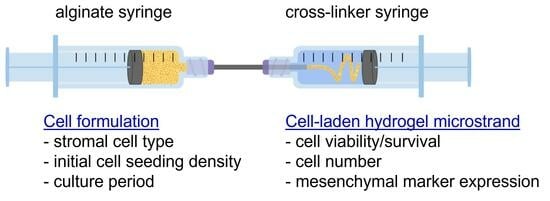
Evaluation of Alginate Hydrogel Microstrands for Stromal Cell Encapsulation and Maintenance
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrogel Solutions and Viscosity Measurement
2.2. Construction of Syringe-in-Syringe (SiS) Device for Fabrication of Alginate Hydrogel Microstrands
2.3. Connective Porosity and Mass Swelling Ratio of Alginate Hydrogel Microstrands
2.4. Cell Culture
2.4.1. Culture of Murine NIH 3T3 Fibroblasts
2.4.2. Isolation and Culture of Murine Primary E16 Salivary Mesenchyme Cells
2.5. Encapsulation of Cells in Alginate Hydrogel Microstrands Using the Syringe-in-Syringe (SiS) Device
2.6. Optical Imaging of Alginate Hydrogel Microstrands
2.7. Trypan Blue Exclusion Assay
2.8. LIVE/DEAD Cell Assay
2.9. AlamarBlue Assay of Viable Cell Growth in Alginate Hydrogel Microstrands
2.10. Immunocytochemistry Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect of the Syringe Volume/Capacity for Construction of Syringe-in-Syringe (SiS) Devices on the Diameter of Hydrogel Microstrands
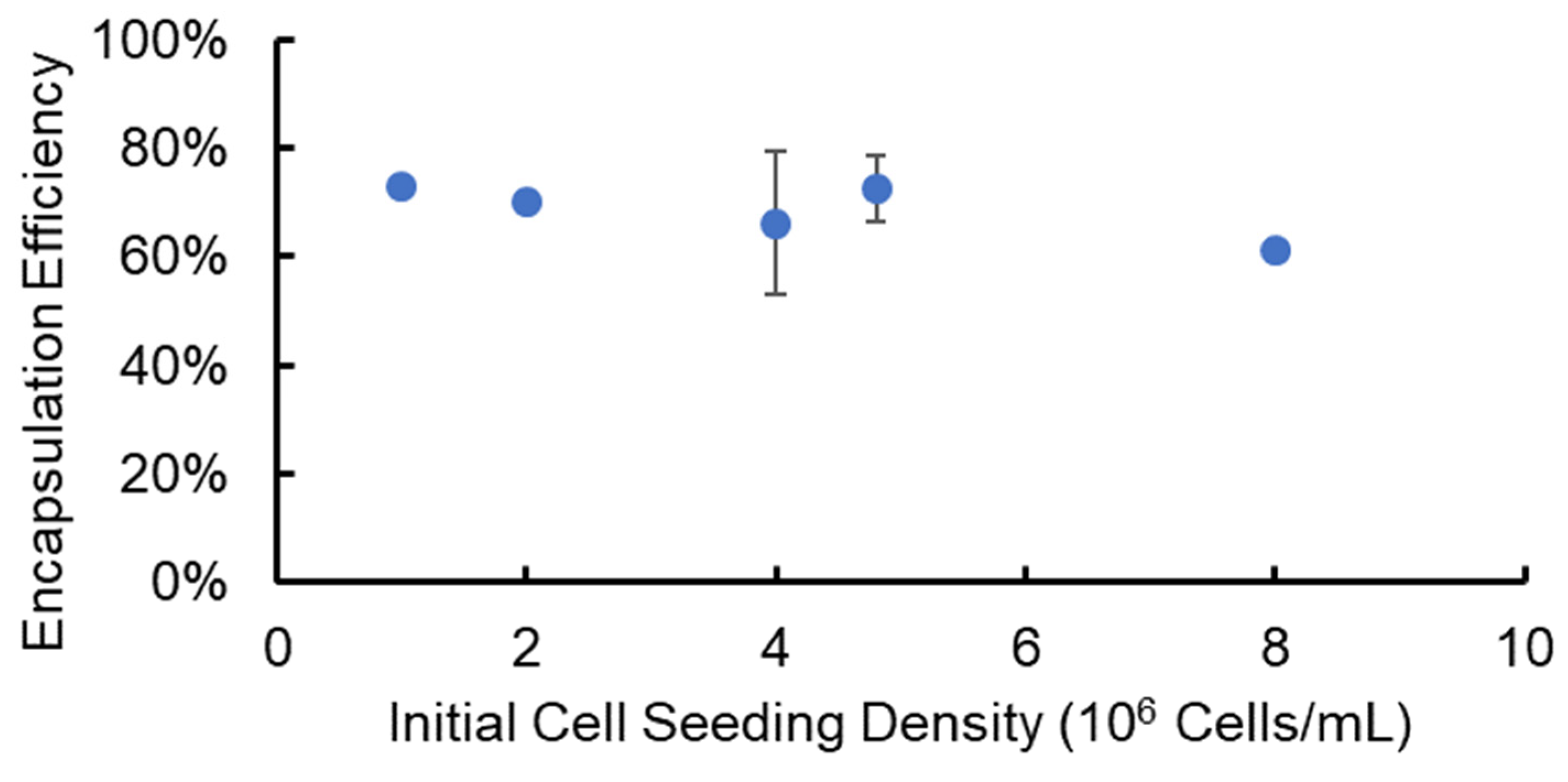
3.2. Cell Encapsulation and Recovery Efficiency from Alginate Hydrogel Microstrands
3.3. Evaluation of Cell Viability in Alginate Hydrogel Microstrands
3.4. Assessment of Cell Growth in Alginate Hydrogel Microstrands
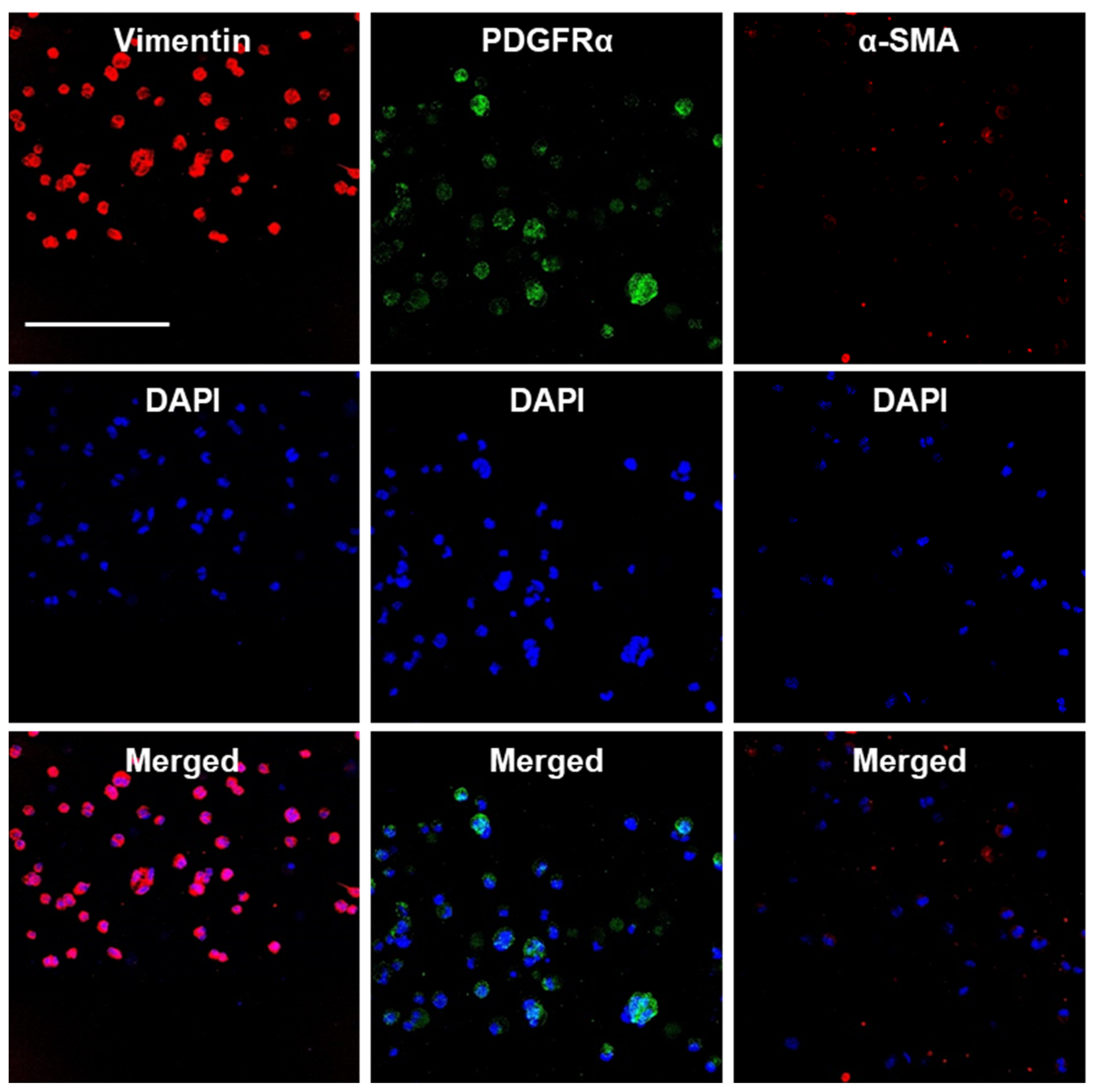
3.5. Culture of MSC-like Cells in Alginate Hydrogel Microstrands and Their Phenotype Maintenance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M.; Kanno, H.; Hoshino, M.; Cho, H.; Matsumoto, N.; Itokazu, Y.; Tajima, N.; Yamada, H.; Sawada, H.; Ishikawa, H.; et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J. Clin. Investig. 2004, 113, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Fukuda, K.; Miyoshi, S.; Konishi, F.; Kodama, H.; Pan, J.; Sano, M.; Takahashi, T.; Hori, S.; Abe, H.; et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Investig. 1999, 103, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-g5 secretion by human mesenchymal stem cells is required to suppress t lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M. Mesenchymal stromal cell ‘licensing’: A multistep process. Leukemia 2011, 25, 1408–1414. [Google Scholar] [CrossRef]
- Rasmusson, I.; Ringdén, O.; Sundberg, B.; Le Blanc, K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes. but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 2003, 76, 1208–1213. [Google Scholar] [CrossRef]
- English, K.; Barry, F.P.; Field-Corbett, C.P.; Mahon, B.P. IFN-γ and TNF-α differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 2007, 110, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; Mazzarello, S.; Zlepnig, J.; Dong, Y.Y.; Montroy, J.; McIntyre, L.; Devereaux, P.J.; Stewart, D.J.; Mazer, C.D.; Barron, C.C.; et al. Safety and efficacy of adult stem cell therapy for acute myocardial infarction and ischemic heart failure (SafeCell Heart): A systematic review and meta-analysis. Stem Cells Transl. Med. 2018, 7, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Rosenthal, N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ito, Y.; Kawano, Y.; Kurozumi, K.; Kobune, M.; Tsuda, H.; Bizen, A.; Honmou, O.; Niitsu, Y.; Hamada, H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004, 11, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S.; Underhill, L.H.; Dvorak, H.F. Tumors: Wounds that do not heal. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.T.; Kubis, N.; Tomita, Y.; Karaszewski, B.; Calando, Y.; Oudina, K.; Petite, H.; Seylaz, J.; Pinard, E. In vivo imaging with cellular resolution of bone marrow cells transplanted into the ischemic brain of a mouse. Neuroimage 2006, 31, 958–967. [Google Scholar] [CrossRef]
