The Impact of Defect Size on Bone Healing in Critical-Size Bone Defects Investigated on a Rat Femur Defect Model Comparing Two Treatment Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Human Acellular Dermis (HAD)—Epiflex®
2.3. Surgery
2.4. The Determination of Bone Union and Bone Mineral Density (BMD)
2.5. Assessment of New Bone Formation
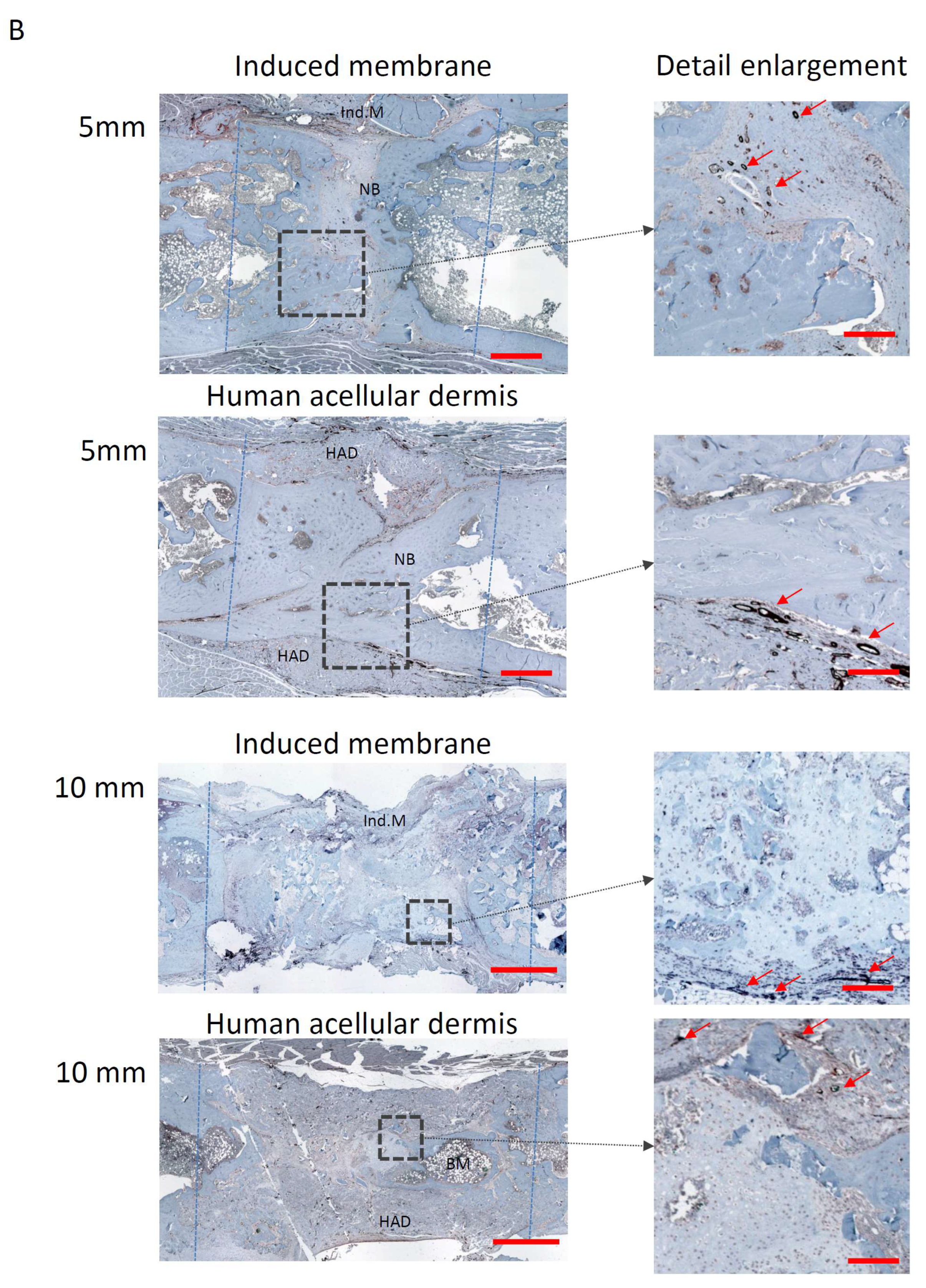
2.6. Assessment of Vascularisation
2.7. Statistics
3. Results
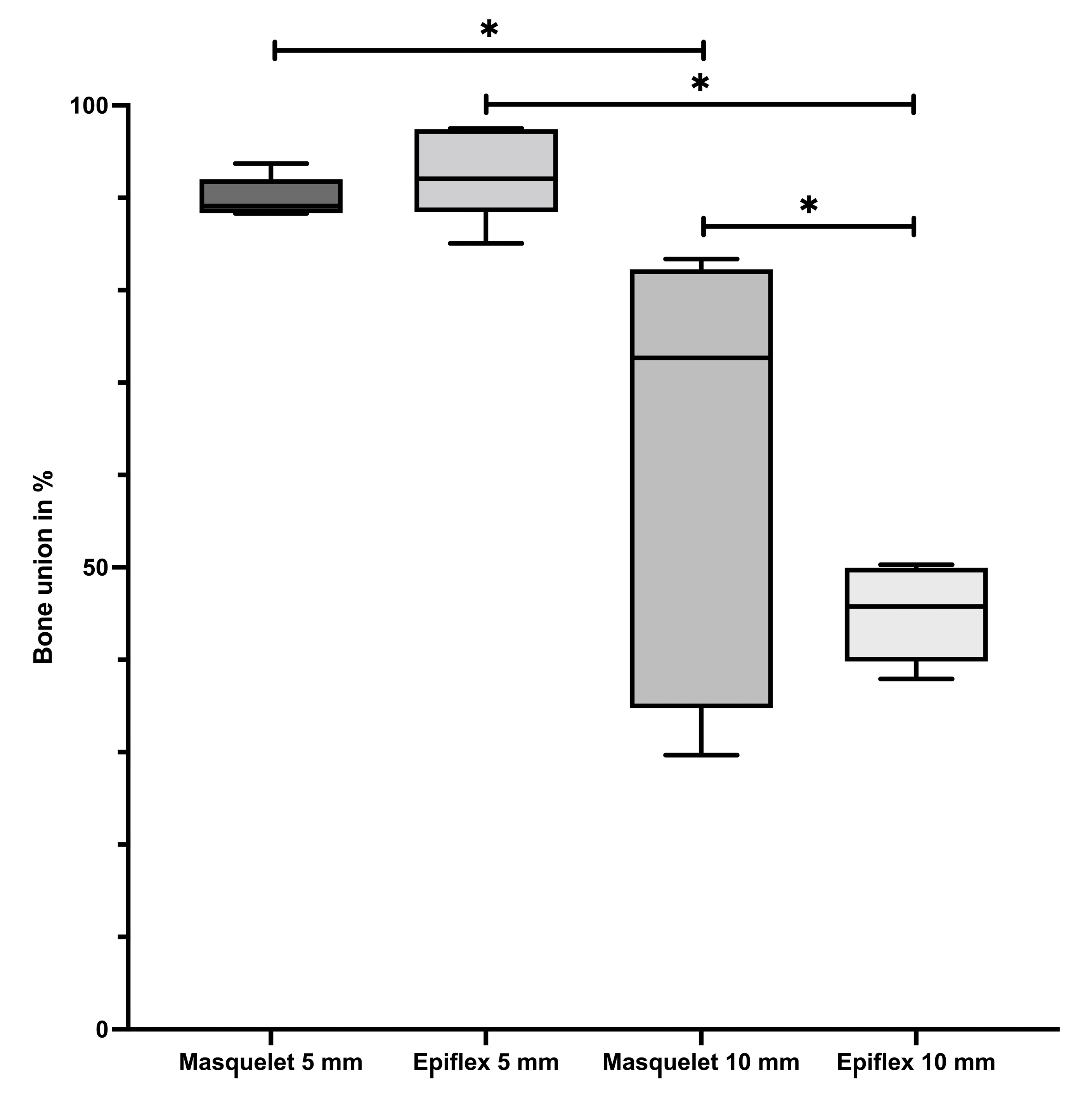
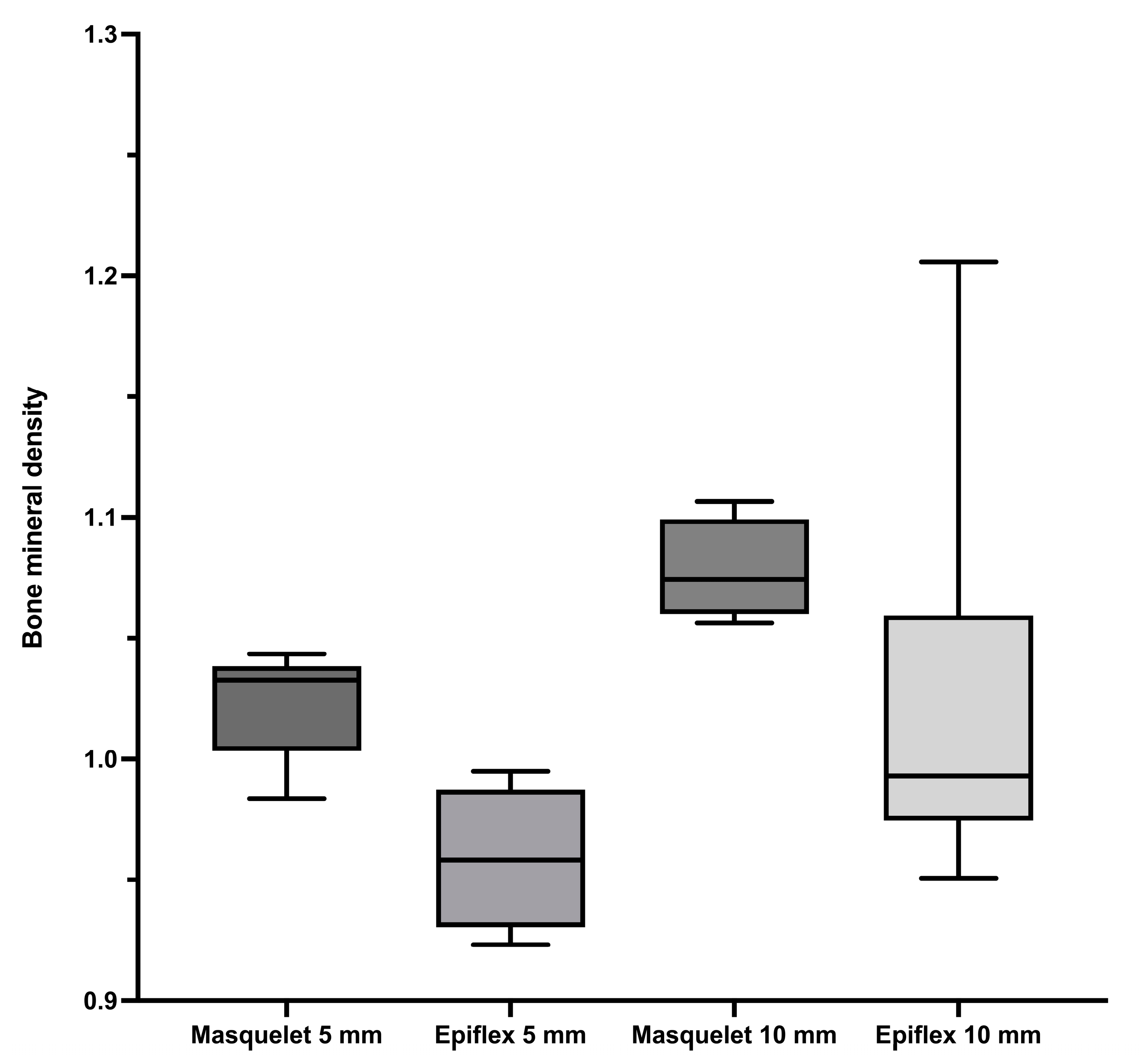
3.1. Bone Union and Bone Mineral Density
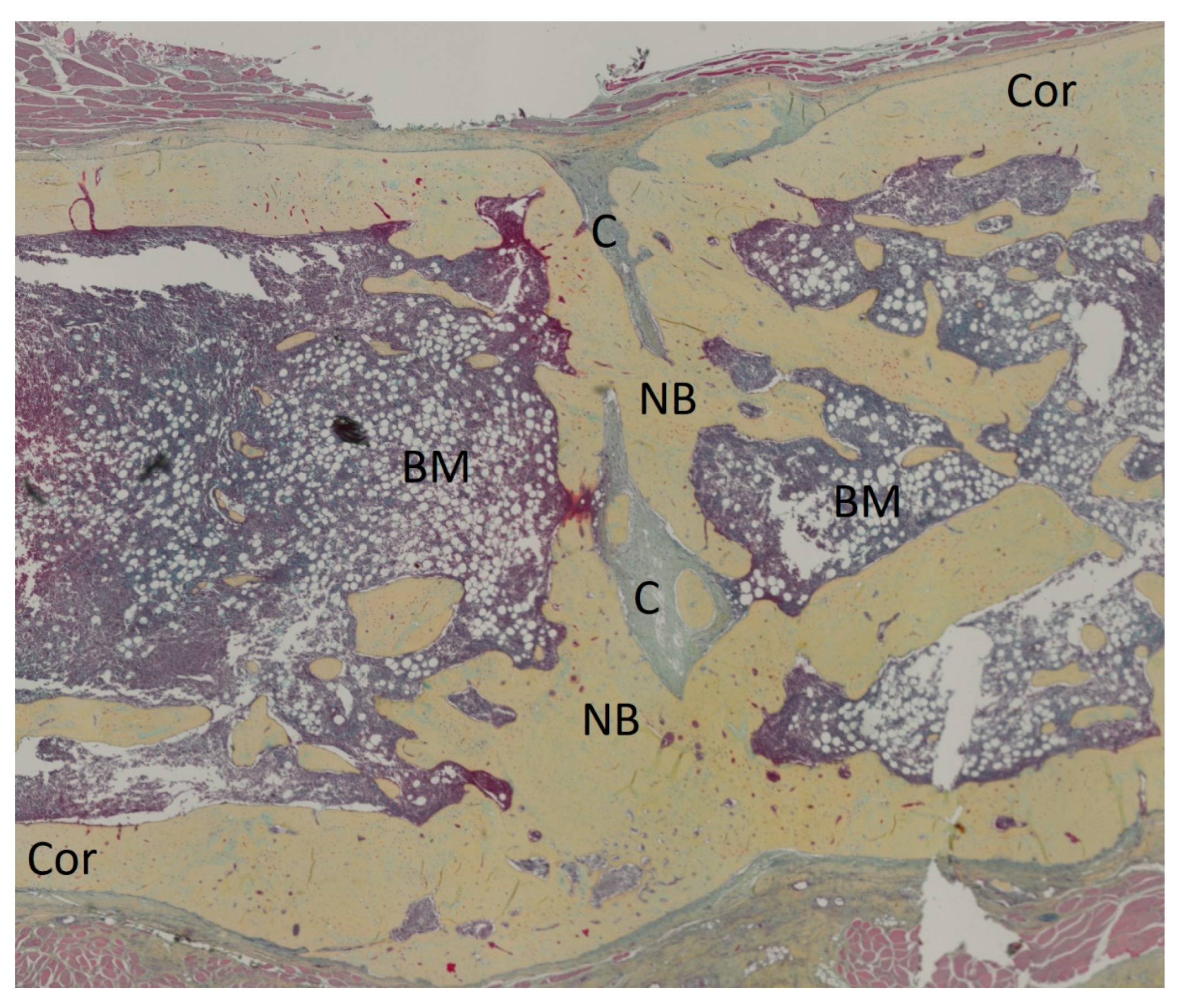
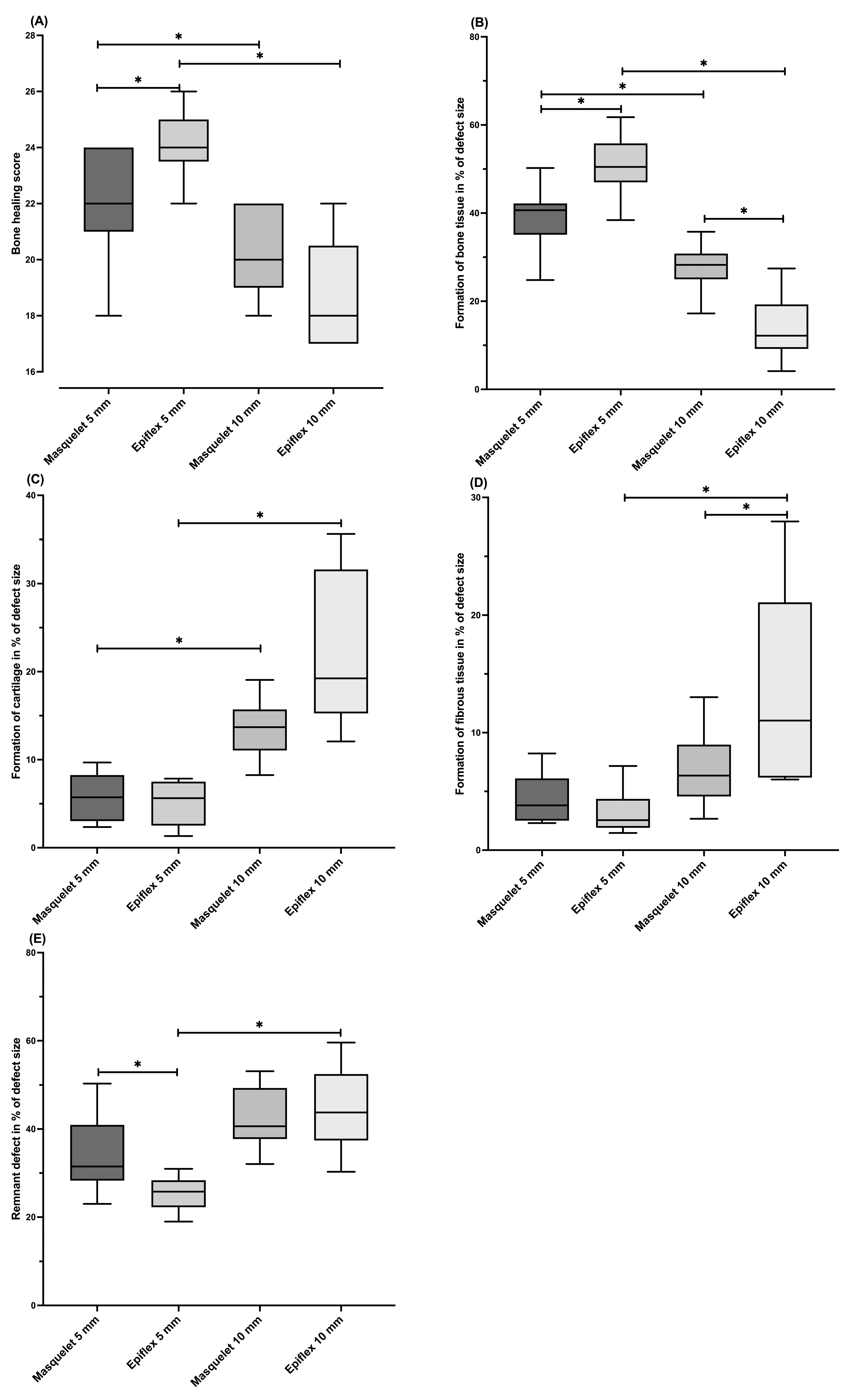
3.2. Histology and Bone Healing Score
3.3. Vascularisation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.F.; Simpson, A.H.; Robinson, C.M. The management of fractures with bone loss. J. Bone Jt. Surg. Br. 2005, 87, 142–150. [Google Scholar] [CrossRef]
- Bosse, M.J.; MacKenzie, E.J.; Kellam, J.F.; Burgess, A.R.; Webb, L.X.; Swiontkowski, M.F.; Sanders, R.W.; Jones, A.L.; McAndrew, M.P.; Patterson, B.M.; et al. A prospective evaluation of the clinical utility of the lower-extremity injury-severity scores. J. Bone Jt. Surg. Am. 2001, 83, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Karger, C.; Kishi, T.; Schneider, L.; Fitoussi, F.; Masquelet, A.C. