Regulation of Oxygen Tension as a Strategy to Control Chondrocytic Phenotype for Cartilage Tissue Engineering and Regeneration
Abstract
1. Introduction
2. Cartilage Repair Methods
3. Tissue Engineering of Cartilage
4. Environmental Factors as Inducers of Cartilage Formation
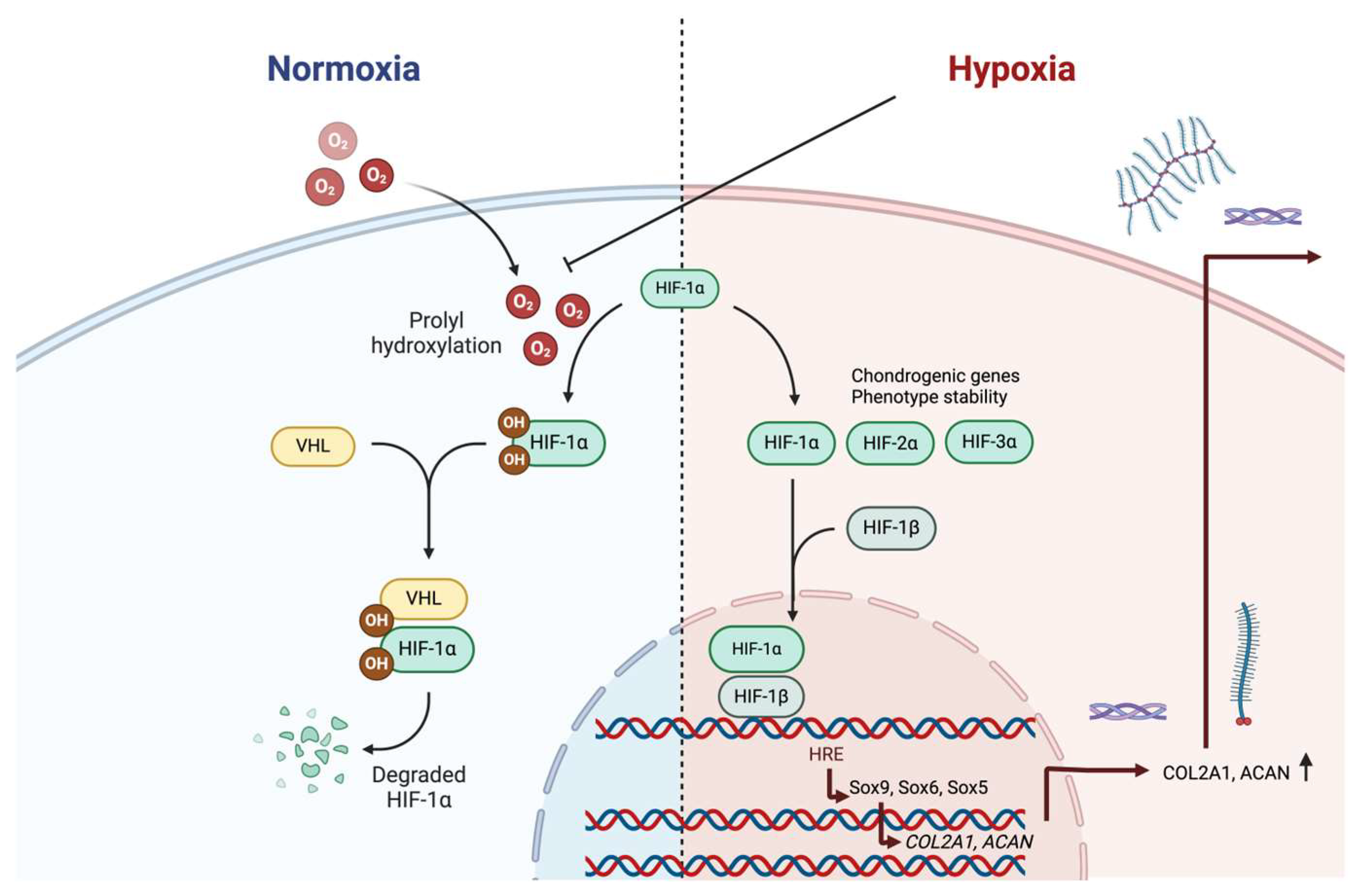
5. Low Oxygen Effects on Chondrocytes and Chondrogenic Phenotype
5.1. Low Oxygen Tension Effects on Chondrocytes of Articular Cartilage
5.2. Low Oxygen Tension Effects on Stem Cells or Progenitor Cells
5.3. Low Oxygen Tension in Cartilage Tissue-Engineering
5.4. Low Oxygen Tension Related Extracellular Vesicles and Non-Coding RNAs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACAN | Aggrecan |
| COL2A1 | Type II collagen |
| ECM | Extracellular matrix |
| DIAS | Dermis-isolated adult stem cells |
| EVs | Extracellular vesicles |
| FAK | Focal adhesion kinase |
| HIF | Hypoxia-inducible transcription factor |
| hiPSC | Human induced pluripotent stem cell |
| HRE | Hypoxia response element |
| MSC | Mesenchymal stem cell |
| OA | Osteoarthritis |
| PCM | Pericellular matrix |
| RhoA | Ras homolog family member A |
| ROCK | Rho associated coiled-coil containing protein kinase |
| Sox5 | Sex-determining region Y-box 5 protein |
| Sox6 | Sex-determining region Y-box 6 protein |
| Sox9 | Sex-determining region Y-box 9 protein |
| TGF-β | Transforming growth factor β |
| VHL | von Hippel-Landau protein |
| YAP | Yes-associated protein |
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Johnson, V.L.; Hunter, D.J. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef]
- Turkiewicz, A.; Petersson, I.F.; Björk, J.; Hawker, G.; Dahlberg, L.E.; Lohmander, L.S.; Englund, M. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthr. Cartil. 2014, 22, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Roos, E.M. A pragmatic approach to prevent post-traumatic osteoarthritis after sport or exercise-related joint injury. Best Pract. Res. Clin. Rheumatol. 2019, 33, 158–171. [Google Scholar] [CrossRef]
- Küpper, J.C.; Zandiyeh, P.; Ronsky, J.L. Empirical joint contact mechanics: A comprehensive review. Proc. Inst. Mech. Eng. H 2023, 237, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Richmond, S.A.; Fukuchi, R.K.; Ezzat, A.; Schneider, K.; Schneider, G.; Emery, C.A. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J. Orthop. Sports Phys. Ther. 2013, 43, 515-B19. [Google Scholar] [CrossRef]
- Allen, K.D.; Choong, P.F.; Davis, A.M.; Dowsey, M.M.; Dziedzic, K.S.; Emery, C.; Hunter, D.J.; Losina, E.; Page, A.E.; Roos, E.M.; et al. Osteoarthritis: Models for appropriate care across the disease continuum. Best Pract. Res. Clin. Rheumatol. 2016, 30, 503–535. [Google Scholar] [CrossRef]