- Castelo-Branco, M.T.L.; Soares, I.D.P.; Lopes, D.V.; Buongusto, F.; Martinusso, C.A.; do Rosario, A.; Souza, S.A.L.; Gutfilen, B.; Fonseca, L.M.B.; Elia, C.; et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS ONE 2012, 7, e33360. [Google Scholar] [CrossRef]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Studeny, M.; Marini, F.C.; Champlin, R.E.; Zompetta, C.; Fidler, I.J.; Andreeff, M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002, 62, 3603–3608. [Google Scholar] [PubMed]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal stem cell-based therapy of inflammatory lung diseases: Current understanding and future perspectives. Stem Cells Int. 2019, 2019, 4236973. [Google Scholar] [CrossRef] [PubMed]
- François, S.; Usunier, B.; Douay, L.; Benderitter, M.; Chapel, A. Long-Term quantitative biodistribution and side effects of human mesenchymal stem cells (HMSCs) engraftment in NOD/SCID mice following irradiation. Stem Cells Int. 2014, 2014, 939275. [Google Scholar] [CrossRef] [PubMed]
- De Becker, A.; Van Riet, I. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells 2016, 8, 73. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Rao, V.V.; Borelli, A.N.; Anseth, K.S. Engineering the MSC Secretome: A hydrogel focused approach. Adv. Healthc. Mater. 2021, 10, 2001948. [Google Scholar] [CrossRef] [PubMed]
- Scarfe, L.; Taylor, A.; Sharkey, J.; Harwood, R.; Barrow, M.; Comenge, J.; Beeken, L.; Astley, C.; Santeramo, I.; Hutchinson, C.; et al. Non-invasive imaging reveals conditions that impact distribution and persistence of cells after in vivo administration. Stem Cell Res. Ther. 2018, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Levit, R.D.; Landázuri, N.; Phelps, E.A.; Brown, M.E.; García, A.J.; Davis, M.E.; Joseph, G.; Long, R.; Safley, S.A.; Suever, J.D.; et al. Cellular encapsulation enhances cardiac repair. J. Am. Heart Assoc. 2013, 2, e000367. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Jabbari, N.; Sharifi, R.; Ahmadi, M.; Vahhabi, A.; Seyedzadeh, S.J.; Nawaz, M.; Szafert, S.; Mahmoodi, M.; Jabbari, E.; et al. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 2020, 249, 117447. [Google Scholar] [CrossRef]
- Denoeud, C.; Luo, G.; Paquet, J.; Boisselier, J.; Wosinski, P.; Moya, A.; Diallo, A.; Larochette, N.; Marinesco, S.; Meiller, A.; et al. Enzyme-controlled, nutritive hydrogel for mesenchymal stromal cell survival and paracrine functions. Commun. Biol. 2023, 6, 1266. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Yang, L. Hydrogel encapsulation: Taking the therapy of mesenchymal stem cells and their derived secretome to the next level. Front. Bioeng. Biotechnol. 2022, 10, 859927. [Google Scholar] [CrossRef] [PubMed]
