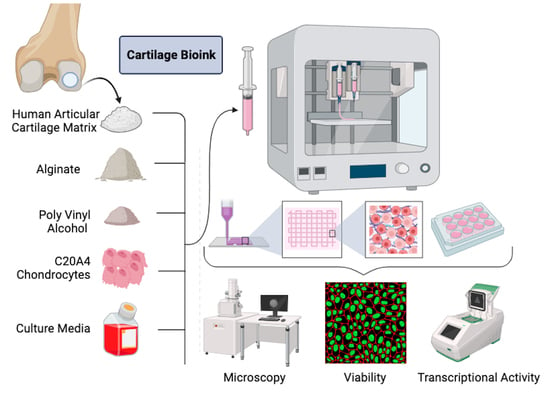
Fabrication of a Novel 3D Extrusion Bioink Containing Processed Human Articular Cartilage Matrix for Cartilage Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
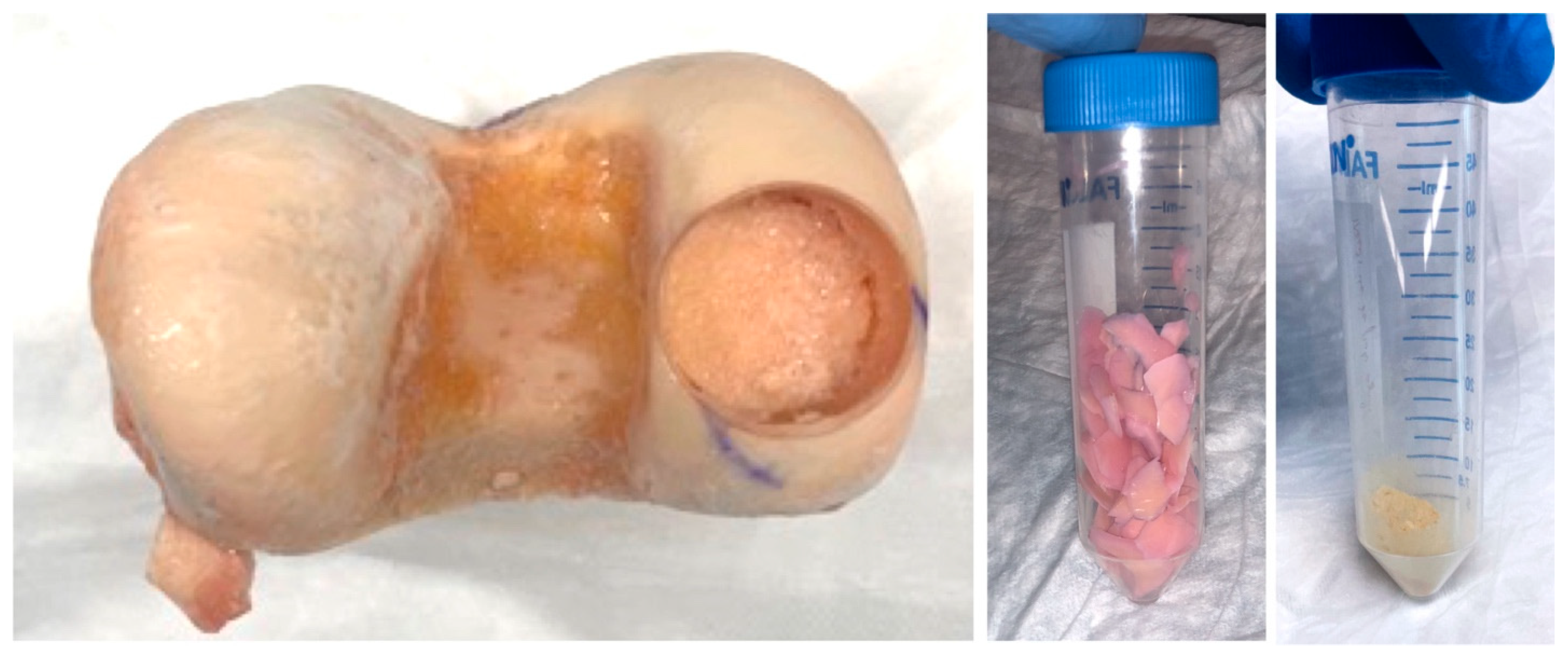
2.1. Formation of Decellularized Articular Cartilage Matrix
2.2. Synthesis of Experimental Bioink Formulations
2.3. Bioprinting Cell Laden Constructs
2.4. Culture of 3D Bioprinted Constructs
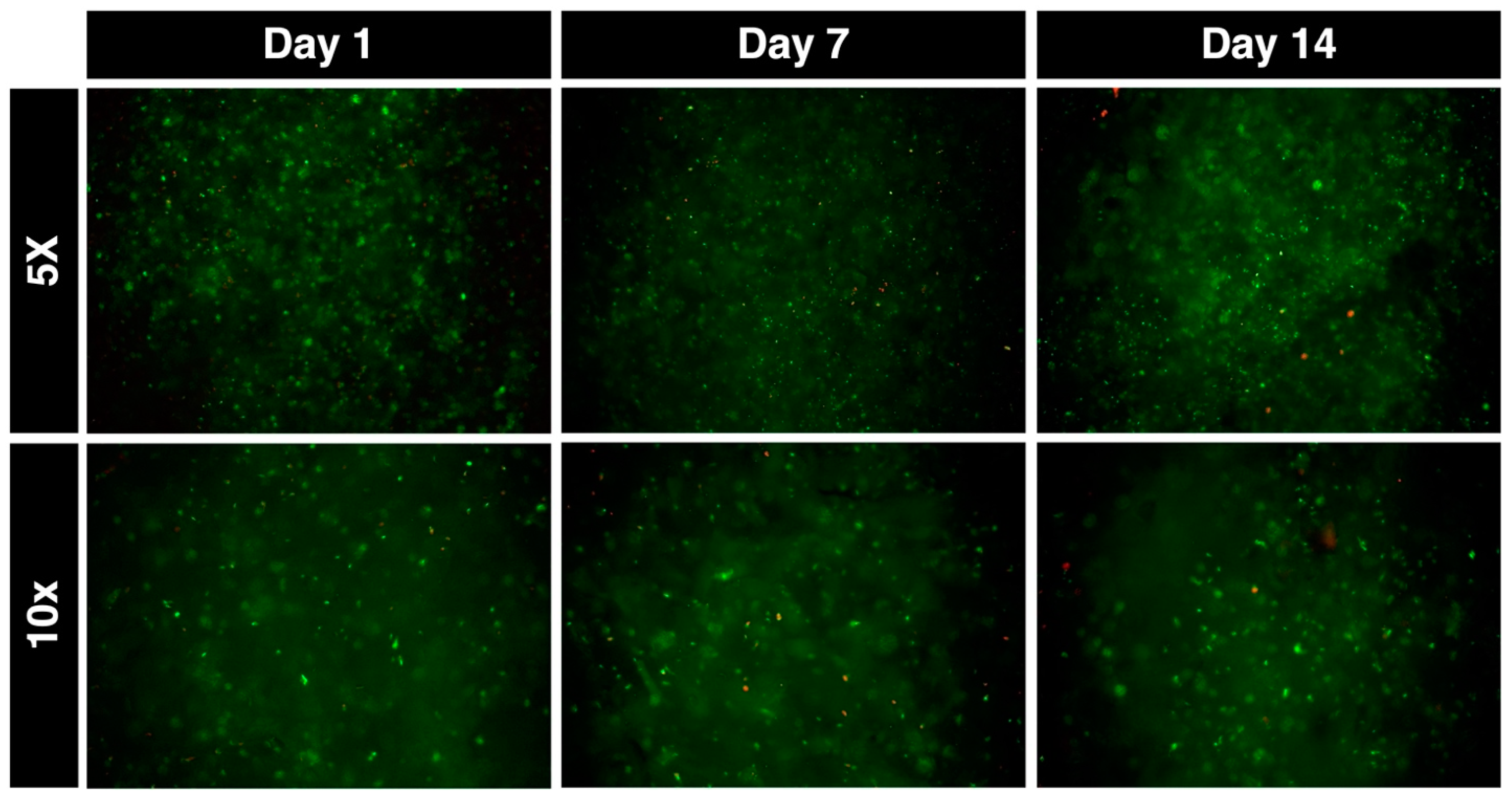
2.5. Cell Survivability in Bioprinted Scaffolds
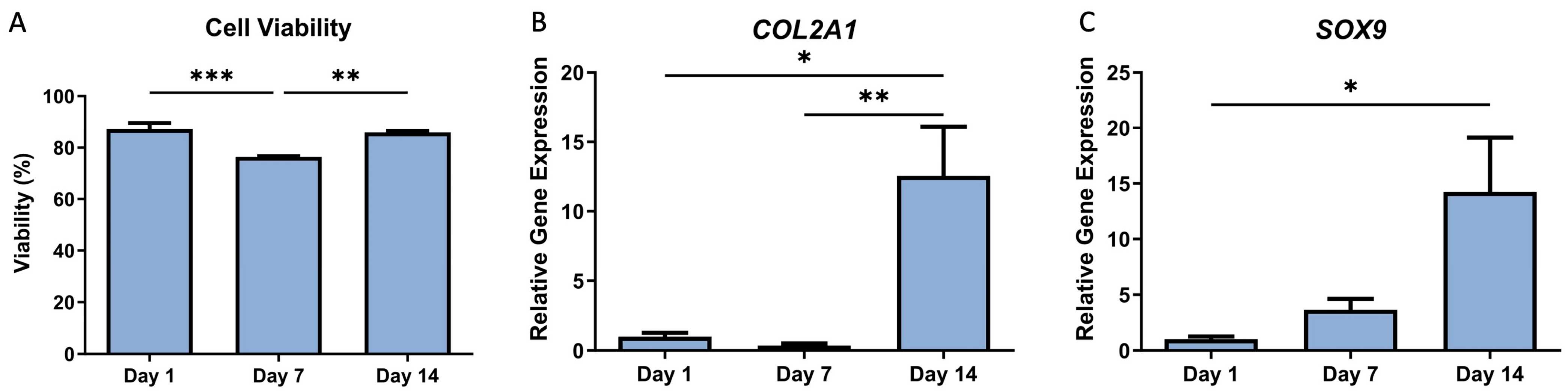
2.6. Real-Time PCR Analysis of Chondrogenic Gene Expression in Bioprinted Scaffolds
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New Insights—Part 1: The Disease and Its Risk Factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Osteoarthritis (OA)|Arthritis|CDC. Available online: https://www.cdc.gov/arthritis/basics/osteoarthritis.htm (accessed on 3 December 2023).
- Buckwalter, J.A.; Mankin, H.J. Articular Cartilage. Part I: Tissue Design and Chondrocyte-Matrix Interactions. J. Bone Jt. Surg. 1997, 79, 600–611. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; Salama, A.; Diab, M.; Beneventi, D.; Dufresne, A. In Situ Mineralization of Nano-Hydroxyapatite on Bifunctional Cellulose Nanofiber/Polyvinyl Alcohol/Sodium Alginate Hydrogel Using 3D Printing. Int. J. Biol. Macromol. 2020, 160, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Jakob, M.; Démarteau, O.; Suetterlin, R.; Heberer, M.; Martin, I. Chondrogenesis of Expanded Adult Human Articular Chondrocytes Is Enhanced by Specific Prostaglandins. Rheumatology 2004, 43, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Alford, J.W.; Cole, B.J. Cartilage Restoration, Part 1: Basic Science, Historical Perspective, Patient Evaluation, and Treatment Options. Am. J. Sports Med. 2005, 33, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular Cartilage. Part II: Degeneration and Osteoarthrosis, Repair, Regeneration, and Transplantation. J. Bone Jt. Surg. 1997, 79, 612–632. [Google Scholar] [CrossRef]
