Application of the Single Source—Detector Separation Algorithm in Wearable Neuroimaging Devices: A Step toward Miniaturized Biosensor for Hypoxia Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
2.3. Measurement Device
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
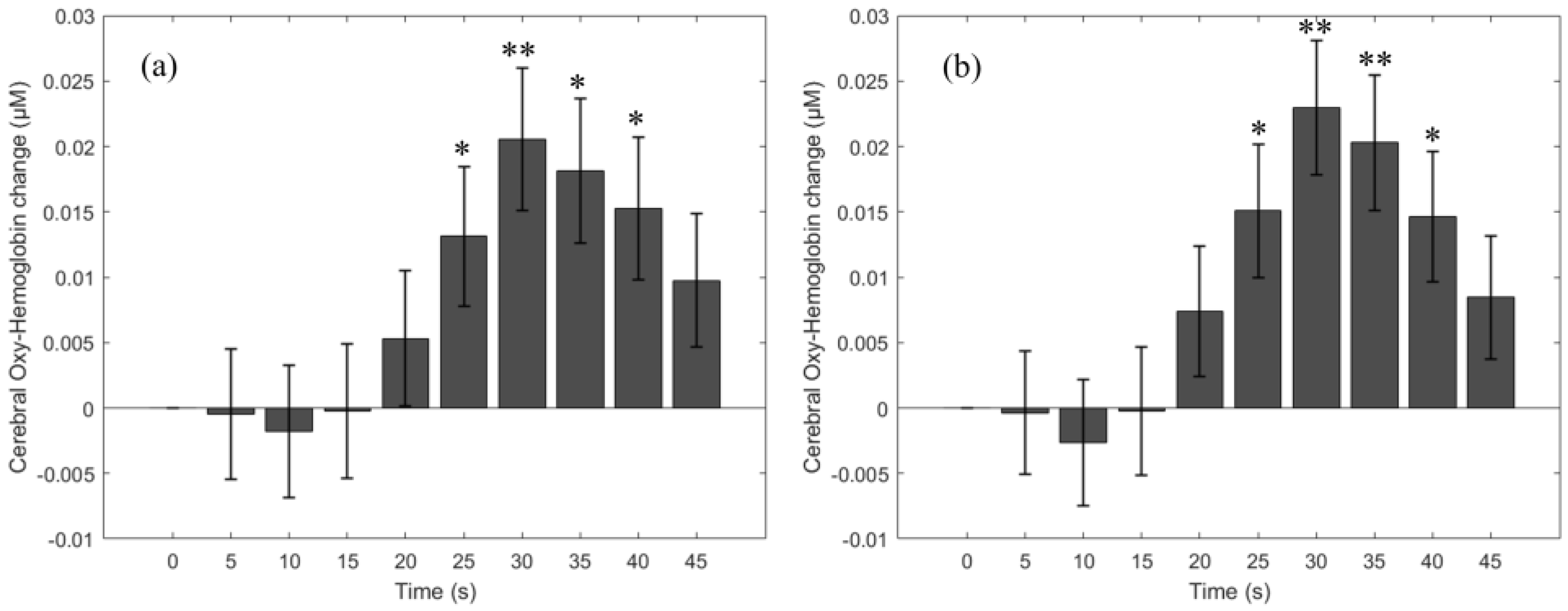
3.1. HbO Change Due to the Breath-Holding Task
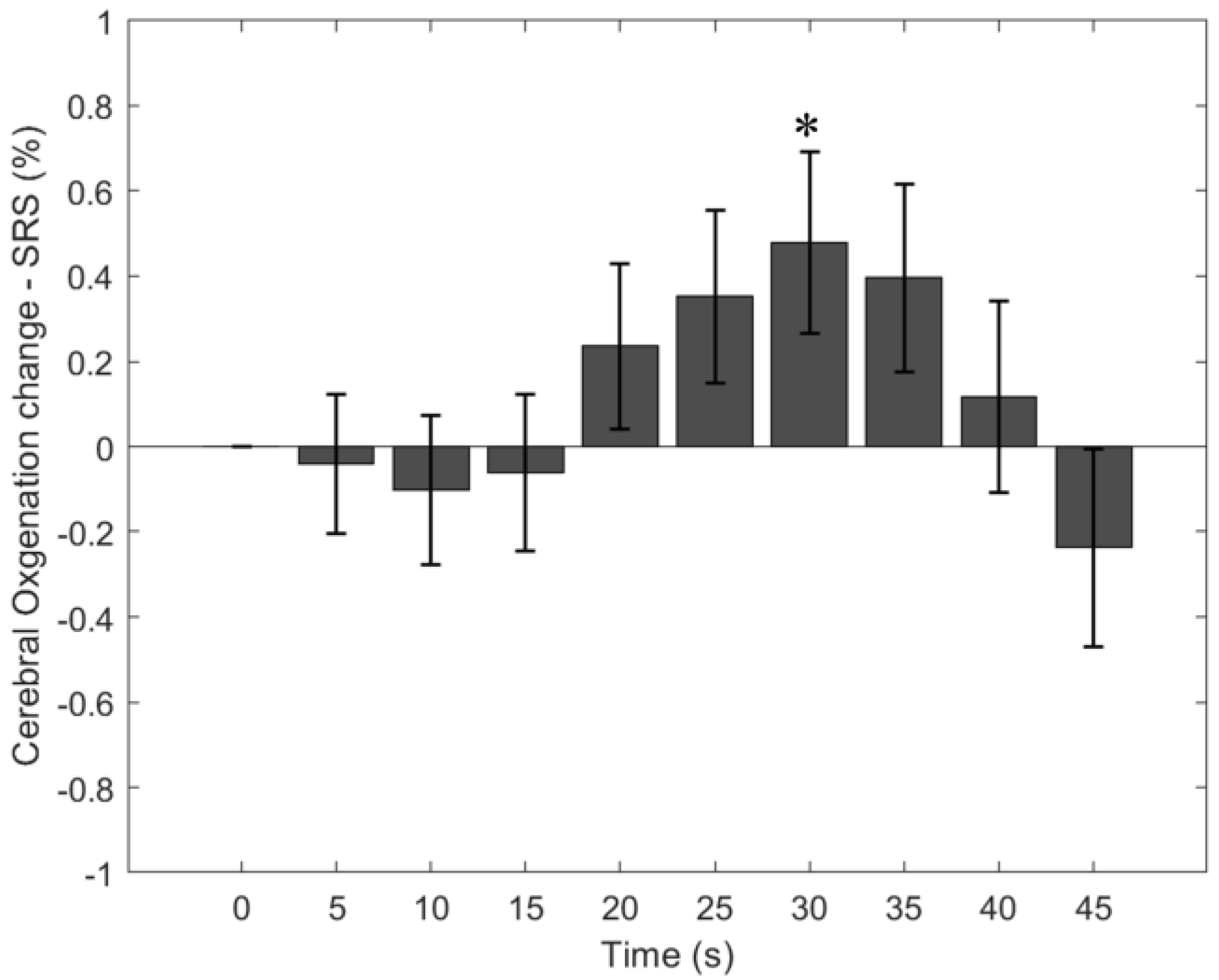
3.2. Cerebral StO2-SRS Change Due to the Breath-Holding Task
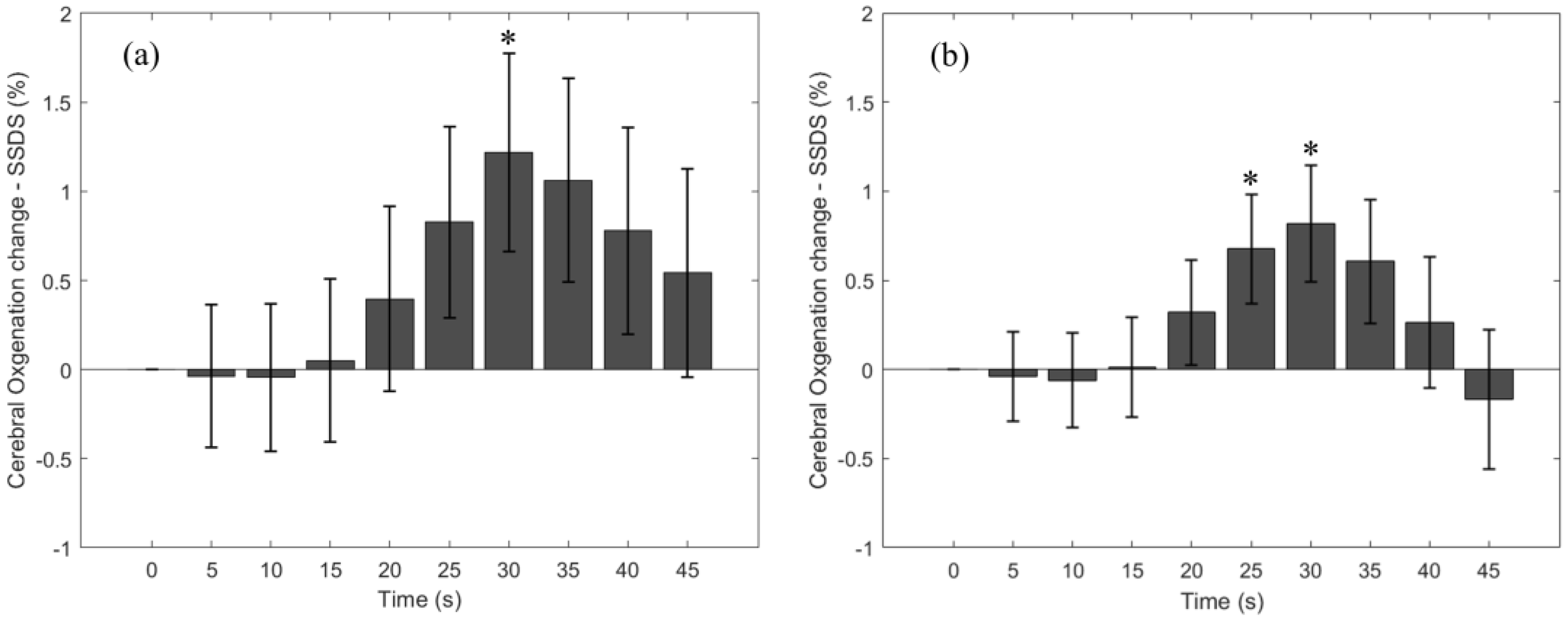
3.3. Cerebral StO2-SSDS Change Due to the Breath-Holding Task
3.4. Cerebral StO2 during a Long versus Short Breath-Holding Task
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhutta, B.S.; Alghoula, F.; Berim, I. Hypoxia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lacerte, M.; Hays Shapshak, A.; Mesfin, F.B. Hypoxic Brain Injury. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Barud, M.; Dabrowski, W.; Siwicka-Gieroba, D.; Robba, C.; Bielacz, M.; Badenes, R. Usefulness of Cerebral Oximetry in TBI by NIRS. J. Clin. Med. 2021, 10, 2938. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Ji, Z.; Sun, C. A Review of Monitoring Methods for Cerebral Blood Oxygen Saturation. Healthcare 2021, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.M.K.; Hsiang, J.N.K.; Poon, W.S. Monitoring of autoregulation using laser Doppler flowmetry in patients with head injury. J. Neurosurg. 1997, 86, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Lara, L.; Zorrilla-Vaca, A.; Geocadin, R.G.; Healy, R.J.; Ziai, W.; Mirski, M.A. Cerebral Autoregulation-oriented Therapy at the Bedside: A Comprehensive Review. Anesthesiology 2017, 126, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.M.; Lee, J.K.; Kibler, K.K.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Koehler, R.C.; Shaffner, D.H. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 2007, 38, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.D.; Kothmann, E.; Giussani, D.A. Detection and response to acute systemic hypoxia. BJA Educ. 2020, 20, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A.; Matthay, M. Understanding blood gas analysis. Intensive Care Med. 2018, 44, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.; Patil, S.M.; Zubair, M.; Keenaghan, M. Arterial Blood Gas. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lima-Oliveira, G.; Lippi, G.; Salvagno, G.L.; Montagnana, M.; Picheth, G.; Guidi, G.C. Different manufacturers of syringes: A new source of variability in blood gas, acid-base balance and related laboratory test? Clin. Biochem. 2012, 45, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Albert, T.J.; Swenson, E.R. Circumstances When Arterial Blood Gas Analysis Can Lead Us Astray. Respir Care 2016, 61, 119–121. [Google Scholar] [CrossRef]
- Haider, M.Z.; Anwer, F. Secondary Polycythemia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Billett, H.H. Hemoglobin and Hematocrit. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworth Publishers, a division of Reed Publishing: Boston, MA, USA, 1990. [Google Scholar]
- Mithoowani, S.; Laureano, M.; Crowther, M.A.; Hillis, C.M. Investigation and management of erythrocytosis. Cmaj 2020, 192, E913–E918. [Google Scholar] [CrossRef]
- Sakisaka, S.; Watanabe, M.; Tateishi, H.; Harada, M.; Shakado, S.; Mimura, Y.; Gondo, K.; Yoshitake, M.; Noguchi, K.; Hino, T.; et al. Erythropoietin production in hepatocellular carcinoma cells associated with polycythemia: Immunohistochemical evidence. Hepatology 1993, 18, 1357–1362. [Google Scholar] [CrossRef]
- Bouzat, P.; Sala, N.; Payen, J.F.; Oddo, M. Beyond intracranial pressure: Optimization of cerebral blood flow, oxygen, and substrate delivery after traumatic brain injury. Ann. Intensive Care 2013, 3, 23. [Google Scholar] [CrossRef]
- Mirza, H.; Hashmi, M.F. Lung Ventilation Perfusion Scan (VQ Scan). In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sarkar, M.; Niranjan, N.; Banyal, P.K. Mechanisms of hypoxemia. Lung India 2017, 34, 47–60. [Google Scholar] [CrossRef]
