Immunomodulation of Cancer Cells Using Autologous Blood Concentrates as a Patient-Specific Cell Culture System: A Comparative Study on Osteosarcoma and Fibrosarcoma Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Cell Lines
2.3. Preparation of PRF
2.4. PRF Treatment of MG63 and HT1080
2.5. Cell Viability Assay
2.6. Immunofluorescence Staining
2.7. Growth Factor and Cytokine Quantification with Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Gene Expression Analyses
2.9. Statistical Analysis
3. Results
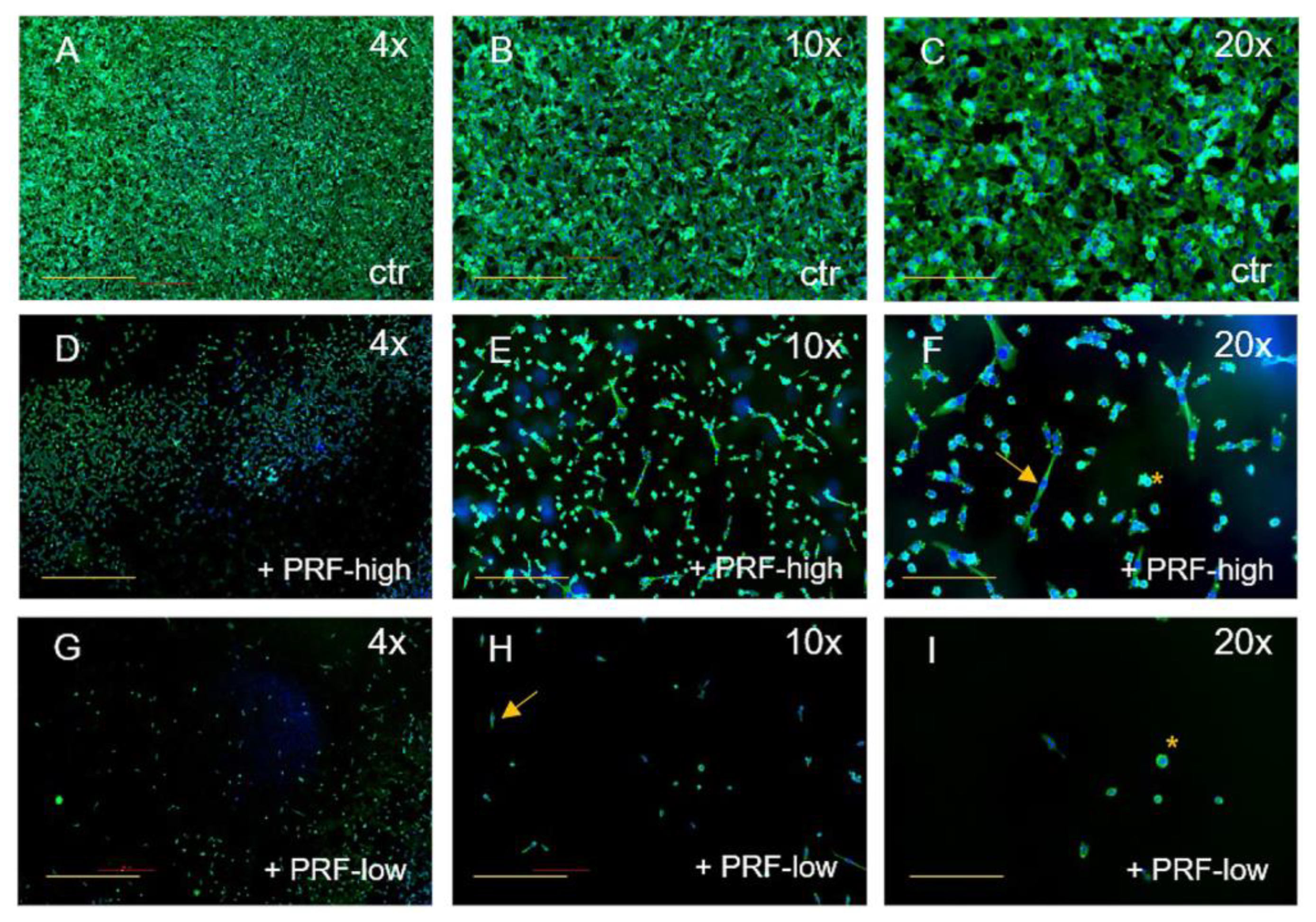
3.1. PRF-Mediated Effect on Cell Viability and Cell Morphology of Tumor Cells
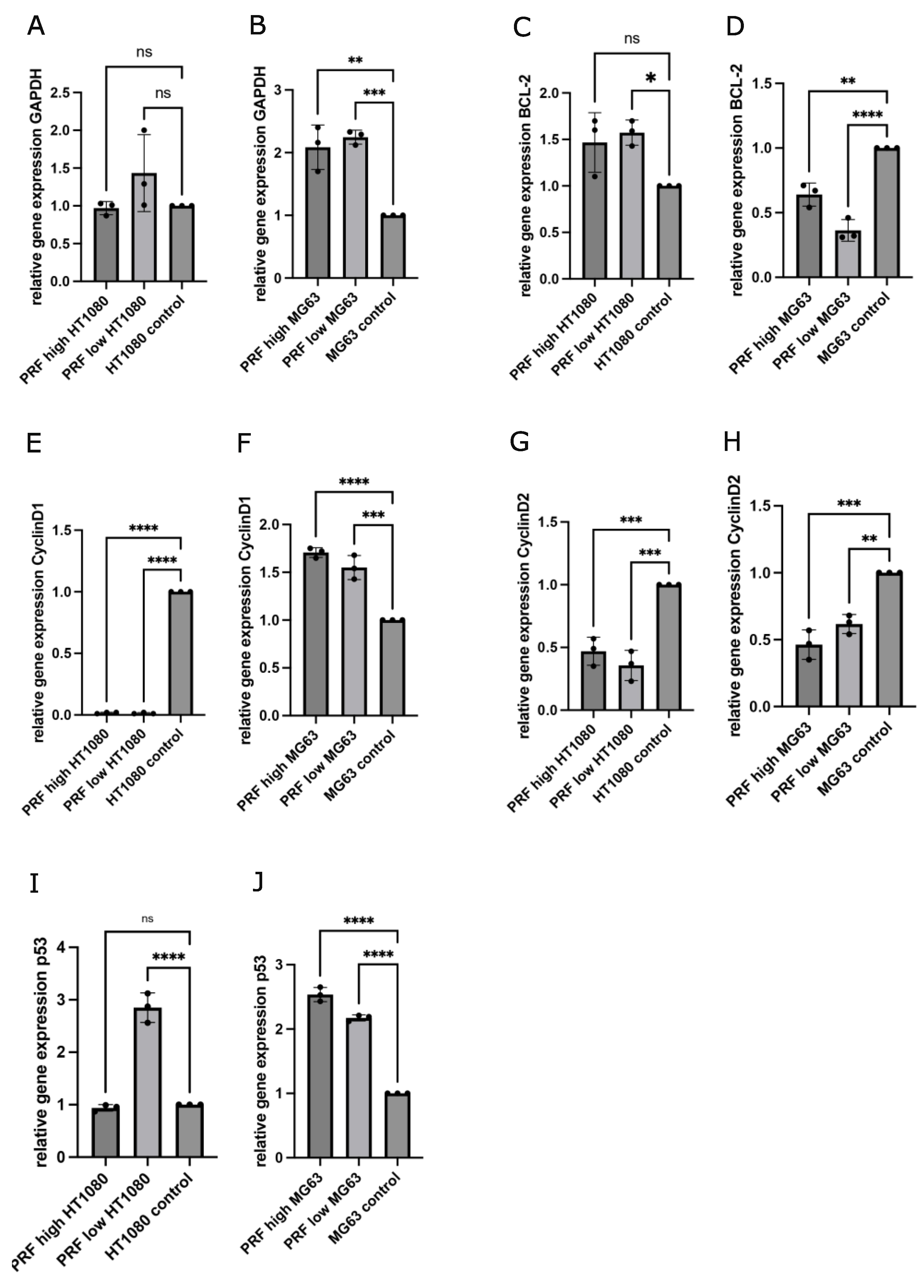
3.2. Gene Expression Analyses of Cell Cycle- and Apoptosis-Associated Factors in MG63 and HT1080 in Response to PRF Treatment Compared to Untreated Controls
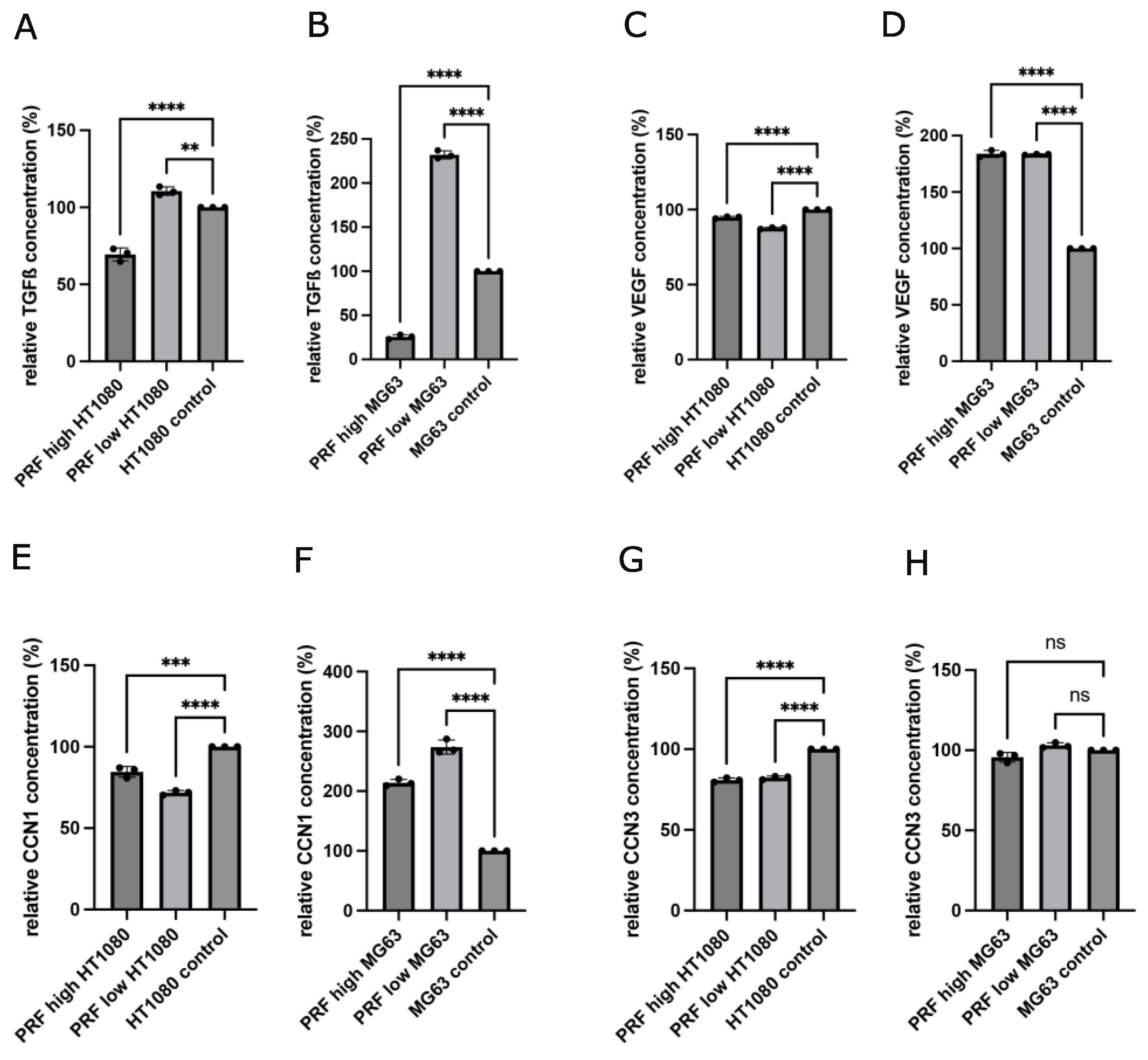
3.3. Determination of Growth Factors and Cytokines in Supernatants of MG63 and HT1080 in Response to Indirect PRF Application
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur. J. Trauma. Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Diss, A.; Odin, G.; Doglioli, P.; Hippolyte, M.P.; Charrier, J.B. In vitro effects of Choukroun’s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Herrera-Vizcaino, C.; Al-Maawi, S.; Lorenz, J.; Miron, R.J.; Nelson, K.; Schwarz, F.; Choukroun, J.; Sader, R. Fifteen Years of Platelet Rich Fibrin in Dentistry and Oromaxillofacial Surgery: How High is the Level of Scientific Evidence? J. Oral Implantol. 2018, 44, 471–492. [Google Scholar] [CrossRef]