- Clair, B.L.; Johnson, A.R.; Howard, T. Cartilage Repair: Current and Emerging Options in Treatment. Foot Ankle Spec. 2009, 2, 179–188. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Pallante, A.L.; Bae, W.C.; Chen, A.C.; Görtz, S.; Bugbee, W.D.; Sah, R.L. Chondrocyte Viability Is Higher after Prolonged Storage at 37 Degrees C than at 4 Degrees C for Osteochondral Grafts. Am. J. Sports Med. 2009, 37 (Suppl. 1), 24S–32S. [Google Scholar] [CrossRef]
- Rzhepakovsky, I.; Anusha Siddiqui, S.; Avanesyan, S.; Benlidayi, M.; Dhingra, K.; Dolgalev, A.; Enukashvily, N.; Fritsch, T.; Heinz, V.; Kochergin, S.; et al. Anti-Arthritic Effect of Chicken Embryo Tissue Hydrolyzate against Adjuvant Arthritis in Rats (X-Ray Microtomographic and Histopathological Analysis). Food Sci. Nutr. 2021, 9, 5648–5669. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A Comparison of Different Bioinks for 3D Bioprinting of Fibrocartilage and Hyaline Cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef] [PubMed]
- Munaz, A.; Vadivelu, R.K.; St. John, J.; Barton, M.; Kamble, H.; Nguyen, N.T. Three-Dimensional Printing of Biological Matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Francis, S.L.; Duchi, S.; Onofrillo, C.; Di Bella, C.; Choong, P.F.M. Adipose-Derived Mesenchymal Stem Cells in the Use of Cartilage Tissue Engineering: The Need for a Rapid Isolation Procedure. Stem Cells Int. 2018, 2018, 8947548. [Google Scholar] [CrossRef] [PubMed]
- Leberfinger, A.N.; Ravnic, D.J.; Dhawan, A.; Ozbolat, I.T. Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication. Stem Cells Transl. Med. 2017, 6, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Cavallo, C.; Desando, G.; Parisi, V.; Petretta, M.; Bartolotti, I.; Grigolo, B. Three-Dimensional Bioprinting of Cartilage by the Use of Stem Cells: A Strategy to Improve Regeneration. Materials 2018, 11, 1749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, Hyaluronan and Chondroitin Sulfate in Tissue Engineering for Cartilage Regeneration: A Review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D Printing of Gelatin Methacrylamide Cell-Laden Tissue-Engineered Constructs with High Cell Viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Ahearne, M.; Kelly, D.J. A Comparison of Fibrin, Agarose and Gellan Gum Hydrogels as Carriers of Stem Cells and Growth Factor Delivery Microspheres for Cartilage Regeneration. Biomed. Mater. 2013, 8, 035004. [Google Scholar] [CrossRef]