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop. Traumatol. Surg. Res. 2012, 98, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Zimmermann, G.; Hammer, K.; Bruckner, T.; Grützner, P.A.; von Recum, J. Cigarette smoking influences the clinical and occupational outcome of patients with tibial shaft fractures. Injury 2011, 42, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Tzioupis, C.; Giannoudis, P.V. Prevalence of long-bone non-unions. Injury 2007, 38, S3–S9. [Google Scholar] [CrossRef]
- Norris, B.L.; Vanderkarr, M.; Sparks, C.; Chitnis, A.S.; Ray, B.; Holy, C.E. Treatments, cost and healthcare utilization of patients with segmental bone defects. Injury 2021, 52, 2935–2940. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: Any specific needs? Injury 2011, 42, S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am. 2010, 41, 27–37, table of contents. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 2000, 45, 346–353. [Google Scholar] [PubMed]
- Taylor, B.C.; French, B.G.; Fowler, T.T.; Russell, J.; Poka, A. Induced membrane technique for reconstruction to manage bone loss. J. Am. Acad. Orthop. Surg. 2012, 20, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, F.; Ceglia, M.J.; Bettini, L.; Bianco, S.; Buzzi, R.; Campanacci, D.A. Induced membrane technique using enriched bone grafts for treatment of posttraumatic segmental long bone defects. J. Orthop. Traumatol. 