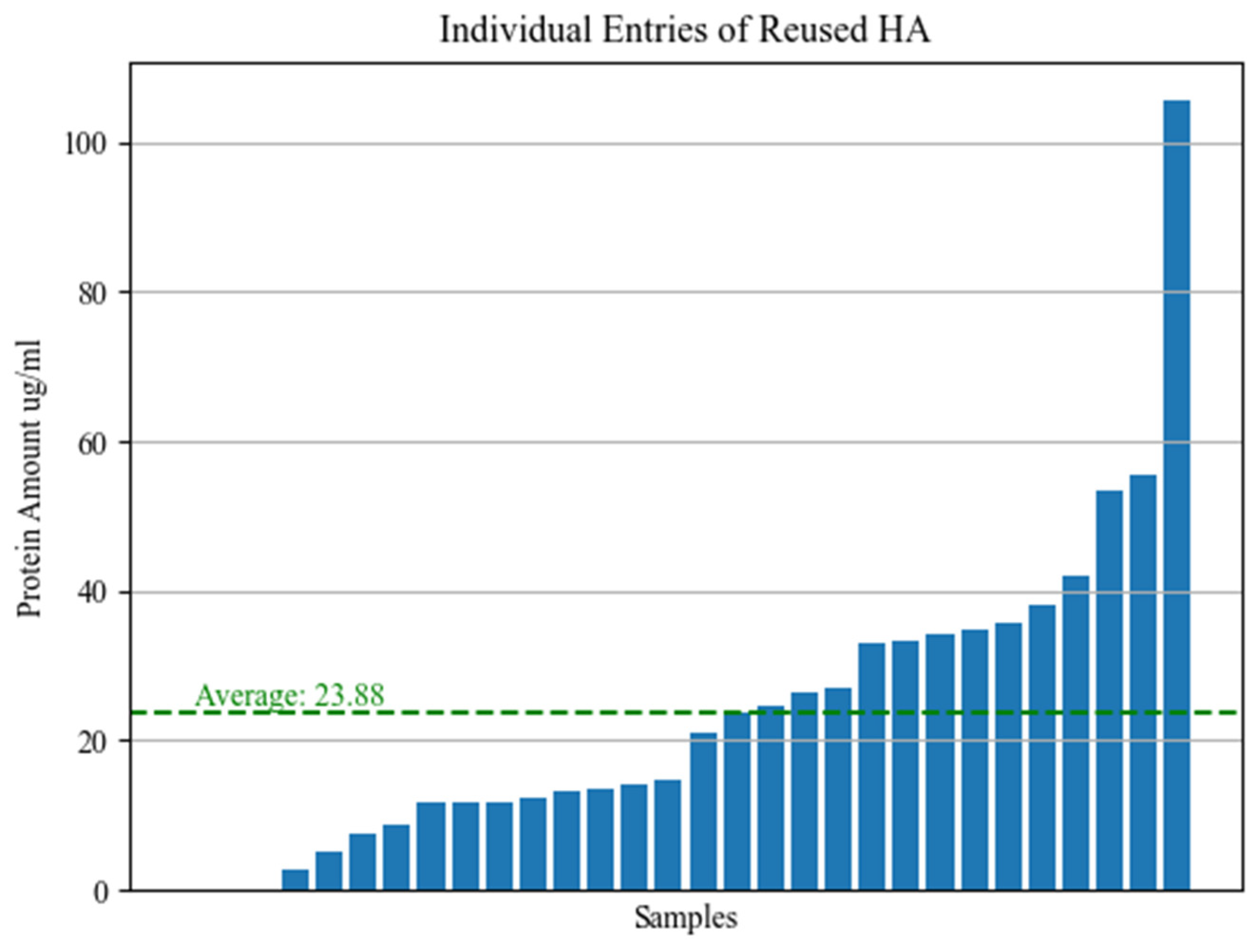
3.2. Surface-Attached Protein Levels
3.3. Statistical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oskarsson, M.; Otsuki, M.; Welander, M.; Abrahamsson, I. Peri-implant tissue healing at implants with different designs and placement protocols: An experimental study in dogs. Clin. Oral Implant. Res. 2018, 29, 873–880. [Google Scholar] [CrossRef]
- Fernandes, D.; Nunes, S.; López-Castro, G.; Marques, T.; Montero, J.; Borges, T. Effect of customized healing abutments on the peri-implant linear and volumetric tissue changes at maxillary immediate implant sites: A 1-year prospective randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 745–757. [Google Scholar] [CrossRef]
- Souza, A.B.; Alshihri, A.; Kämmerer, P.W.; Araújo, M.G.; Gallucci, G.O. Histological and micro-CT analysis of peri-implant soft and hard tissue healing on implants with different healing abutments configurations. Clin. Oral Implant. Res. 2018, 29, 1007–1015. [Google Scholar] [CrossRef]
- Kyaw, T.T.; Abdou, A.; Nakata, H.; Pimkhaokham, A. Dental implant healing abutment decontamination: A systematic review of in vitro studies. Int. J. Oral Implantol. 2022, 15, 311–324. [Google Scholar]
- Paganotto, G.; Zimmer, R.; Klein-Junior, C.A.; Rivaldo, E.G. Reuse of healing abutments: Ethical, biological and professional training implications. J. Clin. Exp. Dent. 2022, 14, e822–e826. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Boniol, M.; Kunjumen, T.; Nair, T.S.; Siyam, A.; Campbell, J.; Diallo, K. The global health workforce stock and distribution in 2020 and 2030: A threat to equity and ‘universal’ health coverage? BMJ Glob. Health 2022, 7, e009316. [Google Scholar] [CrossRef]