- Rowland, C.R.; Lennon, D.P.; Caplan, A.I.; Guilak, F. The Effects of Crosslinking of Scaffolds Engineered from Cartilage ECM on the Chondrogenic Differentiation of MSCs. Biomaterials 2013, 34, 5802–5812. [Google Scholar] [CrossRef] [PubMed]
- Malinauskas, M.; Jankauskaite, L.; Aukstikalne, L.; Dabasinskaite, L.; Rimkunas, A.; Mickevicius, T.; Pockevicius, A.; Krugly, E.; Martuzevicius, D.; Ciuzas, D.; et al. Cartilage Regeneration Using Improved Surface Electrospun Bilayer Polycaprolactone Scaffolds Loaded with Transforming Growth Factor-Beta 3 and Rabbit Muscle-Derived Stem Cells. Front. Bioeng. Biotechnol. 2022, 10, 971294. [Google Scholar] [CrossRef] [PubMed]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Kutlusoy, T.; Oktay, B.; Apohan, N.K.; Süleymanoğlu, M.; Kuruca, S.E. Chitosan-Co-Hyaluronic Acid Porous Cryogels and Their Application in Tissue Engineering. Int. J. Biol. Macromol. 2017, 103, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Rzhepakovsky, I.; Piskov, S.; Avanesyan, S.; Sizonenko, M.; Timchenko, L.; Anfinogenova, O.; Nagdalian, A.; Blinov, A.; Denisova, E.; Kochergin, S.; et al. Composite of Bacterial Cellulose and Gelatin: A Versatile Biocompatible Scaffold for Tissue Engineering. Int. J. Biol. Macromol. 2024, 256, 128369. [Google Scholar] [CrossRef] [PubMed]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef]
- Henrionnet, C.; Pourchet, L.; Neybecker, P.; Messaoudi, O.; Gillet, P.; Loeuille, D.; Mainard, D.; Marquette, C.; Pinzano, A. Combining Innovative Bioink and Low Cell Density for the Production of 3D-Bioprinted Cartilage Substitutes: A Pilot Study. Stem Cells Int. 2020, 2020, 2487072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wehrle, E.; Vetsch, J.R.; Paul, G.R.; Rubert, M.; Müller, R. Alginate Dependent Changes of Physical Properties in 3D Bioprinted Cell-Laden Porous Scaffolds Affect Cell Viability and Cell Morphology. Biomed. Mater. 2019, 14, 065009. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Juste, R.; Larrañaga-Jaurrieta, G.; Larraza, I.; Ramos-Diez, S.; Camarero-Espinosa, S.; Gabilondo, N.; Eceiza, A. Alginate-Waterborne Polyurethane 3D Bioprinted Scaffolds for Articular Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2023, 253, 127070. [Google Scholar] [CrossRef]