- Ghanaati, S.; Smieszek-Wilczewska, J.; Al-Maawi, S.; Neff, P.; Zadeh, H.H.; Sader, R.; Heselich, A.; Rutkowski, J.L. Solid PRF Serves as Basis for Guided Open Wound Healing of the Ridge after Tooth Extraction by Accelerating the Wound Healing Time Course-A Prospective Parallel Arm Randomized Controlled Single Blind Trial. Bioengineering 2022, 9, 661. [Google Scholar] [CrossRef]
- Grecu, A.F.; Reclaru, L.; Ardelean, L.C.; Nica, O.; Ciuca, E.M.; Ciurea, M.E. Platelet-Rich Fibrin and its Emerging Therapeutic Benefits for Musculoskeletal Injury Treatment. Medicina 2019, 55, 141. [Google Scholar] [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef]
- El Bagdadi, K.; Kubesch, A.; Yu, X.; Al-Maawi, S.; Orlowska, A.; Dias, A.; Booms, P.; Dohle, E.; Sader, R.; Kirkpatrick, C.J.; et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: A proof of concept of LSCC (low speed centrifugation concept). Eur. J. Trauma. Emerg. Surg. 2019, 45, 467–479. [Google Scholar] [CrossRef]
- Herrera-Vizcaino, C.; Dohle, E.; Al-Maawi, S.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Choukroun, J.; Ghanaati, S. Platelet-rich fibrin secretome induces three dimensional angiogenic activation in vitro. Eur. Cell Mater. 2019, 37, 250–264. [Google Scholar] [CrossRef]
- Wend, S.; Kubesch, A.; Orlowska, A.; Al-Maawi, S.; Zender, N.; Dias, A.; Miron, R.J.; Sader, R.; Booms, P.; Kirkpatrick, C.J.; et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J. Mater. Sci. Mater. Med. 2017, 28, 188. [Google Scholar] [CrossRef]
- Gupta, S.; Dutta, S.; Hui, S.P. Regenerative Potential of Injured Spinal Cord in the Light of Epigenetic Regulation and Modulation. Cells 2023, 12, 1694. [Google Scholar] [CrossRef]
- Broadhead, M.L.; Clark, J.C.; Myers, D.E.; Dass, C.R.; Choong, P.F. The molecular pathogenesis of osteosarcoma: A review. Sarcoma 2011, 2011, 959248. [Google Scholar] [CrossRef]