- Li, S.; Cao, P.; Chen, T.; Ding, C. Latest insights in disease-modifying osteoarthritis drugs development. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X1169839. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xi, L.; Yu, M.; Fan, Z.; Wang, W.; Ju, A.; Liang, Z.; Zhou, G.; Ren, W. Regeneration of articular cartilage defects: Therapeutic strategies and perspectives. J. Tissue Eng. 2023, 14, 20417314231164765. [Google Scholar] [CrossRef] [PubMed]
- Stefanik, J.J.; Guermazi, A.; Roemer, F.W.; Peat, G.; Niu, J.; Segal, N.A.; Lewis, C.E.; Nevitt, M.; Felson, D.T. Changes in patellofemoral and tibiofemoral joint cartilage damage and bone marrow lesions over 7 years: The Multicenter Osteoarthritis Study. Osteoarthr. Cartil. 2016, 24, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Gress, K.; Charipova, K.; An, D.; Hasoon, J.; Kaye, A.D.; Paladini, A.; Varrassi, G.; Viswanath, O.; Abd-Elsayed, A.; Urits, I. Treatment recommendations for chronic knee osteoarthritis. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 369–382. [Google Scholar] [CrossRef]
- Price, A.J.; Alvand, A.; Troelsen, A.; Katz, J.N.; Hooper, G.; Gray, A.; Carr, A.; Beard, D. Knee replacement. Lancet 2018, 392, 1672–1682. [Google Scholar] [CrossRef]
- Rönn, K.; Reischi, N.; Gautier, E.; Jacobi, M. Current surgical treatment of knee osteoarthritis. Arthritis 2011, 2011, 454873. [Google Scholar] [CrossRef]
- Hafkamp, F.J.; Gosens, T.; de Vries, J.; den Oudsten, B.L. Do dissatisfied patients have unrealistic expectations? A systematic review and best-evidence synthesis in knee and hip arthroplasty patients. EFORT Open Rev. 2020, 5, 226–240. [Google Scholar] [CrossRef]
- Pettersson, M.; Kelk, P.; Belibasakis, G.N.; Bylund, D.; Molin Thorén, M.; Johansson, A. Titanium ions form particles that activate and execute interleukin-1β release from lipopolysaccharide-primed macrophages. J. Periodontal Res. 2017, 52, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and complimentary therapies in osteoarthritis and cartilage repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.L.; Kuiper, N.J. Regenerative medicine: A review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Ossendorff, R.; Walter, S.G.; Schildberg, F.A.; Spang, J.; Obudzinski, S.; Preiss, S.; Schneider, S.; Salzmann, G.M. Biologic principles of minced cartilage implantation: A narrative review. Arch. Ortop. Trauma Surg. 2023, 143, 3259–3269. [Google Scholar] [CrossRef] [PubMed]
- Hangody, L.; Kish, G.; Kárpáti, Z.; Szerb, I.; Udvarhelyi, I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg. Sports Traumatol. Arthrosc. 1997, 5, 262–267. [Google Scholar] [CrossRef]
