Direct Current Stimulation over the Primary Motor Cortex, Cerebellum, and Spinal Cord to Modulate Balance Performance: A Randomized Placebo-Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Assessments
2.5. Analysis
3. Results
4. Discussion
4.1. Stimulated Area Specific Modulation
4.2. Leg-Specific Modulation
4.3. Balance Task-Specific Modulation
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.I.; Bikson, M.; Datta, A.; Minhas, P.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: A neurophysiological study. Brain Stimul. 2013, 6, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Giordano, J.; Bikson, M.; Kappenman, E.S.; Clark, V.P.; Coslett, H.B.; Hamblin, M.R.; Hamilton, R.; Jankord, R.; Kozumbo, W.J.; McKinley, R.A.; et al. Mechanisms and Effects of Transcranial Direct Current Stimulation. Dose Response 2017, 15, 1559325816685467. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Rach, S.; Neuling, T.; Strüber, D. Transcranial alternating current stimulation: A review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci. 2013, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, P.; Nitsche, M.A.; Ekhtiari, H. A framework for categorizing electrode montages in transcranial direct current stimulation. Front. Hum. Neurosci. 2015, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Halakoo, S.; Ehsani, F.; Hosnian, M.; Kheirkhahan, A.; Samaei, A.; Emadi, A. The comparative effects of anodal and cathodal trans-cranial direct current stimulation on balance and posture: A systematic review of literature and meta-analysis. J. Clin. Neurosci. 2023, 107, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.N.; Porto, A.B.; Marcori, A.J.; Lage, G.M.; Altimari, L.R.; Alves Okazaki, V.H. Motor learning and tDCS: A systematic review on the dependency of the stimulation effect on motor task characteristics or tDCS assembly specifications. Neuropsychologia 2023, 179, 108463. [Google Scholar] [CrossRef] [PubMed]
- Im, C.H.; Park, J.H.; Shim, M.; Chang, W.H.; Kim, Y.H. Evaluation of local electric fields generated by transcranial direct current stimulation with an extracephalic reference electrode based on realistic 3D body modeling. Phys. Med. Biol. 2012, 57, 2137–2150. [Google Scholar] [CrossRef]
- Guidetti, M.; Maria Bianchi, A.; Parazzini, M.; Maiorana, N.; Bonato, M.; Ferrara, R.; Libelli, G.; Montemagno, K.; Ferrucci, R.; Priori, A.; et al. Monopolar tDCS might affect brainstem reflexes: A computational and neurophysiological study. Clin. Neurophysiol. 2023, 155, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, M.; Giannoni-Luza, S.; Bocci, T.; Pacheco-Barrios, K.; Bianchi, A.M.; Parazzini, M.; Ionta, S.; Ferrucci, R.; Maiorana, N.V.; Verde, F.; et al. Modeling Electric Fields in Transcutaneous Spinal Direct Current Stimulation: A Clinical Perspective. Biomedicines 2023, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, B.W.; Bekkers, E.M.J.; Gilat, M.; de Rond, V.; Hardwick, R.M.; Nieuwboer, A. Functional neuroimaging of human postural control: A systematic review with meta-analysis. Neurosci. Biobehav. Rev. 2020, 115, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Surgent, O.J.; Dadalko, O.I.; Pickett, K.A.; Travers, B.G. Balance and the brain: A review of structural brain correlates of postural balance and balance