2019, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Pelissier, P.; Masquelet, A.C.; Bareille, R.; Pelissier, S.M.; Amedee, J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J. Orthop. Res. 2004, 22, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Gessmann, J.; Rosteius, T.; Baecker, H.; Sivalingam, K.; Peter, E.; Schildhauer, T.A.; Köller, M. Is the bioactivity of induced membranes time dependent? Eur. J. Trauma Emerg. Surg. 2022, 48, 3051–3061. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S.; Rissanen, T.T.; Vajanto, I.; Hartikainen, J. Vascular endothelial growth factors: Biology and current status of clinical applications in cardiovascular medicine. J. Am. Coll. Cardiol. 2007, 49, 1015–1026. [Google Scholar] [CrossRef]
- Verboket, R.D.; Leiblein, M.; Janko, M.; Schaible, A.; Brune, J.C.; Schröder, K.; Heilani, M.; Fremdling, C.; Busche, Y.; Irrle, T.; et al. From two stages to one: Acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur. J. Trauma Emerg. Surg. 2020, 46, 317–327. [Google Scholar] [CrossRef]
- Leiblein, M.; Kolb, T.; Christian, L.; Schröder, K.; Yaman, C.; Schaible, A.; Marzi, I.; Henrich, D.; Janko, M. Introduction of a New Surgical Method to Improve Bone Healing in a Large Bone Defect by Replacement of the Induced Membrane by a Human Decellularized Dermis Repopulated with Bone Marrow Mononuclear Cells in Rat. Materials 2020, 13, 2629. [Google Scholar] [CrossRef]