- Soye, J.A.; Loughrey, C.B.; Hanley, P.D. Computed tomography pulmonary angiography: A sample of experience at a District General Hospital. Ulster Med. J. 2008, 77, 175–180. [Google Scholar]
- Bickler, P.E.; Feiner, J.R.; Severinghaus, J.W. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. J. Am. Soc. Anesthesiol. 2005, 102, 715–719. [Google Scholar] [CrossRef]
- Feiner, J.R.; Severinghaus, J.W.; Bickler, P.E. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: The effects of oximeter probe type and gender. Anesth. Analg. 2007, 105, S18–S23. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.-K.I.; Charpignon, M.; Kim, H.; Josef, C.; De Hond, A.A.; Fojas, J.J.; Tabaie, A.; Liu, X.; Mireles-Cabodevila, E.; Carvalho, L. Analysis of discrepancies between pulse oximetry and arterial oxygen saturation measurements by race and ethnicity and association with organ dysfunction and mortality. JAMA Netw. Open 2021, 4, e2131674. [Google Scholar] [CrossRef] [PubMed]
- Crooks, C.J.; West, J.; Morling, J.R.; Simmonds, M.; Juurlink, I.; Briggs, S.; Cruickshank, S.; Hammond-Pears, S.; Shaw, D.; Card, T.R. Pulse oximeter measurement error of oxygen saturation in patients with SARS-CoV-2 infection stratified by smoking status. Eur. Respir. J. 2022, 60, 2201190. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat-Yazdi, F.; Torres, A., Jr.; Fortuna, R.; Geiss, D.M. Pulse oximeter accuracy and precision affected by sensor location in cyanotic children. Pediatr. Crit. Care Med. 2008, 9, 393–397. [Google Scholar] [CrossRef]
- Foglia, E.E.; Whyte, R.K.; Chaudhary, A.; Mott, A.; Chen, J.; Propert, K.J.; Schmidt, B. The effect of skin pigmentation on the accuracy of pulse oximetry in infants with hypoxemia. J. Pediatr. 2017, 182, 375–377.e372. [Google Scholar] [CrossRef]