- Gimigliano, F.; Negrini, S. The World Health Organization “Rehabilitation 2030: A call for action”. Eur. J. Phys. Rehabil. Med. 2017, 53, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.S.; Schramm, S.T.J.; Siddiqui, D.A.; Huo, W.; Palmer, K.L.; Wilson, T.G., Jr.; Rodrigues, D.C. Effects of multiple implantations of titanium healing abutments: Surface characteristics and microbial colonization. Dent. Mater. 2020, 36, e279–e291. [Google Scholar] [CrossRef]
- Sahin, S.C.; Dere, K.A. Evaluation of residual contamination on reused healing abutments. Clin. Oral Investig. 2021, 25, 5889–5895. [Google Scholar] [CrossRef]
- Barreiros, P.; Braga, J.; Faria-Almeida, R.; Coelho, C.; Teughels, W.; Souza, J.C.M. Remnant oral biofilm and microorganisms after autoclaving sterilization of retrieved healing abutments. J. Periodontal Res. 2021, 56, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Cleaning and Sterilization Instructions for Astra Tech Implant System® EV Dentsply Sirona Implants. 2017. Available online: https://assets.dentsplysirona.com/dentsply/pim/manufacturer/Implants/Implant_systems/Astra_Tech_Implant_System_EV/Instruments/Accessories/32671332-USX-1711%20Cleaning%20and%20sterilization%20instructions%20for%20Astra%20Tech%20Implant%20System%20EV_LR.pdf (accessed on 1 April 2024).