- Nau, C.; Simon, S.; Schaible, A.; Seebach, C.; Schröder, K.; Marzi, I.; Henrich, D. Influence of the induced membrane filled with syngeneic bone and regenerative cells on bone healing in a critical size defect model of the rat’s femur. Injury 2018, 49, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Leiblein, M.; Koch, E.; Winkenbach, A.; Schaible, A.; Nau, C.; Büchner, H.; Schröder, K.; Marzi, I.; Henrich, D. Size matters: Effect of granule size of the bone graft substitute (Herafill®) on bone healing using Masquelet’s induced membrane in a critical size defect model in the rat’s femur. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Rössner, E.; Smith, M.D.; Petschke, B.; Schmidt, K.; Vitacolonna, M.; Syring, C.; von Versen, R.; Hohenberger, P. Epiflex(®) a new decellularised human skin tissue transplant: Manufacture and properties. Cell Tissue Bank. 2011, 12, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Bhavsar, M.; Leppik, L.; Oliveira, K.M.C.; Barker, J.H. Histological Scoring Method to Assess Bone Healing in Critical Size Bone Defect Models. Tissue Eng. Part. C Methods 2018, 24, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Morelli, I.; Drago, L.; George, D.A.; Gallazzi, E.; Scarponi, S.; Romanò, C.L. Masquelet technique: Myth or reality? A systematic review and meta-analysis. Injury 2016, 47, S68–S76. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Marcucio, R.; Humphrey, C.; Colnot, C.; Knobe, M.; Harvey, E.J. Trauma-induced inflammation and fracture healing. J. Orthop. Trauma 2010, 24, 522–525. [Google Scholar] [CrossRef]