- Rasheed, S.; Nelson-Rees, W.A.; Toth, E.M.; Arnstein, P.; Gardner, M.B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer 1974, 33, 1027–1033. [Google Scholar] [CrossRef]
- Nicholls, C.; Li, H.; Liu, J.P. GAPDH: A common enzyme with uncommon functions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef]
- Vaux, D.L.; Cory, S.; Adams, J.M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988, 335, 440–442. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef]
- Wang, X.W.; Harris, C.C. p53 tumor-suppressor gene: Clues to molecular carcinogenesis. J. Cell Physiol. 1997, 173, 247–255. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- McGregor, A.D.; MacDonald, D.G. Patterns of spread of squamous cell carcinoma within the mandible. Head. Neck 1989, 11, 457–461. [Google Scholar] [CrossRef]
- Ames, B.N.; Gold, L.S. The causes and prevention of cancer: The role of environment. Biotherapy 1998, 11, 205–220. [Google Scholar] [CrossRef]
- Gerrard, T.L.; Cohen, D.J.; Kaplan, A.M. Human neutrophil-mediated cytotoxicity to tumor cells. J. Natl. Cancer Inst. 1981, 66, 483–488. [Google Scholar]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105 (Suppl. S1), S13–S33. [Google Scholar] [CrossRef]
- Nurden, A.T. Qualitative disorders of platelets and megakaryocytes. J. Thromb. Haemost. 2005, 3, 1773–1782. [Google Scholar] [CrossRef]
- Nurden, A.T. Platelets and tissue remodeling: Extending the role of the blood clotting system. Endocrinology 2007, 148, 3053–3055. [Google Scholar] [CrossRef]