- Grecchi, F.; DIGirolamo, M.; Cura, F.; Candotto, V.; Carinci, F. A new system of implant abutment connection: How to improve a two-piece implant system sealing. Oral Implantol. 2017, 10, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.; Roffel, S.; Meyer, M.; Gasser, A. Biology of soft tissue repair: Gingival epithelium in wound healing and attachment to the tooth and abutment surface. Eur. Cells Mater. 2019, 38, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Lashkarizadeh, N.; Foroudisefat, M.; Abyari, S.; Mohammadi, M.; Lashkarizadeh, L. Is It Safe to Reuse Healing Abutments? An Experimental Study on IL-1β and TNF-α Cytokine Levels in Peri-Implant Crevicular Fluid. J. Prosthodont. 2022, 31, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Browne, V.; Flewelling, M.; Wierenga, M.; Wilson, A.; Aprecio, R.; Richardson, P.; Angelov, N.; Johnson, N. Sterilization analysis of contaminated healing abutments and impression copings. J. Calif. Dent. Assoc. 2012, 40, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Menini, M.; Santori, G.; Rakic, M.; Sculean, A.; Pesce, P. Titanium abutment surface modifications and peri-implant tissue behavior: A systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 1113–1124. [Google Scholar] [CrossRef]
- Borisenko, A.; Antonenko, M.; Zelinsky, N.; Stolyar, V.; Popov, R. Early Postoperative Complications in Dental Implant Patients. Georgian Med. News 2020, 302, 23–28. [Google Scholar]
- Naseri, R.; Yaghini, J.; Feizi, A. Levels of smoking and dental implants failure: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Al Ansari, Y.; Shahwan, H.; Chrcanovic, B.R. Diabetes Mellitus and Dental Implants: A Systematic Review and Meta-Analysis. Materials 2022, 15, 3227. [Google Scholar] [CrossRef]
- Dawson, D.R., 3rd; Jasper, S. Key systemic and environmental risk factors for implant failure. Dent. Clin. 2015, 59, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Aaboe, M.; Araujo, M.; Carrión, J.B.; Cavalcanti, R.; Cionca, N.; Cochran, D.; Darby, I.; Funakoshi, E.; Gierthmuehlen, P.C.; et al. Group 4 ITI Consensus Report: Risks and biologic complications associated with implant dentistry. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 351–358. [Google Scholar] [CrossRef] [PubMed]
- Halpern, L.R.; Adams, D.R. Medically Complex Dental Implant Patients: Controversies About Systemic Disease and Dental Implant Success/Survival. Dent. Clin. 2021, 65, 1–19. [Google Scholar] [CrossRef]
- Rotim, Ž.; Pelivan, I.; Sabol, I.; Sušić, M.; Ćatić, A.; Bošnjak, A.P. The effect of local and systemic factors on dental implant failure—analysis of 670 patients with 1260 implants. Acta Clin. Croat. 2022, 60, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, T.T.; Abdou, A.; Arunjaroensuk, S.; Nakata, H.; Kanazawa, M.; Pimkhaokham, A. Effect of chemical and electrochemical decontamination protocols on single and multiple-used healing abutments: A comparative analysis of contact surface area, micro-gap, micro-leakage, and surface topography. Clin. Implant. Dent. Relat. Res. 2023, 25, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.A.; Paula, O.F.; Silva, C.R.; Leão, M.V.; Santos, S.S. Stability of antimicrobial activity of peracetic acid solutions used in the final disinfection process. Braz. Oral Res. 2015, 29, S1806-83242015000100239. [Google Scholar] [CrossRef] [PubMed]
- Nemchenko, U.M.; Kungurtseva, E.A.; Grigorova, E.V.; Belkova, N.L.; Markova, Y.A.; Noskova, O.A.; Chemezova, N.N.; Savilov, E.D. Simulation of bacterial biofilms and estimation of the sensitivity of healthcare-associated infection pathogens to bactericide Sekusept active. Klin. Lab. Diagn. 2020, 65, 652–658. (In English) [Google Scholar] [CrossRef] [PubMed]