- Joddar, B.; Tasnim, N.; Thakur, V.; Kumar, A.; McCallum, R.; Chattopadhyay, M. Delivery of mesenchymal stem cells from gelatin–alginate hydrogels to stomach lumen for treatment of gastroparesis. Bioengineering 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, M.; Tran, O.N.; Wang, H.; Abdul-Azees, P.; Dean, D.D.; Chen, X.-D.; Yeh, C.-K. Autologous mesenchymal stem cells offer a new paradigm for salivary gland regeneration. Int. J. Oral Sci. 2023, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.K.; Vissers, C.B.; Leach, J.K. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl. Med. 2016, 5, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; del Barrio, J.; Loh, X.J.; Scherman, O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef]
- Donati, I.; Holtan, S.; Mørch, Y.A.; Borgogna, M.; Dentini, M.; Skjåk-Bræk, G. New hypothesis on the role of alternating sequences in calcium−alginate gels. Biomacromolecules 2005, 6, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef]
- Xie, Y.; Kollampally, S.C.R.; Jorgensen, M.; Zhang, X. Alginate microfibers as therapeutic delivery scaffolds and tissue mimics. Exp. Biol. Med. 2022, 247, 2103–2118. [Google Scholar] [CrossRef]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell culture in alginate hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behavior. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Re’em, T.; Tsur-Gang, O.; Cohen, S. The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGFβ1-induced chondrogenesis of human mesenchymal stem cells. Biomaterials 2010, 31, 6746–6755. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bölükbas, D.A.; Prithiviraj, S.; Da Silva, I.A.N.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv. Mater. 2021, 33, 2005476. [Google Scholar] [CrossRef]
- Berg, J.; Hiller, T.; Kissner, M.S.; Qazi, T.H.; Duda, G.N.; Hocke, A.C.; Hippenstiel, S.; Elomaa, L.; Weinhart, M.; Fahrenson, C.; et al. Optimization of cell-laden bioinks for 3D bioprinting and efficient infection with influenza A virus. Sci. Rep. 2018, 8, 13877. [Google Scholar] [CrossRef] [PubMed]
- Compaan, A.M.; Christensen, K.; Huang, Y. Inkjet bioprinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomater. Sci. Eng. 2017, 3, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.A.; Soon, C.F.; Ma, N.L.; Tee, K.S.; Lim, G.P.; Morsin, M.; Ahmad, M.K.; Abdulmaged, A.I.; Cheong, S.C. Alginate-gelatin bioink for bioprinting of hela spheroids in alginate-gelatin hexagon shaped scaffolds. Polym. Bull. 2021, 78, 6115–6135. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Taketa, H.; Sathi, G.A.; Farahat, M.; Rahman, K.A.; Sakai, T.; Hirano, Y.; Kuboki, T.; Torii, Y.; Matsumoto, T. Peptide-modified substrate for modulating gland tissue growth and morphology in vitro. Sci. Rep. 2015, 5, 11468. [Google Scholar] [CrossRef]
- Wang, C.-C.; Yang, K.-C.; Lin, K.-H.; Liu, Y.-L.; Liu, H.-C.; Lin, F.-H. Cartilage regeneration in SCID mice using a highly organized three-dimensional alginate scaffold. Biomaterials 2012, 33, 120–127. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, K.B.; Gomes, D.B.; Lee, K.; Santos, S.G.; Sousa, A.; Silva, E.A.; Mooney, D.J.; Granja, P.L.; Barrias, C.C. Injectable MMP-sensitive alginate hydrogels as hMSC delivery systems. Biomacromolecules 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.; Villafuertes, E.; Haeck, J.; Koevoet, W.; Fernandez-Gutierrez, B.; Hoogduijn, M.; Verhaar, J.; Bernsen, M.; van Buul, G.; van Osch, G. Encapsulation of allogeneic mesenchymal stem cells in alginate extends local presence and therapeutic function. Eur. Cell Mater. 2017, 33, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, H.; Tang, Z.; Long, G.; Huang, W. Wound dressing model of human umbilical cord mesenchymal stem cells-alginates complex promotes skin wound healing by paracrine signaling. Stem Cells Int. 2016, 2016, 3269267. [Google Scholar] [CrossRef] [PubMed]