- Harris, B.U.; Char, D.S.; Feinstein, J.A.; Verma, A.; Shiboski, S.C.; Ramamoorthy, C. Accuracy of pulse oximeters intended for hypoxemic pediatric patients. Pediatr. Crit. Care Med. 2016, 17, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Palmer, G.M.; Dewhirst, M.W. Chapter 46—Imaging Hypoxia. In Molecular Imaging, 2nd; Ross, B.D., Gambhir, S.S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 869–895. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Helias, M.; Raia, L.; Nicolas, E.; Jaubert, P.; Benghanem, S.; Ait Hamou, Z.; Dupland, P.; Charpentier, J.; Pène, F. Impact of COVID-19 on the association between pulse oximetry and arterial oxygenation in patients with acute respiratory distress syndrome. Sci. Rep. 2022, 12, 1462. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.J.; Shen, Y.; Lu, L.; Zhang, R.Y.; Zhang, Q.; Shen, W.F. Increased blood glycohemoglobin A1c levels lead to overestimation of arterial oxygen saturation by pulse oximetry in patients with type 2 diabetes. Cardiovasc. Diabetol. 2012, 11, 110. [Google Scholar] [CrossRef]
- Wilson, B.J.; Cowan, H.J.; Lord, J.A.; Zuege, D.J.; Zygun, D.A. The accuracy of pulse oximetry in emergency department patients with severe sepsis and septic shock: A retrospective cohort study. BMC Emerg. Med. 2010, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Newth, C.J.; Khemani, R.G. Accuracy of pulse oximetry in children. Pediatrics 2014, 133, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.; Bhattacharyya, A.; Ramos, J.A.; Tonelli, A.R. Pulse oximetry and arterial oxygen saturation during cardiopulmonary exercise testing. Med. Sci. Sports Exerc. 2018, 50, 1992. [Google Scholar] [CrossRef] [PubMed]
- Petterson, M.T.; Begnoche, V.L.; Graybeal, J.M. The effect of motion on pulse oximetry and its clinical significance. Anesth. Analg. 2007, 105, S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.I.; Chung, P.C.; Lee, C.W.; Yu, H.P. Cerebral perfusion monitoring in acute care surgery: Current and perspective use. Expert. Rev. Med. Devices 2016, 13, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, K.; Zhang, L.; Zong, H.; Meng, L.; Han, R. Cerebral near-infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. Cochrane Database Syst. Rev. 2018, 1, CD010947. [Google Scholar] [CrossRef]