- Deng, X.H.; Sun, Z.; Su, J. [Current status of disinfection and sterilization for dental handpieces in the hospitals]. Zhonghua Yu Fang Yi Xue Za Zhi. 2004, 38, 365–368. [Google Scholar]
- Eswaramurthy, P.; Aras, M.; DSouza, K.M.; Nagarsekar, A.; Gaunkar, R.B. Contemporary Sterilization Protocols of Healing Abutments for Reusability: A Systematic Review. JDR Clin. Transl. Res. 2022, 7, 352–359. [Google Scholar] [CrossRef]
- Johani, K.; Abualsaud, D.; Costa, D.M.; Hu, H.; Whiteley, G.; Deva, A.; Vickery, K. Characterization of microbial community composition, antimicrobial resistance and biofilm on intensive care surfaces. J. Infect. Public Health 2018, 11, 418–424. [Google Scholar] [CrossRef]
- Kuzmin, A.N.; Pliss, A.; Prasad, P.N. Ramanomics: New Omics Disciplines Using Micro Raman Spectrometry with Biomolecular Component Analysis for Molecular Profiling of Biological Structures. Biosensors 2017, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Abreu, O.J.; Estepa, A.V.; Naqvi, A.R.; Nares, S.; Narvekar, A. Assessment of Detoxification Strategies for Used Dental Implant Healing Abutments: Macroscopic and Biological Implications. Int. J. Oral Maxillofac. Implant. 2023, 1–42. [Google Scholar] [CrossRef]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994, 32, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ríos, J.; Zárate, A.M.; Figueroa, J.D.; Medina, J.; Fuentes-Lemus, E.; Rodríguez-Fernández, M.; Aliaga, M.; López-Alarcón, C. Protein quantification by bicinchoninic acid (BCA) assay follows complex kinetics and can be performed at short incubation times. Anal. Biochem. 2020, 608, 113904. [Google Scholar] [CrossRef]
- Demant, E.J. Bovine serum albumin-(7-hydroxycoumarin-4-acetic acid) complex: Applications to the fluorometric measurement of fatty acid concentrations. Anal. Biochem. 1999, 267, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.I.; Imazato, S. Autoclave sterilization of dental handpieces: A literature review. J. Prosthodont. Res. 2020, 64, 239–242. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, C.; Barbosa, R.E.; Issa, J.P.; Watanabe, E.; Ito, I.Y.; de Albuquerque Junior, R.F. Use of checkerboard DNA-DNA hybridization to evaluate the internal contamination of dental implants and comparison of bacterial leakage with cast or pre-machined abutments. Clin. Oral Implant. Res. 2009, 20, 571–577. [Google Scholar] [CrossRef]
- Barbosa, R.E.; do Nascimento, C.; Issa, J.P.; Watanabe, E.; Ito, I.Y.; de Albuquerque, R.F., Jr. Bacterial culture and DNA Checkerboard for the detection of internal contamination in dental implants. J. Prosthodont. 2009, 18, 376–381. [Google Scholar] [CrossRef]
- Narvekar, A.; Valverde Estepa, A.; Naqvi, A.; Nares, S. Used dental implant healing abutments elicit immune responses: A comparative analysis of detoxification strategies. Clin. Implant. Dent. Relat. Res. 2020, 22, 730–738. [Google Scholar] [CrossRef]
- Park, K.H.; Song, H.J.; Park, Y.J. Albumin adsorption on microwave-treated titanium dioxide for dental implant materials. Colloids Surf. B Biointerfaces 2021, 208, 112124. [Google Scholar] [CrossRef] [PubMed]