- Critchley, S.; Sheehy, E.J.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S.F.; Jeon, O.; Alsberg, E.; Brama, P.A.J.; Kelly, D.J. 3D Printing of Fibre-Reinforced Cartilaginous Templates for the Regeneration of Osteochondral Defects. Acta Biomater. 2020, 113, 130–143. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, R.; Chen, T.; Zhang, K.; Gui, J. 3D Bioprinting Modified Autologous Matrix-Induced Chondrogenesis(AMIC) Technique for Repair of Cartilage Defects. Mater. Des. 2021, 203, 109621. [Google Scholar] [CrossRef]
- Han, W.; Singh, N.K.; Kim, J.J.; Kim, H.; Kim, B.S.; Park, J.Y.; Jang, J.; Cho, D.W. Directed Differential Behaviors of Multipotent Adult Stem Cells from Decellularized Tissue/Organ Extracellular Matrix Bioinks. Biomaterials 2019, 224, 119496. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zheng, Y.-W.; Lan, Q.-H.; Kou, L.; Xu, H.-L.; Zhao, Y.-Z. Recent Development and Biomedical Applications of Decellularized Extracellular Matrix Biomaterials. Mater. Sci. Eng. C 2019, 104, 109942. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Almalla, A.; Keshi, E.; Hillebrandt, K.H.; Sauer, I.M.; Weinhart, M. Rise of Tissue- and Species-Specific 3D Bioprinting Based on Decellularized Extracellular Matrix-Derived Bioinks and Bioresins. Biomater. Biosyst. 2023, 12, 100084. [Google Scholar] [CrossRef] [PubMed]
- Sahranavard, M.; Sarkari, S.; Safavi, S.; Ghorbani, F. Three-Dimensional Bio-Printing of Decellularized Extracellular Matrix-Based Bio-Inks for Cartilage Regeneration: A Systematic Review. Biomater. Transl. 2022, 3, 105. [Google Scholar] [CrossRef]
- Cartilage Repair/Cartilage Regeneration Market Worth $2.8 Billion|MarketsandMarkets—MarketWatch. Available online: https://www.marketwatch.com/press-release/cartilage-repair-cartilage-regeneration-market-worth-2-8-billion-marketsandmarkets-18d280fc (accessed on 29 December 2023).