- Richter, D.L.; Tanksley, J.A.; Miller, M.D. Osteochondral autograft transplantation: A review of the surgical technique and outcomes. Sports Med. Arthrosc. Rev. 2016, 24, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, G.E.; Mankin, H.J. Transplantation of osteochondral allografts. Annu. Rev. Med. 1984, 35, 311–324. [Google Scholar] [CrossRef]
- Beer, A.J.; Tauro, T.M.; Redondo, M.L.; Christian, D.R.; Cole, B.J.; Frank, R.M. Use of allografts in orthopaedic surgery: Safety, procurement, storage, and outcomes. Orthop. J. Sports Med. 2019, 7, 2325967119891435. [Google Scholar] [CrossRef]
- Fisher, J.N.; Tessaro, I.; Bertocco, T.; Peretti, G.M.; Mangiavini, L. The application of stem cells from different tissues to cartilage repair. Stem Cells Int. 2017, 2017, 2761678. [Google Scholar] [CrossRef]
- Thompson, M.; Mei, S.H.J.; Wolfe, D.; Champagne, J.; Fergusson, D.; Stewart, D.J.; Sullivan, K.J.; Doxtator, E.; Lalu, M.; English, S.W.; et al. Cell therapy with intravascular administration of mesenchymal stem cells continues to be safe: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 19, 100249. [Google Scholar] [CrossRef]
- Barry, F. MSC therapy for osteoarthritis: An unfinished story. J. Orthop. Res. 2019, 37, 1229–1235. [Google Scholar] [CrossRef]
- Razak, H.R.B.A.; Corona, K.; Totlis, T.; Chan, L.Y.T.; Salreta, J.F.; Sleiman, O.; Vasso, M.; Baums, M.H. Mesenchymal stem cell implantation provides short-term clinical improvement and satisfactory cartilage restoration in patients with knee osteoarthritis but the evidence is limited: A systematic review performed by the early-osteoarthritis group of ESSKA-European knee associates section. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5306–5318. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent stem cell-based cell therapy—Promise and challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Deinsberger, J.; Reisinger, D.; Weber, B. Global trends in clinical trials involving pluripotent stem cells: A systematic multi-database analysis. NPJ Regen. Med. 2020, 5, 15. [Google Scholar] [CrossRef]
- Vats, A.; Bielby, R.C.; Tolley, N.; Dickinson, S.C.; Boccaccini, A.R.; Hollander, A.P.; Bishop, A.E.; Polak, J.M. Chondrogenic differentiation of human embryonic stem cells: The effect of the micro-environment. Tissue Eng. 2006, 12, 1687–1697. [Google Scholar] [CrossRef]
- Qu, C.; Lammi, M.J. Chondrocyte-specific gene expressions in human embryonic stem cells differentiated under feeder-free culture conditions. Curr. Regen. Med. 2017, 7, 54–63. [Google Scholar] [CrossRef]
- Qu, C.; Puttonen, K.A.; Lindeberg, H.; Ruponen, M.; Hovatta, O.; Koistinaho, J.; Lammi, M.J. Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture. Int. J. Biochem. Cell Biol. 2013, 45, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354 (Suppl. 1), SI32–SI34. [Google Scholar] [CrossRef] [PubMed]