training in humans. Gait Posture 2019, 71, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Taube, W.; Gruber, M.; Gollhofer, A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol. 2008, 193, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Zhou, S. Soleus H-reflex and its relation to static postural control. Gait Posture 2011, 33, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Baharlouei, H.; Ali Salehinejad, M.; Talimkhani, A.; Nitsche, M.A. The Effect of Non-invasive Brain Stimulation on Gait in Healthy Young and Older Adults: A Systematic Review of the Literature. Neuroscience 2023, 516, 125–140. [Google Scholar] [CrossRef]
- Guo, Z.; Bao, D.; Manor, B.; Zhou, J. The Effects of Transcranial Direct Current Stimulation (tDCS) on Balance Control in Older Adults: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 275. [Google Scholar] [CrossRef]
- de Moura, M.C.D.S.; Hazime, F.A.; Marotti Aparicio, L.V.; Grecco, L.A.C.; Brunoni, A.R.; Hasue, R.H. Effects of transcranial direct current stimulation (tDCS) on balance improvement: A systematic review and meta-analysis. Somatosens. Mot. Res. 2019, 36, 122–135. [Google Scholar] [CrossRef]
- Priori, A.; Ciocca, M.; Parazzini, M.; Vergari, M.; Ferrucci, R. Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists. J. Physiol. 2014, 592, 3345–3369. [Google Scholar] [CrossRef]
- Manto, M.; Argyropoulos, G.P.D.; Bocci, T.; Celnik, P.A.; Corben, L.A.; Guidetti, M.; Koch, G.; Priori, A.; Rothwell, J.C.; Sadnicka, A.; et al. Consensus Paper: Novel Directions and Next Steps of Non-invasive Brain Stimulation of the Cerebellum in Health and Disease. Cerebellum 2022, 21, 1092–1122. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, M.; Ferrucci, R.; Vergari, M.; Aglieco, G.; Naci, A.; Versace, S.; Pacheco-Barrios, K.; Giannoni-Luza, S.; Barbieri, S.; Priori, A.; et al. Effects of Transcutaneous Spinal Direct Current Stimulation (tsDCS) in Patients With Chronic Pain: A Clinical and Neurophysiological Study. Front. Neurol. 2021, 12, 695910. [Google Scholar] [CrossRef] [PubMed]
- Keel, J.C.; Smith, M.J.; Wassermann, E.M. A safety screening questionnaire for transcranial magnetic stimulation. Clin. Neurophysiol. 2001, 112, 720. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, E.; Hoff, M.; Rjosk, V.; Steele, C.J.; Gundlach, C.; Sehm, B.; Villringer, A.; Ragert, P. Anodal Transcranial Direct Current Stimulation Does Not Facilitate Dynamic Balance Task Learning in Healthy Old Adults. Front. Hum. Neurosci. 2017, 11, 16. [Google Scholar] [CrossRef]
- Veldema, J.; Gharabaghi, A. Non-invasive brain stimulation for improving gait, balance, and lower limbs motor function in stroke. J. Neuroeng. Rehabil. 2022, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.; Salvador, R.; Wenger, C.; de Carvalho, M.; Miranda, P.C. Transcutaneous spinal direct current stimulation of the lumbar and sacral spinal cord: A modelling study. J. Neural Eng. 2018, 15, 036008. [Google Scholar] [CrossRef] [PubMed]
- Herwig, U.; Satrapi, P.; Schönfeldt-Lecuona, C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003, 16, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Chakraverty, R.; Pynsent, P.; Isaacs, K. Which spinal levels are identified by palpation of the iliac crests and the posterior superior iliac spines? J. Anat. 2007, 210, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Cooperstein, R.; Haneline, M.T. Spinous process palpation using the scapular tip as a landmark vs a radiographic criterion standard. J. Chiropr. Med. 2007, 6, 87–93. [Google Scholar] [CrossRef][Green Version]