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital Light Processing 3D Printed Silk Fibroin Hydrogel for Cartilage Tissue Engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and Bioprinting Technologies to Make Heterogeneous and Biomimetic Tissue Constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-Inspired Hydrogel Composed of Hyaluronic Acid and Alginate as a Potential Bioink for 3D Bioprinting of Articular Cartilage Engineering Constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mandal, B.B.; Bhardwaj, N. 3D Bioprinting of Photo-Crosslinkable Silk Methacrylate (SilMA)-Polyethylene Glycol Diacrylate (PEGDA) Bioink for Cartilage Tissue Engineering. J. Biomed. Mater. Res. A 2022, 110, 884–898. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Semon, J.A.; Bromet, B.; Day, D.E.; Leu, M.C. Bioprinting with Human Stem Cell-Laden Alginate-Gelatin Bioink and Bioactive Glass for Tissue Engineering. Int. J. Bioprinting 2019, 5, 204. [Google Scholar] [CrossRef]
- Lee, H.; Kim, W.; Lee, J.; Park, K.S.; Yoo, J.J.; Atala, A.; Kim, G.H.; Lee, S.J. Self-Aligned Myofibers in 3D Bioprinted Extracellular Matrix-Based Construct Accelerate Skeletal Muscle Function Restoration. Appl. Phys. Rev. 2021, 8, 021405. [Google Scholar] [CrossRef] [PubMed]
- Visscher, D.O.; Lee, H.; van Zuijlen, P.P.M.; Helder, M.N.; Atala, A.; Yoo, J.J.; Lee, S.J. A Photo-Crosslinkable Cartilage-Derived Extracellular Matrix Bioink for Auricular Cartilage Tissue Engineering. Acta Biomater. 2021, 121, 193–203. [Google Scholar] [CrossRef]
- Stone, R.N.; Reeck, J.C.; Oxford, J.T. Advances in Cartilage Tissue Engineering Using Bioinks with Decellularized Cartilage and Three-Dimensional Printing. Int. J. Mol. Sci. 2023, 24, 5526. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Han, Y.; Fu, Q.; Hong, Y.; Li, L.; Cao, J.; Li, H.; Liu, Y.; Chen, Y.; Zhu, J.; et al. Application of Tissue-Derived Bioink for Articular Cartilage Lesion Repair. Chem. Eng. J. 2022, 450, 138292. [Google Scholar] [CrossRef]
- Beck, E.C.; Barragan, M.; Tadros, M.H.; Gehrke, S.H.; Detamore, M.S. Approaching the Compressive Modulus of Articular Cartilage with a Decellularized Cartilage-Based Hydrogel. Acta Biomater. 2016, 38, 94–105. [Google Scholar] [CrossRef]
- Merkely, G.; Ackermann, J.; Farina, E.M.; VanArsdale, C.; Lattermann, C.; Gomoll, A.H. Shorter Storage Time Is Strongly Associated With Improved Graft Survivorship at 5 Years After Osteochondral Allograft Transplantation. Am. J. Sports Med. 2020, 48, 3170–3176. [Google Scholar] [CrossRef]