- Schon, L.C.; Gill, N.; Thorpe, M.; Davis, J.; Nadaud, J.; Kim, J.; Molligan, J.; Zhang, Z. Efficacy of a mesenchymal stem cell loaded surgical mesh for tendon repair in rats. J. Transl. Med. 2014, 12, 110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashemibeni, B.; Goharian, V.; Esfandiari, E.; Sadeghi, F.; Fasihi, F.; Alipur, R.; Valiani, A.; Ghorbani, M.; Emami, Z.M.; Shabani, F.; et al. An animal model study for repair of tracheal defects with autologous stem cells and differentiated chondrocytes from adipose-derived stem cells. J. Pediatr. Surg. 2012, 47, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, Z.; Chai, Y.; Zhang, S.; Wu, X.; Huang, S.; Su, J.; Jiang, J. Synthesis of cell composite alginate microfibers by microfluidics with the application potential of small diameter vascular grafts. Biofabrication 2017, 9, 025030. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, C.; Wang, P.; Wang, L.; Bao, C.; Weir, M.D.; Reynolds, M.A.; Ren, K.; Zhao, L.; Xu, H.H.K. Engineering bone regeneration with novel cell-laden hydrogel microfiber-injectable calcium phosphate scaffold. Mater. Sci. Eng. C 2017, 75, 895–905. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, M.; Sharma, N.K.; Barhoumi, Z.; Chen, X. Printability of 3D printed hydrogel scaffolds: Influence of hydrogel composition and printing parameters. Appl. Sci. 2019, 10, 292. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef]
- Park, J.H.; Chung, B.G.; Lee, W.G.; Kim, J.; Brigham, M.D.; Shim, J.; Lee, S.; Hwang, C.; Durmus, N.G.; Demirci, U.; et al. Microporous cell-laden hydrogels for engineered tissue constructs. Biotechnol. Bioeng. 2010, 106, 138–148. [Google Scholar] [CrossRef]
- Shin, S.-J.; Park, J.-Y.; Lee, J.-Y.; Park, H.; Park, Y.-D.; Lee, K.-B.; Whang, C.-M.; Lee, S.-H. “On the Fly” Continuous generation of alginate fibers using a microfluidic device. Langmuir 2007, 23, 9104–9108. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jeong, G.S.; Choi, Y.Y.; Lee, K.H.; Khademhosseini, A.; Lee, S.-H. Digitally tunable physicochemical coding of material composition and topography in continuous microfibers. Nat. Mater. 2011, 10, 877–883. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Liu, X.; Guo, Z.; He, Y.; Wei, D.; Zhong, M.; Guo, L.; Fan, H.; Zhang, X. Wet-spinning fabrication of shear-patterned alginate hydrogel microfibers and the guidance of cell alignment. Regen. Biomater. 2017, 4, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Unser, A.M.; Mooney, B.; Corr, D.T.; Tseng, Y.-H.; Xie, Y. 3D brown adipogenesis to create “Brown-Fat-in-Microstrands”. Biomaterials 2016, 75, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.; Gibbons, A.; Sui, K.; Carpenter, R.; Zhang, X.; Xie, Y. Predictable fabrication of pre-made alginate hydrogel microtubes for stem cell aggregation using needle-in-needle devices. Biofabrication 2021, 13, 035043. [Google Scholar] [CrossRef]