- Plisky, P.; Schwartkopf-Phifer, K.; Huebner, B.; Garner, M.B.; Bullock, G. Systematic Review and Meta-Analysis of the Y-Balance Test Lower Quarter: Reliability, Discriminant Validity, and Predictive Validity. Int. J. Sports Phys. Ther. 2021, 16, 1190–1209. [Google Scholar] [CrossRef]
- Lynall, R.C.; Campbell, K.R.; Mauntel, T.C.; Blackburn, J.T.; Mihalik, J.P. Single-Legged Hop and Single-Legged Squat Balance Performance in Recreational Athletes With a History of Concussion. J. Athl. Train. 2020, 55, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Lodge, C.; Wallace, J. Test-Retest Reliability of Single-Leg Time to Stabilization Following a Drop-Landing Task in Healthy Individuals. J. Sport Rehabil. 2021, 30, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Troester, J.C.; Jasmin, J.G.; Duffield, R. Reliability of Single-Leg Balance and Landing Tests in Rugby Union; Prospect of Using Postural Control to Monitor Fatigue. J. Sports Sci. Med. 2018, 17, 174–180. [Google Scholar]
- Mengarelli, A.; Verdini, F.; Cardarelli, S.; Di Nardo, F.; Burattini, L.; Fioretti, S. Balance assessment during squatting exercise: A comparison between laboratory grade force plate and a commercial, low-cost device. J. Biomech. 2018, 71, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Schneiders, A.G.; Sullivan, S.J.; O’Malley, K.J.; Clarke, S.V.; Knappstein, S.A.; Taylor, L.J. A valid and reliable clinical determination of footedness. PM&R 2010, 2, 835–841. [Google Scholar]
- Takakusaki, K.; Takahashi, M.; Obara, K.; Chiba, R. Neural substrates involved in the control of posture. Adv. Robot. 2017, 31, 2–23. [Google Scholar] [CrossRef]
- Dutta, S.; Parihar, A.; Khanna, A.; Gomez, J.; Chakraborty, W.; Jerry, M.; Grisafe, B.; Raychowdhury, A.; Datta, S. Programmable coupled oscillators for synchronized locomotion. Nat. Commun. 2019, 10, 3299. [Google Scholar] [CrossRef] [PubMed]
- Dzeladini, F.; van den Kieboom, J.; Ijspeert, A. The contribution of a central pattern generator in a reflex-based neuromuscular model. Front. Hum. Neurosci. 2014, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.L. Central pattern generators. Curr. Biol. 2000, 10, R176. [Google Scholar] [CrossRef]
- Geyer, H.; Herr, H. A muscle-reflex model that encodes principles of legged mechanics produces human walking dynamics and muscle activities. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 263–273. [Google Scholar] [CrossRef]
- Ramadan, R.; Geyer, H.; Jeka, J.; Schöner, G.; Reimann, H. A neuromuscular model of human locomotion combines spinal reflex circuits with voluntary movements. Sci. Rep. 2022, 12, 8189. [Google Scholar] [CrossRef]
- Koser, D.E.; Moeendarbary, E.; Hanne, J.; Kuerten, S.; Franze, K. CNS cell distribution and axon orientation determine local spinal cord mechanical properties. Biophys. J. 2015, 108, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bestmann, S.; Evans, C. A future of current flow modelling for transcranial electrical stimulation? Curr. Behav. Neurosci. Rep. 2021, 8, 150–159. [Google Scholar] [CrossRef]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. 2018, 9, 5092. [Google Scholar] [CrossRef]