- Bexkens, R.; Ogink, P.T.; Doornberg, J.N.; Kerkhoffs, G.M.M.J.; Eygendaal, D.; Oh, L.S.; van den Bekerom, M.P.J. Donor-Site Morbidity after Osteochondral Autologous Transplantation for Osteochondritis Dissecans of the Capitellum: A Systematic Review and Meta-Analysis. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2237–2246. [Google Scholar] [CrossRef]
- McAllister, D.R.; Joyce, M.J.; Mann, B.J.; Vangsness, C.T. Allograft Update: The Current Status of Tissue Regulation, Procurement, Processing, and Sterilization. Am. J. Sports Med. 2007, 35, 2148–2158. [Google Scholar] [CrossRef]

| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| Collagen type 2 alpha 1 (COL2A1) | CCCTGGTCTTGGTGGAG | CCATCATCACCAGGCTTTC |
| Sex-determining region Y-box 9 (SOX9) | TCTGAACGAGAGCGAGAA | GCGGCTGGTACTTGTAATC |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitchison, A.H.; Allen, N.B.; Shaffrey, I.R.; O’Neill, C.N.; Abar, B.; Anastasio, A.T.; Adams, S.B. Fabrication of a Novel 3D Extrusion Bioink Containing Processed Human Articular Cartilage Matrix for Cartilage Tissue Engineering. Bioengineering 2024, 11, 329. https://doi.org/10.3390/bioengineering11040329
Aitchison AH, Allen NB, Shaffrey IR, O’Neill CN, Abar B, Anastasio AT, Adams SB. Fabrication of a Novel 3D Extrusion Bioink Containing Processed Human Articular Cartilage Matrix for Cartilage Tissue Engineering. Bioengineering. 2024; 11(4):329. https://doi.org/10.3390/bioengineering11040329
Chicago/Turabian StyleAitchison, Alexandra Hunter, Nicholas B. Allen, Isabel R. Shaffrey, Conor N. O’Neill, Bijan Abar, Albert T. Anastasio, and Samuel B. Adams. 2024. "Fabrication of a Novel 3D Extrusion Bioink Containing Processed Human Articular Cartilage Matrix for Cartilage Tissue Engineering" Bioengineering 11, no. 4: 329. https://doi.org/10.3390/bioengineering11040329
APA StyleAitchison, A. H., Allen, N. B., Shaffrey, I. R., O’Neill, C. N., Abar, B., Anastasio, A. T., & Adams, S. B. (2024). Fabrication of a Novel 3D Extrusion Bioink Containing Processed Human Articular Cartilage Matrix for Cartilage Tissue Engineering. Bioengineering, 11(4), 329. https://doi.org/10.3390/bioengineering11040329