- Yeo, M.; Ha, J.; Lee, H.; Kim, G. Fabrication of hASCs-laden structures using extrusion-based cell printing supplemented with an electric field. Acta Biomater. 2016, 38, 33–43. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, W.; Zhu, J.-M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 2013, 34, 130–139. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Sands, R.W.; Bhatta, D.; Arany, P.; Verbeke, C.S.; Edwards, D.A.; Mooney, D.J. Injectable preformed scaffolds with shape-memory properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. [Google Scholar] [CrossRef]
- Abune, L.; Lee, K.; Wang, Y. Development of a biomimetic extracellular matrix with functions of protein sequestration and cell attachment using dual aptamer-functionalized hydrogels. ACS Biomater. Sci. Eng. 2022, 8, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Poon, C. Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. J. Mech. Behav. Biomed. Mater. 2022, 126, 105024. [Google Scholar] [CrossRef] [PubMed]
- Kaklamani, G.; Cheneler, D.; Grover, L.M.; Adams, M.J.; Bowen, J. Mechanical properties of alginate hydrogels manufactured using external gelation. J. Mech. Behav. Biomed. Mater. 2014, 36, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Koslow, M.; O’Keefe, K.J.; Hosseini, Z.F.; Nelson, D.A.; Larsen, M. ROCK inhibitor increases proacinar cells in adult salivary gland organoids. Stem Cell Res. 2019, 41, 101608. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Z.F.; Nelson, D.A.; Moskwa, N.; Larsen, M. Generating embryonic salivary gland organoids. Curr. Protoc. Cell Biol. 2019, 83, e76. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Z.F.; Nelson, D.A.; Moskwa, N.; Sfakis, L.M.; Castracane, J.; Larsen, M. FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids. J. Cell Sci. 2018, 131, jcs208728. [Google Scholar] [CrossRef]
- Erikstein, B.S.; Hagland, H.R.; Nikolaisen, J.; Kulawiec, M.; Singh, K.K.; Gjertsen, B.T.; Tronstad, K.J. Cellular stress induced by resazurin leads to autophagy and cell death via production of reactive oxygen species and mitochondrial impairment. J. Cell Biochem. 2010, 111, 574–584. [Google Scholar] [CrossRef]
- Gloeckner, H.; Jonuleit, T.; Lemke, H.-D. Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar BlueTM. J. Immunol. Methods 2001, 252, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Tascher, G.; Damm, G.; Nüssler, A.K.; Heinzle, E.; Noor, F. Real-time in situ viability assessment in a 3D bioreactor with liver cells using resazurin assay. Cytotechnology 2013, 65, 297–305. [Google Scholar] [CrossRef]
- Place, T.L.; Domann, F.E.; Case, A.J. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic. Biol. Med. 2017, 113, 311–322. [Google Scholar] [CrossRef]
- Wang, S.; Qu, X.; Zhao, R.C. Clinical applications of mesenchymal stem cells. J. Hematol. Oncol. 2012, 5, 19. [Google Scholar] [CrossRef]
- Sanchez-Diaz, M.; Quiñones-Vico, M.I.; Sanabria de la Torre, R.; Montero-Vílchez, T.; Sierra-Sánchez, A.; Molina-Leyva, A.; Arias-Santiago, S. Biodistribution of mesenchymal stromal cells after administration in animal models and humans: A systematic review. J. Clin. Med. 2021, 10, 2925. [Google Scholar] [CrossRef]