- Morouço, P.; Fernandes, C.; Lattanzi, W. Challenges and innovations in osteochondral regeneration: Insights from biology and inputs from bioengineering toward the optimization of tissue engineering strategies. J. Funct. Biomater. 2012, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Lammi, M.J.; Piltti, J.; Prittinen, J.; Qu, C. Challenges in fabrication of tissue-engineered cartilage with correct cellular colonization and extracellular matrix assembly. Int. J. Mol. Sci. 2018, 19, 2700. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef] [PubMed]
- Benya, P.D.; Schaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Benya, P.D. Alterations in chondrocyte cytoskeletal architecture during phenotypic modulation by retinoic acid and dihydrocytochalasin B-induced reexpression. J. Cell Biol. 1988, 106, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Paschos, N.K.; Hu, J.C.; Athanasiou, K. Articular cartilage tissue engineering: The role of signaling molecules. Cell. Mol. Life Sci. 2016, 73, 1173–1194. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Jin, Y.-Q.; Liu, W.; Zhang, W.J.; Hong, T.-H.; Zhou, G.; Baggett, L.S.; Mikos, A.G.; Cao, Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008, 9, 60. [Google Scholar] [CrossRef]
- Vasara, A.I.; Hyttinen, M.M.; Pulliainen, O.; Lammi, M.J.; Jurvelin, J.S.; Peterson, L.; Lindahl, A.; Helminen, H.J.; Kiviranta, I. Immature porcine knee cartilage lesions show good healing with or without autologous chondrocyte transplantation. Osteoarthr. Cartil. 2006, 14, 1066–1074. [Google Scholar] [CrossRef][Green Version]
- Lammi, M.J. Cellular signaling in cartilage tissue engineering. Curr. Signal Transd. Ther. 2007, 2, 41–48. [Google Scholar] [CrossRef]
- Lefebvre, V.; Angelozzi, M.; Haseeb, A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef]
- Kim, B.; Bonassar, L.J. Understanding the influence of local physical stimuli on chondrocyte behavior. Adv. Exp. Med. Biol. 2023, 1402, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.T.; Bader, D.L.; Shelton, J.C.; Lee, D.A. Temporal regulation of chondrocyte metabolism in agarose constructs subjected to dynamic compression. Arch. Biochem. Biophys. 2003, 417, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Glatt, V.; Evans, C.H.; Stoddart, M.J. Regenerative rehabilitation: The role of mechanotransduction in orthopaedic regenerative medicine. J. Orthop. Res. 2019, 37, 1263–1269. [Google Scholar] [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cell: Implications for cartilage tissue engineering. J. Orthop. Res. 2018, 36, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; McNulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122. [Google Scholar] [CrossRef] [PubMed]