- Nguyen, T.; Park, S.; Hill, B.; Gandjbakhche, A.H. Single Source-Detector Separation Approach to Calculate Tissue Oxygen Saturation Using Continuous Wave Near-infrared Spectroscopy. IEEE Open J. Eng. Med. Biol. 2023, 4, 79–84. [Google Scholar] [CrossRef]
- Zijlstra, W.G.; Buursma, A.; der Roest, W.P.M.-V. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Bouten, J.; Bourgois, J.G.; Boone, J. Hold your breath: Peripheral and cerebral oxygenation during dry static apnea. Eur. J. Appl. Physiol. 2020, 120, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Parkes, M.J. Breath-holding and its breakpoint. Exp. Physiol. 2006, 91, 1–15. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.; Park, S.; Park, J.; Sodager, A.; George, T.; Gandjbakhche, A. Application of the Single Source—Detector Separation Algorithm in Wearable Neuroimaging Devices: A Step toward Miniaturized Biosensor for Hypoxia Detection. Bioengineering 2024, 11, 385. https://doi.org/10.3390/bioengineering11040385
Nguyen T, Park S, Park J, Sodager A, George T, Gandjbakhche A. Application of the Single Source—Detector Separation Algorithm in Wearable Neuroimaging Devices: A Step toward Miniaturized Biosensor for Hypoxia Detection. Bioengineering. 2024; 11(4):385. https://doi.org/10.3390/bioengineering11040385
Chicago/Turabian StyleNguyen, Thien, Soongho Park, Jinho Park, Asma Sodager, Tony George, and Amir Gandjbakhche. 2024. "Application of the Single Source—Detector Separation Algorithm in Wearable Neuroimaging Devices: A Step toward Miniaturized Biosensor for Hypoxia Detection" Bioengineering 11, no. 4: 385. https://doi.org/10.3390/bioengineering11040385
APA StyleNguyen, T., Park, S., Park, J., Sodager, A., George, T., & Gandjbakhche, A. (2024). Application of the Single Source—Detector Separation Algorithm in Wearable Neuroimaging Devices: A Step toward Miniaturized Biosensor for Hypoxia Detection. Bioengineering, 11(4), 385. https://doi.org/10.3390/bioengineering11040385