- Sathyendra, V.; Darowish, M. Basic science of bone healing. Hand Clin. 2013, 29, 473–481. [Google Scholar] [CrossRef]
- Alford, A.I.; Nicolaou, D.; Hake, M.; McBride-Gagyi, S. Masquelet’s induced membrane technique: Review of current concepts and future directions. J. Orthop. Res. 2021, 39, 707–718. [Google Scholar] [CrossRef]
- Cuthbert, R.J.; Churchman, S.M.; Tan, H.B.; McGonagle, D.; Jones, E.; Giannoudis, P.V. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone 2013, 57, 484–492. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Wilhelm, K.; Barker, J.H.; Marzi, I. Endothelial progenitor cells improve directly and indirectly early vascularization of mesenchymal stem cell-driven bone regeneration in a critical bone defect in rats. Cell Transpl. 2012, 21, 1667–1677. [Google Scholar] [CrossRef]
- Nau, C.; Henrich, D.; Seebach, C.; Schröder, K.; Barker, J.H.; Marzi, I.; Frank, J. Tissue engineered vascularized periosteal flap enriched with MSC/EPCs for the treatment of large bone defects in rats. Int. J. Mol. Med. 2017, 39, 907–917. [Google Scholar] [CrossRef][Green Version]
- Verboket, R.D.; Irrle, T.; Busche, Y.; Schaible, A.; Schröder, K.; Brune, J.C.; Marzi, I.; Nau, C.; Henrich, D. Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells 2021, 10, 1249. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Meier, S.; Nau, C.; Bonig, H.; Marzi, I. Safety and feasibility of cell-based therapy of autologous bone marrow-derived mononuclear cells in plate-stabilized proximal humeral fractures in humans. J. Transl. Med. 2016, 14, 314. [Google Scholar] [CrossRef]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schroder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2018, 12, 653–666. [Google Scholar] [CrossRef]


| Group Number: | Histology | µ-CT | α-SMA | BMD |
|---|---|---|---|---|
| (1). Masq5 (Masquelet + 5 mm defect) | 11 | 5 | 12 | 5 |
| (2). HAD5 (HAD + 5 mm defect) | 10 | 5 | 11 | 4 |
| (3). Masq10 (Masquelet + 10 mm defect) | 13 | 8 | 7 | 4 |
| (4). HAD10 (HAD + 10 mm defect) | 6 | 7 | 7 | 6 |
| Score Value | Newly Built Bone | Newly Built Cartilage | Newly Built Fibrous Tissue | Remaining Defect Size |
|---|---|---|---|---|
| 0 | No visible formation | No visible formation | Fully filled with fibrous tissue | 100% |
| 1 | <10% | <10% | <90% | <90% |
| 2 | <20% | <20% | <80% | <80% |
| 3 | <30% | <30% | <70% | <70% |
| 4 | <40% | <40% | <60% | <60% |
| 5 | <50% | <50% | <50% | <50% |
| 6 | <60% | <60% | <40% | <40% |
| 7 | <70% | <70% | <30% | <30% |
| 8 | <80% | <80% | <20% | <20% |
| 9 | <90% | <90% | <10% | <10% |