- Chew, M.; Tompkins, G.; Tawse-Smith, A.; Waddell, J.N.; Ma, S. Reusing titanium healing abutments: Comparison of two decontamination methods. Int. J. Prosthodont. 2018, 31, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Bidra, A.S.; Kejriwal, S.; Bhuse, K. Should healing abutments and cover screws for dental implants be reused? A systematic review. J. Prosthodont. 2020, 29, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, C.; Schonnenbaum, T.R.; Audia, F.; Chung, K.H. In-Vitro Study of the Contamination Remaining on Used Healing Abutments after Cleaning and Sterilizing in Dental Practice. Clin. Implant. Dent. Relat. Res. 2016, 18, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Berton, F.; Porrelli, D.; Lombardi, T. Reuse of implant healing abutments: Comparative evaluation of the efficacy of two cleaning procedures. Int. J. Prosthodont. 2018, 31, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Garcés, M.A.; Jorba, M.; Ciurana, J.; Vinas, M.; Vinuesa, M.T. Is the re-use of sterilized implant abutments safe enough? (implant abutment safety). Med. Oral Patol. Oral Cir. Bucal 2019, 24, e583–e587. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, L.; Lekholm, U.; Ericson, L.E. Soft-tissue response to clinically retrieved titanium cover screws reimplanted in the rat abdominal wall. Int. J. Oral Maxillofac. Implant. 1989, 4, 233–239. [Google Scholar]
- Cakan, U.; Delilbasi, C.; Er, S.; Kivanc, M. Is it safe to reuse dental implant healing abutments sterilized and serviced by dealers of dental implant manufacturers? An in vitro sterility analysis. Implant Dent. 2015, 24, 174–179. [Google Scholar] [CrossRef]



| Morphology | Cylindrical screw geometry with cylindrical head for screwed prosthesis |  |
| Diameter | 3.8 mm | |
| Height | 3 mm | |
| Material | Titanium alloy Ti6Al4V grade 5 ELI ref. standard ASTM F136-13 | |
| Sterilization | Non-sterile, autoclavable | |
| CE classification | Class IIb, rule 8 |
| Composition | ||
| Active ingredients | Sodium percarbonate | 20% |
| Tetraacetylethylenediamine | 15% | |
| Excipients | Washing agents, non-ionic surfactants, stabilizers and co-formulants | |
| Chemical-physical characteristics | ||
| Appearance | White to yellowish granulate | |
| Specific weight | 1 g/mL | |
| pH | 10 | |
| Flash point | >100 °C | |
| Storage temperature | 0 °C < T < 25 °C | |
| Properties | Concentration | Time of contact |
| Mycobactericidal activity | 2% | 15 min |
| Sporicidal activity | 2% | 30 min |
| Virucidal activity | 2% | 15 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burioni, R.; Silvestrini, L.; D’Orto, B.; Tetè, G.; Nagni, M.; Polizzi, E.; Gherlone, E.F. Could Dental Material Reuse Play a Significant Role in Preservation of Raw Materials, Water, Energy, and Costs? Microbiological Analysis of New versus Reused Healing Abutments: An In Vitro Study. Bioengineering 2024, 11, 387. https://doi.org/10.3390/bioengineering11040387
Burioni R, Silvestrini L, D’Orto B, Tetè G, Nagni M, Polizzi E, Gherlone EF. Could Dental Material Reuse Play a Significant Role in Preservation of Raw Materials, Water, Energy, and Costs? Microbiological Analysis of New versus Reused Healing Abutments: An In Vitro Study. Bioengineering. 2024; 11(4):387. https://doi.org/10.3390/bioengineering11040387
Chicago/Turabian StyleBurioni, Roberto, Lucia Silvestrini, Bianca D’Orto, Giulia Tetè, Matteo Nagni, Elisabetta Polizzi, and Enrico Felice Gherlone. 2024. "Could Dental Material Reuse Play a Significant Role in Preservation of Raw Materials, Water, Energy, and Costs? Microbiological Analysis of New versus Reused Healing Abutments: An In Vitro Study" Bioengineering 11, no. 4: 387. https://doi.org/10.3390/bioengineering11040387
APA StyleBurioni, R., Silvestrini, L., D’Orto, B., Tetè, G., Nagni, M., Polizzi, E., & Gherlone, E. F. (2024). Could Dental Material Reuse Play a Significant Role in Preservation of Raw Materials, Water, Energy, and Costs? Microbiological Analysis of New versus Reused Healing Abutments: An In Vitro Study. Bioengineering, 11(4), 387. https://doi.org/10.3390/bioengineering11040387