- DaSilva, A.F.; Truong, D.Q.; DosSantos, M.F.; Toback, R.L.; Datta, A.; Bikson, M. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front. Neuroanat. 2015, 9, 89. [Google Scholar] [CrossRef]
- Rice, L.C.; D’Mello, A.M.; Stoodley, C.J. Differential Behavioral and Neural Effects of Regional Cerebellar tDCS. Neuroscience 2021, 462, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Kapreli, E.; Athanasopoulos, S.; Papathanasiou, M.; Van Hecke, P.; Strimpakos, N.; Gouliamos, A.; Peeters, R.; Sunaert, S. Lateralization of brain activity during lower limb joints movement. An fMRI study. Neuroimage 2006, 32, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- Palesi, F.; De Rinaldis, A.; Castellazzi, G.; Calamante, F.; Muhlert, N.; Chard, D.; Tournier, J.D.; Magenes, G.; D’Angelo, E.; Gandini Wheeler-Kingshott, C.A.M. Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: Implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci. Rep. 2017, 7, 12841. [Google Scholar] [CrossRef] [PubMed]
- Lüdemann-Podubecká, J.; Bösl, K.; Theilig, S.; Wiederer, R.; Nowak, D.A. The Effectiveness of 1 Hz rTMS Over the Primary Motor Area of the Unaffected Hemisphere to Improve Hand Function After Stroke Depends on Hemispheric Dominance. Brain Stimul. 2015, 8, 823–830. [Google Scholar] [CrossRef]
- Ziemann, U.; Hallett, M. Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: Further evidence for motor dominance. Clin. Neurophysiol. 2001, 112, 107–113. [Google Scholar] [CrossRef]
- Kimura, D. Acquisition of a motor skill after left-hemisphere damage. Brain 1977, 100, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Muehlbauer, T.; Besemer, C.; Wehrle, A.; Gollhofer, A.; Granacher, U. Relationship between strength, balance and mobility in children aged 7-10 years. Gait Posture 2013, 37, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Ringhof, S.; Stein, T. Biomechanical assessment of dynamic balance: Specificity of different balance tests. Hum. Mov. Sci. 2018, 58, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–4988. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lee, J.H. Comparison of the Amplitudes of the H-reflex of Post-stroke Hemiplegia Patients and Normal Adults during Walking. J. Phys. Ther. Sci. 2013, 25, 729–732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawaishi, Y.; Matsumoto, N.; Nishiwaki, T.; Hirano, T. Postactivation depression of soleus H-reflex increase with recovery of lower extremities motor functions in patients with subacute stroke. J. Phys. Ther. Sci. 2017, 29, 1539–1542. [Google Scholar] [CrossRef] [PubMed]
- Anastasopoulos, D. Tremor in Parkinson’s Disease May Arise from Interactions of Central Rhythms with Spinal Reflex Loop Oscillations. J. Park. Dis. 2020, 10, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.; Lawal, T.; Abboud, H. Spinal dystonia and other spinal movement disorders. Dystonia 2023, 2, 11303. [Google Scholar] [CrossRef]
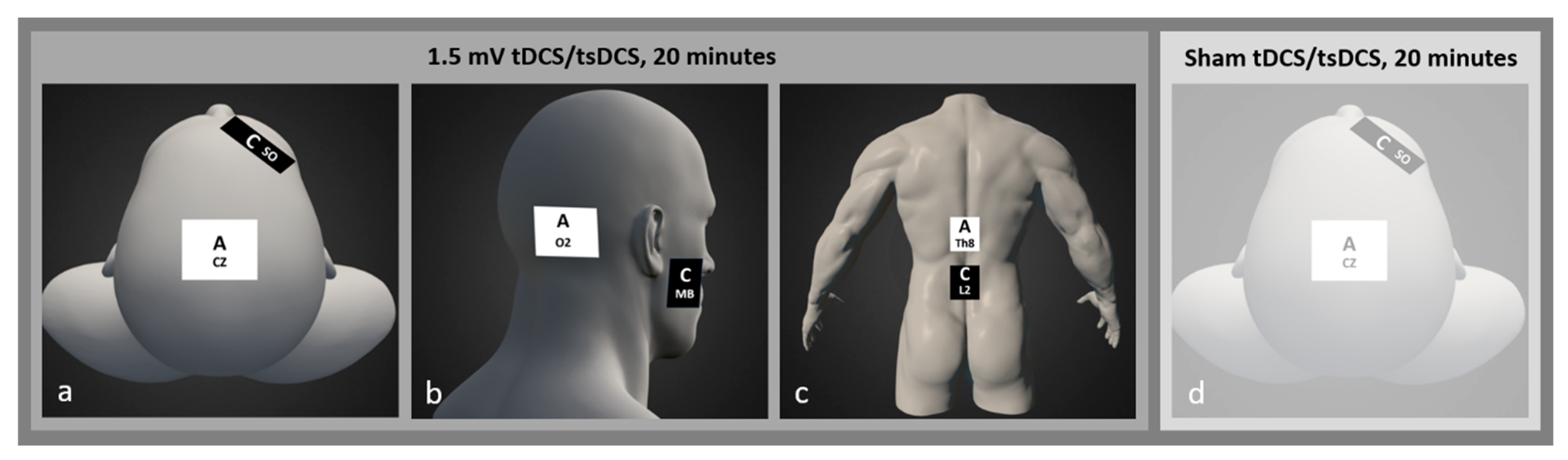
 = sham;
= sham;  = M1;
= M1;  = cerebellum;
= cerebellum;  = spinal.
= spinal.
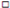 = sham;
= sham;  = M1;
= M1;  = cerebellum;
= cerebellum;  = spinal.