- Xie, R.; Liang, Z.; Ai, Y.; Zheng, W.; Xiong, J.; Xu, P.; Liu, Y.; Ding, M.; Gao, J.; Wang, J.; et al. Composable microfluidic spinning platforms for facile production of biomimetic perfusable hydrogel microtubes. Nat. Protoc. 2021, 16, 937–964. [Google Scholar] [CrossRef]
- Jun, Y.; Kim, M.J.; Hwang, Y.H.; Jeon, E.A.; Kang, A.R.; Lee, S.-H.; Lee, D.Y. Microfluidics-generated pancreatic islet microfibers for enhanced immunoprotection. Biomaterials 2013, 34, 8122–8130. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shang, L.; Guo, J.; Wang, J.; Zhao, Y. Design of capillary microfluidics for spinning cell-laden microfibers. Nat. Protoc. 2018, 13, 2557–2579. [Google Scholar] [CrossRef]
- Sun, T.; Li, X.; Shi, Q.; Wang, H.; Huang, Q.; Fukuda, T. Microfluidic spun alginate hydrogel microfibers and their application in tissue engineering. Gels 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, L.; Gu, L.; Yang, H.; Luo, Y.; Ma, L. High-resolution 3D bioprinting system for fabricating cell-laden hydrogel scaffolds with high cellular activities. Procedia CIRP 2017, 65, 219–224. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.S.; Jung, B.; Won, C.; Hwang, C. Coaxial bioprinting of cell-laden vascular constructs using a gelatin–tyramine bioink. Biomater. Sci. 2019, 7, 4578–4587. [Google Scholar] [CrossRef]
- Huang, J.; Fu, H.; Wang, Z.; Meng, Q.; Liu, S.; Wang, H.; Zheng, X.; Dai, J.; Zhang, Z. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting. RSC Adv. 2016, 6, 108423–108430. [Google Scholar] [CrossRef]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. 2009, 4, 1168–1177. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Li, Z.; Fu, X.; Huang, S. Advances in 3D skin bioprinting for wound healing and disease modeling. Regen. Biomater. 2023, 10, rbac105. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.-J.; Kang, H.-W.; Lee, S.J.; Atala, A.; Yoo, J.J. Bioprinting technology and its applications. Eur. J. Cardiothorac. Surg. 2014, 46, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. A Perspective on bioprinting ethics. Artif. Organs 2016, 40, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Alge, D.L.; Gaharwar, A.K. Bioprinting 101: Design, fabrication, and evaluation of cell-laden 3D bioprinted scaffolds. Tissue Eng. Part A 2020, 26, 318–338. [Google Scholar] [CrossRef] [PubMed]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3d bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.A.; Krizman, D.B.; Ringeisen, B.R. Laser printing of single cells: Statistical analysis, cell viability, and stress. Ann. Biomed. Eng. 2005, 33, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Pepper, M.E.; Seshadri, V.; Burg, T.; Booth, B.W.; Burg, K.J.L.; Groff, R.E. Cell settling effects on a thermal inkjet bioprinter. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 3609–3612. [Google Scholar] [CrossRef]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Dawson, J.I.; Oreffo, R.O.C. The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine. Biomaterials 2019, 209, 10–24. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Metallo, S.J.; Whitesides, G.M. Fabrication of a configurable, single-use microfluidic device. Anal. Chem. 2001, 73, 5645–5650. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R. When PDMS isn’t the best. Anal. Chem. 2007, 79, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.W.; Yang, S.S. The optimization of PDMS-PMMA bonding process using silane primer. Biochip. J. 2010, 4, 148–154. [Google Scholar] [CrossRef]