- Wuest, S.L.; Caliò, M.; Wernas, T.; Tanner, S.; Giger-Lange, C.; Wyss, F.; Ille, F.; Gantenbein, B.; Egli, M. Influence of mechanical unloading on articular chondrocyte dedifferentiation. Int. J. Mol. Sci. 2018, 19, 1289. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhou, Y.; Chen, X.; Han, L.; Wang, L.; Lu, X.L. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: Roles of calcium sources and cell membrane ion channels. J. Orthop. Res. 2018, 36, 730–738. [Google Scholar] [CrossRef]
- Fujii, Y.; Liu, L.; Yagasaki, L.; Inotsume, M.; Chiba, T.; Asahara, H. Cartilage homeostasis and osteoarthritis. Int. J. Mol. Sci. 2022, 23, 6316. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.B.; Looi, Q.H.; How, C.W.; Lee, S.H.; Al-Masawa, M.E.; Chong, P.P.; Law, J.X. Mesenchymal stem cell-derived exosomes and microRNAs in cartilage regeneration: Biogenesis, efficacy, miRNA enrichment and delivery. Pharmaceuticals 2021, 14, 1093. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wang, M.; Zhao, S.; Zhao, Z.; Fang, J. Mechanotransduction pathways in the regulation of cartilage chondrocyte homoestasis. J. Cell. Mol. Med. 2020, 24, 5408–5419. [Google Scholar] [CrossRef]
- Guilak, F.; Nims, R.J.; Dicks, A.; Wu, C.L.; Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018, 71–72, 40–50. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Bonnet, C.S.; Blain, E.J. Mechanical cues: Bidirectional reciprocity in the extracellular matrix drives mechano-signalling in articular cartilage. Int. J. Mol. Sci. 2021, 22, 13595. [Google Scholar] [CrossRef]
- Parkkinen, J.J.; Lammi, M.J.; Pelttari, A.; Helminen, H.J.; Tammi, M.; Virtanen, I. Altered Golgi apparatus in hydrostatically loaded articular cartilage chondrocytes. Ann. Rheum. Dis. 1993, 52, 192–198. [Google Scholar] [CrossRef]
- Parkkinen, J.J.; Lammi, M.J.; Inkinen, R.; Jortikka, M.; Tammi, M.; Virtanen, I.; Helminen, H.J. Influence of short-term hydrostatic pressure on organization of stress fibers in cultured chondrocytes. J. Orthop. Res. 1995, 13, 495–502. [Google Scholar] [CrossRef]
- Jortikka, M.O.; Parkkinen, J.J.; Inkinen, R.I.; Kärner, J.; Järveläinen, H.T.; Nelimarkka, L.O.; Tammi, M.I.; Lammi, M.J. The role of microtubules in the regulation proteoglycan synthesis in chondrocytes under hydrostatic pressure. Arch. Biochem. Biophys. 2000, 374, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.M.; Toyoda, T.; Lee, D.A.; Bader, D.L. Mechanical compression and hydrostatic pressure induce reversible changes in chondrocytes in agarose. J. Biomech. 2006, 39, 1547–1551. [Google Scholar] [CrossRef]