| 10 | Fully healed | Fully healed | No visible formation | No remaining defect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kammerer, A.; Hartmann, F.A.; Nau, C.; Leiblein, M.; Schaible, A.; Neijhoft, J.; Henrich, D.; Verboket, R.; Janko, M. The Impact of Defect Size on Bone Healing in Critical-Size Bone Defects Investigated on a Rat Femur Defect Model Comparing Two Treatment Methods. Bioengineering 2024, 11, 287. https://doi.org/10.3390/bioengineering11030287
Kammerer A, Hartmann FA, Nau C, Leiblein M, Schaible A, Neijhoft J, Henrich D, Verboket R, Janko M. The Impact of Defect Size on Bone Healing in Critical-Size Bone Defects Investigated on a Rat Femur Defect Model Comparing Two Treatment Methods. Bioengineering. 2024; 11(3):287. https://doi.org/10.3390/bioengineering11030287
Chicago/Turabian StyleKammerer, Andreas, Frederik Alexander Hartmann, Christoph Nau, Maximilian Leiblein, Alexander Schaible, Jonas Neijhoft, Dirk Henrich, René Verboket, and Maren Janko. 2024. "The Impact of Defect Size on Bone Healing in Critical-Size Bone Defects Investigated on a Rat Femur Defect Model Comparing Two Treatment Methods" Bioengineering 11, no. 3: 287. https://doi.org/10.3390/bioengineering11030287
APA StyleKammerer, A., Hartmann, F. A., Nau, C., Leiblein, M., Schaible, A., Neijhoft, J., Henrich, D., Verboket, R., & Janko, M. (2024). The Impact of Defect Size on Bone Healing in Critical-Size Bone Defects Investigated on a Rat Femur Defect Model Comparing Two Treatment Methods. Bioengineering, 11(3), 287. https://doi.org/10.3390/bioengineering11030287