- Askari-Sedeh, M.; Baghani, M. pH-Sensitive hydrogel bilayers: Investigation on transient swelling-induced bending through analytical and FEM approaches. Gels 2023, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Askari-Sedeh, M.; Baghani, M. Coupled chemo-mechanical swelling behavior of ph-sensitive hollow cylinder hydrogels under extension–torsion and internal pressure: Analytical and 3D FEM solutions. Int. J. Appl. Mech. 2023, 15, 2350030. [Google Scholar] [CrossRef]
- Xu, H.Q.; Liu, J.C.; Zhang, Z.Y.; Xu, C.X. A review on cell damage, viability, and functionality during 3D bioprinting. Mil. Med. Res. 2022, 9, 70. [Google Scholar] [CrossRef]
- Xu, C.; Chai, W.; Huang, Y.; Markwald, R.R. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol. Bioeng. 2012, 109, 3152–3160. [Google Scholar] [CrossRef]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Gibson, J.; Halliday, J.A.; Ewert, K.; Robertson, S. A controlled release pilocarpine buccal insert in the treatment of Sjögren’s syndrome. Br. Dent. J. 2007, 202, E17. [Google Scholar] [CrossRef]
- Rocchi, C.; Emmerson, E. Mouth-watering results: Clinical need, current approaches, and future directions for salivary gland regeneration. Trends Mol. Med. 2020, 26, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, D.; Liu, D.; Fan, Z.; Zhang, H.; Liu, O.; Ding, G.; Gao, R.; Zhang, C.; Ding, Y.; et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood 2012, 120, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Liu, Y.; Kornete, M.; Roescher, N.; Kodama, S.; Peterson, A.; Piccirillo, C.A.; Tran, S.D. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with sjögren’s-like disease. PLoS ONE 2012, 7, e38615. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow–derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, B.; Pramanik, K. Key aspects of the mesenchymal stem cells (MSCs) in tissue engineering for in vitro skeletal muscle regeneration. Biotechnol. Mol. Biol. Rev. 2012, 7, 5–15. [Google Scholar] [CrossRef]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Albareda, J.; Prades, M.; Gosálvez, J.; Roy, R.; Zaragoza, P.; Martín-Burriel, I.; et al. Priming equine bone marrow-derived mesenchymal stem cells with proinflammatory cytokines: Implications in immunomodulation–immunogenicity balance, cell viability, and differentiation potential. Stem Cells Dev. 2017, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Ryan, A.E.; Alagesan, S.; Lohan, P.; Treacy, O.; Ritter, T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol. Cell Biol. 2013, 91, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; O’Connell, B.C.; He, X.; Lancaster, H.E.; O’Connell, A.C.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA 1997, 94, 3268–3273. [Google Scholar] [CrossRef]
- Samuni, Y.; Baum, B.J. Gene delivery in salivary glands: From the bench to the clinic. Biochim. Biophys. Acta 2011, 1812, 1515–1521. [Google Scholar] [CrossRef]