- Barnum, K.J.; O’Connell, M.J. Cell cycle regulation by checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Regulation of Cell Cycle Progression by Growth Factor-Induced Cell Signaling. Cells 2021, 10, 3327. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Pintucci, G.; Seghezzi, G.; Hyman, K.; Galloway, A.C.; Mignatti, P. VEGF, a prosurvival factor, acts in concert with TGF-beta1 to induce endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2006, 103, 17260–17265. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Briggs, J.; Park, S.; Niu, G.; Kortylewski, M.; Zhang, S.; Gritsko, T.; Turkson, J.; Kay, H.; Semenza, G.L.; et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 2005, 24, 5552–5560. [Google Scholar] [CrossRef]
- Kamata, H.; Honda, S.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, E.; Rapp, U.R. High-intensity Raf signals convert mitotic cell cycling into cellular growth. Cancer Res. 1998, 58, 1636–1640. [Google Scholar] [PubMed]
- Demarse, N.A.; Ponnusamy, S.; Spicer, E.K.; Apohan, E.; Baatz, J.E.; Ogretmen, B.; Davies, C. Direct binding of glyceraldehyde 3-phosphate dehydrogenase to telomeric DNA protects telomeres against chemotherapy-induced rapid degradation. J. Mol. Biol. 2009, 394, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.W.; Saunders, P.A.; Wei, H.; Li, Z.; Seth, P.; Chuang, D.M. Involvement of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and p53 in neuronal apoptosis: Evidence that GAPDH is upregulated by p53. J. Neurosci. 1999, 19, 9654–9662. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massague, J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Newland, A.C.; Jia, L. Bax conformational change is a crucial step for PUMA-mediated apoptosis in human leukemia. Biochem. Biophys. Res. Commun. 2003, 310, 956–962. [Google Scholar] [CrossRef]