= spinal.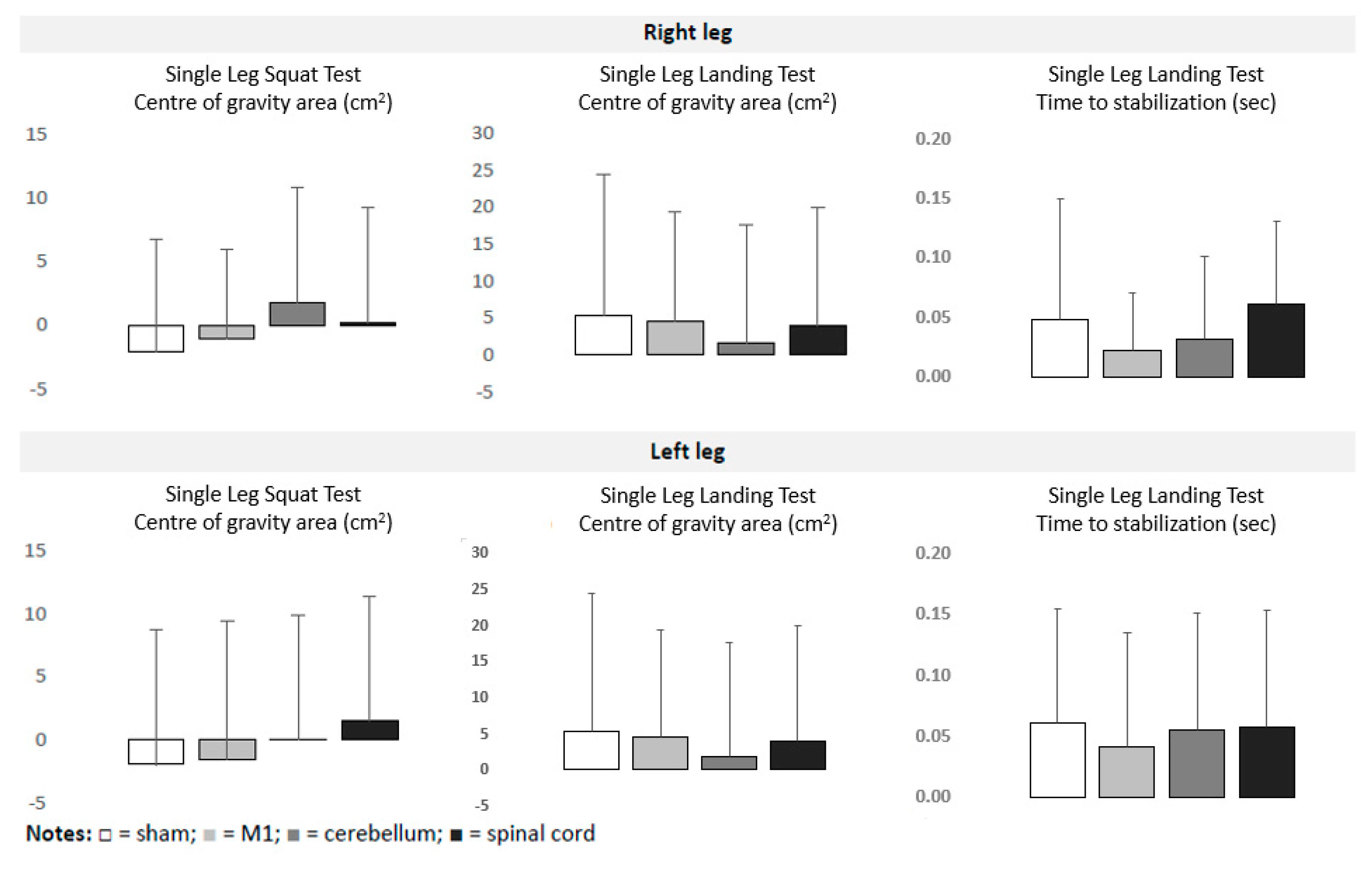
| Sham tDCS | M1 tDCS | Cerebellar tDCS | Spinal tDCS | ||||
|---|---|---|---|---|---|---|---|
| Y Balance Test | Right leg | Anterior direction (cm) | pre | 57.29 ± 7.41 | 56.10 ± 5.57 | 57.54 ± 7.45 | 56.42 ± 5.31 |
| post | 57.53 ± 7.80 | 56.43 ± 5.80 | 58.36 ± 7.76 | 56.91 ± 5.54 | |||
| Posterolateral direction (cm) | pre | 105.21 ± 12.07 | 103.72 ± 10.54 | 104.45 ± 12.76 | 104.00 ± 11.09 | ||
| post | 106.26 ± 12.47 | 106.48 ± 12.31 | 107.14 ± 13.35 | 107.06 ± 12.47 ** | |||
| Posteromedial direction (cm) | pre | 101.19 ± 12.76 | 99.18 ± 13.42 | 102.17 ± 13.99 | 99.47 ± 11.23 | ||
| post | 103.03 ± 13.05 | 103.48 ± 14.42 | 105.53 ± 14.03 | 103.45 ± 11.70 ** | |||
| Left leg | Anterior direction (cm) | pre | 57.76 ± 7.02 | 56.96 ± 5.77 | 56.98 ± 5.87 | 57.02 ± 4.85 | |
| post | 57.75 ± 7.08 | 56.92 ± 5.57 | 57.77 ± 5.92 | 57.60 ± 5.27 | |||
| Posterolateral direction (cm) | pre | 103.78 ± 11.17 | 102.50 ± 10.41 | 103.58 ±± 12.24 | 102.54 ± 10.14 | ||
| post | 104.68 ± 11.12 | 106.54 ± 12.50 ** | 106.56 ± 12.74 ** | 106.06 ± 11.24 *** | |||
| Posteromedial direction (cm) | pre | 101.58 ± 11.15 | 99.88 ± 13.66 | 101.48 ± 14.02 | 100.30 ± 11.15 | ||
| post | 102.99 ± 11.12 | 103.9 ± 14.11 *** | 104.98 ± 14.27 *** | 103.75 ± 10.65 ** | |||
| Single Leg Landing Test | Right leg | Center of gravity area (mm2) | pre | 5163 ± 1486 | 5363 ± 1470 | 5363 ± 1678 | 5406 ± 1690 |
| post | 5619 ± 1975 | 5530 ± 1665 | 5752 ± 1910 | 5938 ± 1764 | |||
| Time to stabilization (ms) | pre | 1.196 ± 0.180 | 1.211 ± 0.185 | 1.141 ± 0.154 | 1.191 ± 0.154 | ||
| post | 1.219 ± 0.188 | 1.243 ± 0.206 | 1.203 ± 0.147 | 1.240 ± 0.160 | |||
| Left leg | Center of gravity area (mm2) | pre | 5437 ± 1224 | 5257 ± 1017 | 5017 ± 1173 | 5305 ± 1282 | |
| post | 5997 ± 1580 | 5154 ± 1281 | 5574 ± 1546 | 6133 ± 1501 | |||
| Time to stabilization (ms) | pre | 1.232 ± 0.153 | 1.273 ± 0.177 | 1.227 ± 0.187 | 1.264 ± 0.170 | ||