- Alevizos, I.; Zheng, C.; Cotrim, A.; Goldsmith, C.; McCullagh, L.; Berkowitz, T.; Strobl, S.; Malyguine, A.; Kopp, W.; Chiorini, J.; et al. Immune reactivity after adenoviral-mediated aquaporin-1 cDNA transfer to human parotid glands. Oral Dis. 2017, 23, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, T.; Fowler, E.W.; Hao, Y.; Ravikrishnan, A.; Harrington, D.A.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S.; Jia, X. Biomaterials-based strategies for salivary gland tissue regeneration. Biomater. Sci. 2016, 4, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Hajiabbas, M.; D’Agostino, C.; Simińska-Stanny, J.; Tran, S.D.; Shavandi, A.; Delporte, C. Bioengineering in salivary gland regeneration. J. Biomed. Sci. 2022, 29, 35. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Liu, W.; Zhang, Y.; Pang, B.; Liu, H.; Zhang, Y.; Zhang, H.; Zhang, L.; Liao, H.; Ren, C.; et al. Continuous microfluidic encapsulation of single mesenchymal stem cells using alginate microgels as injectable fillers for bone regeneration. Acta Biomater. 2020, 111, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef]
- Ansari, S.; Diniz, I.M.; Chen, C.; Sarrion, P.; Tamayol, A.; Wu, B.M.; Moshaverinia, A. Human periodontal ligament- and gingiva-derived mesenchymal stem cells promote nerve regeneration when encapsulated in alginate/hyaluronic acid 3D scaffold. Adv. Healthc. Mater. 2017, 6, 1700670. [Google Scholar] [CrossRef]
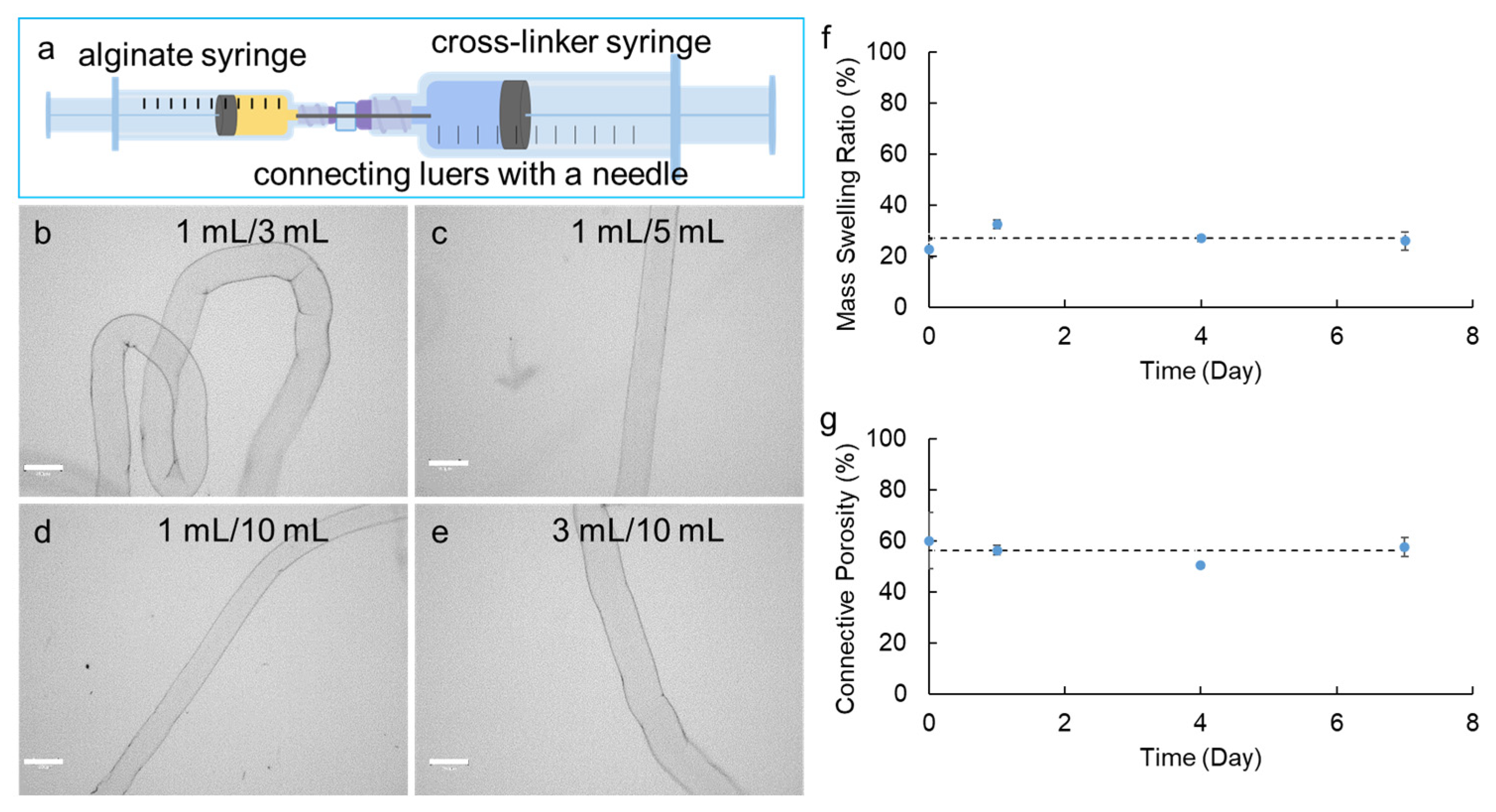



| Device No. | Alginate Syringe Capacity (mL) | Cross-Linker Syringe Capacity (mL) | Needle Gauge (G) | Diameter of Microstrands (µm) a,*** | ||||
|---|---|---|---|---|---|---|---|---|
| Set 1 | Set 2 | Set 3 | Set 4 | Average | ||||
| 1 | 1 | 1 | 30 | not feasible | ||||
| 2 | 1 | 3 | 30 | 290.7 ± 13.7 | 271.0 ± 7.4 | 295.0 ± 4.4 | 310.4 ± 9.5 | 291.8 ± 16.2 |
| 3 | 1 | 5 | 30 | 253.5 ± 8.6 | 213.4 ± 7.4 | 208.4 ± 1.7 | 211.2 ± 4.7 | 221.6 ± 21.3 |
| 4 | 1 | 10 | 30 | 202.3 ± 3.1 | 189.0 ± 1.7 | 183.8 ± 5.2 | 187.6 ± 4.4 | 190.7 ± 8.1 |
| 5 | 3 | 3 | 30 | not feasible | ||||
| 6 | 3 | 5 | 30 | not feasible | ||||
| 7 | 3 | 10 | 30 | 309.0 ± 3.6 | 311.9 ± 1.5 | 299.7 ± 3.6 | 299.7 ± 7.8 | 303.8 ± 5.7 |
| 8 | 5 | 5 | 30 | not feasible | ||||
| 9 | 5 | 10 | 30 | not feasible | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kollampally, S.C.R.; Zhang, X.; Moskwa, N.; Nelson, D.A.; Sharfstein, S.T.; Larsen, M.; Xie, Y. Evaluation of Alginate Hydrogel Microstrands for Stromal Cell Encapsulation and Maintenance. Bioengineering 2024, 11, 375. https://doi.org/10.3390/bioengineering11040375
Kollampally SCR, Zhang X, Moskwa N, Nelson DA, Sharfstein ST, Larsen M, Xie Y. Evaluation of Alginate Hydrogel Microstrands for Stromal Cell Encapsulation and Maintenance. Bioengineering. 2024; 11(4):375. https://doi.org/10.3390/bioengineering11040375
Chicago/Turabian StyleKollampally, Sujith Chander Reddy, Xulang Zhang, Nicholas Moskwa, Deirdre A. Nelson, Susan T. Sharfstein, Melinda Larsen, and Yubing Xie. 2024. "Evaluation of Alginate Hydrogel Microstrands for Stromal Cell Encapsulation and Maintenance" Bioengineering 11, no. 4: 375. https://doi.org/10.3390/bioengineering11040375
APA StyleKollampally, S. C. R., Zhang, X., Moskwa, N., Nelson, D. A., Sharfstein, S. T., Larsen, M., & Xie, Y. (2024). Evaluation of Alginate Hydrogel Microstrands for Stromal Cell Encapsulation and Maintenance. Bioengineering, 11(4), 375. https://doi.org/10.3390/bioengineering11040375