- Rocha, S.; Martin, A.M.; Meek, D.W.; Perkins, N.D. p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-kappaB subunit with histone deacetylase 1. Mol. Cell Biol. 2003, 23, 4713–4727. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dohle, E.; Parkhoo, K.; Bennardo, F.; Schmeinck, L.; Sader, R.; Ghanaati, S. Immunomodulation of Cancer Cells Using Autologous Blood Concentrates as a Patient-Specific Cell Culture System: A Comparative Study on Osteosarcoma and Fibrosarcoma Cell Lines. Bioengineering 2024, 11, 303. https://doi.org/10.3390/bioengineering11040303
Dohle E, Parkhoo K, Bennardo F, Schmeinck L, Sader R, Ghanaati S. Immunomodulation of Cancer Cells Using Autologous Blood Concentrates as a Patient-Specific Cell Culture System: A Comparative Study on Osteosarcoma and Fibrosarcoma Cell Lines. Bioengineering. 2024; 11(4):303. https://doi.org/10.3390/bioengineering11040303
Chicago/Turabian StyleDohle, Eva, Kamelia Parkhoo, Francesco Bennardo, Lena Schmeinck, Robert Sader, and Shahram Ghanaati. 2024. "Immunomodulation of Cancer Cells Using Autologous Blood Concentrates as a Patient-Specific Cell Culture System: A Comparative Study on Osteosarcoma and Fibrosarcoma Cell Lines" Bioengineering 11, no. 4: 303. https://doi.org/10.3390/bioengineering11040303
APA StyleDohle, E., Parkhoo, K., Bennardo, F., Schmeinck, L., Sader, R., & Ghanaati, S. (2024). Immunomodulation of Cancer Cells Using Autologous Blood Concentrates as a Patient-Specific Cell Culture System: A Comparative Study on Osteosarcoma and Fibrosarcoma Cell Lines. Bioengineering, 11(4), 303. https://doi.org/10.3390/bioengineering11040303