| post | 1.274 ± 0.196 | 1.329 ± 0.192 | 1.285 ± 0.210 | 1.326 ± 0.161 | |||
| Single Leg Squat Test | Right leg | Center of gravity area (mm2) | pre | 3020 ± 1246 | 2967 ± 1312 | 3006 ± 988 | 3232 ± 1057 |
| post | 2950 ± 1071 | 3147 ± 1089 | 3085 ± 1201 | 3043 ± 1073 | |||
| Left leg | Center of gravity area (mm2) | pre | 3241 ± 1274 | 3223 ± 1159 | 3179 ± 987 | 3298 ± 973 | |
| post | 3087 ± 1299 | 3223 ± 1302 | 3329 ± 817 | 3093 ± 898 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veldema, J.; Steingräber, T.; von Grönheim, L.; Wienecke, J.; Regel, R.; Schack, T.; Schütz, C. Direct Current Stimulation over the Primary Motor Cortex, Cerebellum, and Spinal Cord to Modulate Balance Performance: A Randomized Placebo-Controlled Trial. Bioengineering 2024, 11, 353. https://doi.org/10.3390/bioengineering11040353
Veldema J, Steingräber T, von Grönheim L, Wienecke J, Regel R, Schack T, Schütz C. Direct Current Stimulation over the Primary Motor Cortex, Cerebellum, and Spinal Cord to Modulate Balance Performance: A Randomized Placebo-Controlled Trial. Bioengineering. 2024; 11(4):353. https://doi.org/10.3390/bioengineering11040353
Chicago/Turabian StyleVeldema, Jitka, Teni Steingräber, Leon von Grönheim, Jana Wienecke, Rieke Regel, Thomas Schack, and Christoph Schütz. 2024. "Direct Current Stimulation over the Primary Motor Cortex, Cerebellum, and Spinal Cord to Modulate Balance Performance: A Randomized Placebo-Controlled Trial" Bioengineering 11, no. 4: 353. https://doi.org/10.3390/bioengineering11040353
APA StyleVeldema, J., Steingräber, T., von Grönheim, L., Wienecke, J., Regel, R., Schack, T., & Schütz, C. (2024). Direct Current Stimulation over the Primary Motor Cortex, Cerebellum, and Spinal Cord to Modulate Balance Performance: A Randomized Placebo-Controlled Trial. Bioengineering, 11(4), 353. https://doi.org/10.3390/bioengineering11040353