- Grandy, C.; Port, F.; Pfeil, J.; Azevedo Gonzalez-Oliva, M.; Vassalli, M.; Gottschalk, K.-E. Cell shape and tension alter focal adhesion structure. Biomater. Adv. 2023, 145, 213277. [Google Scholar] [CrossRef] [PubMed]
- Borbiro, I.; Rohacs, T. Regulation of Piezo channels by cellular signaling pathways. Curr. Top. Membr. 2017, 79, 245–261. [Google Scholar] [CrossRef]
- Yamashiro, S.; Rutkowski, D.M.; Lynch, K.A.; Liu, Y.; Vavylonis, D.; Watanabe, N. Force transmission by retrograde actin flow-induced dynamic molecular stretching of talin. Nat. Commun. 2023, 14, 8468. [Google Scholar] [CrossRef]
- Zhen, G.; Guo, Q.; Li, Y.; Wu, C.; Zhu, S.; Wang, R.; Guo, X.E.; Kim, B.C.; Huang, J.; Hu, Y.; et al. Mechanical stress determines the configuration of TGFβ activation in articular cartilage. Nat. Commun. 2021, 12, 1706. [Google Scholar] [CrossRef]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng. Part B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef] [PubMed]
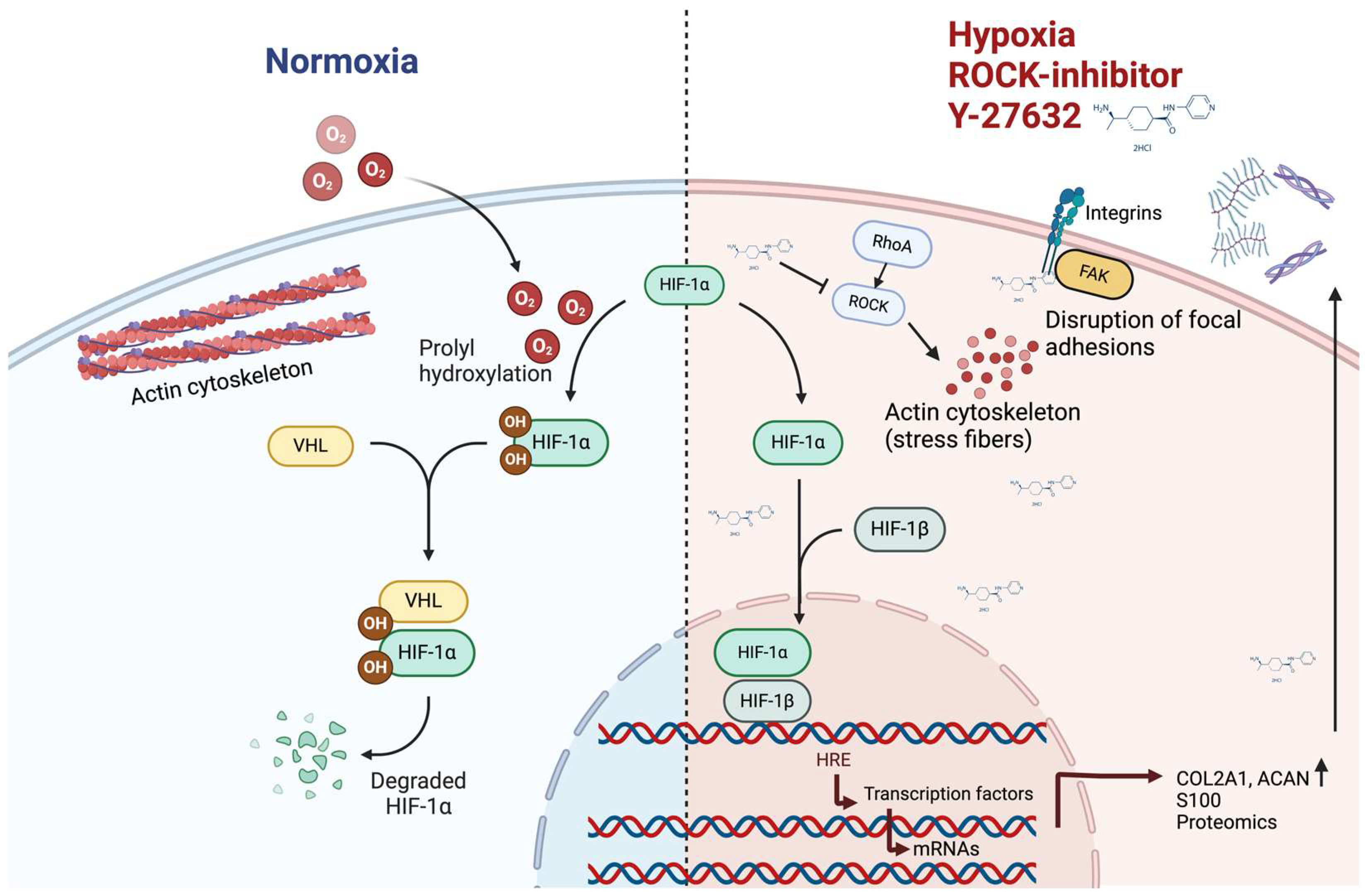
- Woods, A.; Wang, G.; Beier, F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 2005, 280, 11626–11634. [Google Scholar] [CrossRef]
- Matsumoto, E.; Furumatsu, T.; Kanazawa, T.; Tamura, M.; Ozaki, T. ROCK inhibitor prevents the dedifferentiation of human articular chondrocytes. Biochem. Biophys. Res. Commun. 2012, 420, 124–129. [Google Scholar] [CrossRef]
- Furumatsu, T.; Matsumoto-Ogawa, E.; Tanaka, T.; Lu, Z.; Ozaki, T. ROCK inhibition enhances aggrecan deposition and suppresses matrix metalloproteinase-3 production in human articular chondrocytes. Connect. Tissue Res. 2014, 55, 89–95. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Y.; Cheng, P.; Zhang, J.; Yang, C.; Pi, B.; Ye, Y.; You, H.; Chen, A.; Xu, T.; et al. YAP and ERK mediated mechanical strain-induced cell cycle progression through RhoA and cytoskeletal dynamics in rat growth plate chondrocytes. J. Orthop. Res. 2016, 34, 1121–1129. [Google Scholar] [CrossRef]
- Ruhlen, R.; Marberry, K. The chondrocyte primary cilium. Osteoarthr. Cartil. 2014, 22, 1071–1076. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, S.; Ling, H.; Zhang, C.; Kong, Y. Primary cilia: A cellular regulator of articular cartilage degeneration. Stem Cells Int. 2022, 2022, 2560441. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Leong, D.J.; Zhuo, Z.; Majeska, R.J.; Cardoso, L.; Spray, D.C.; Goldring, M.B.; Cobelli, N.J.; Sun, H.B. Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and downregulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthr. Cartil. 2016, 24, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Fu, S.; Ferreira, M.B.; Hou, Y.; Pearce, O.M.; Gavara, N.; Knight, M.M. YAP activation inhibits inflammatory signalling and cartilage breakdown associated with reduced primary cilia expression. Osteoarthr. Cartil. 2023, 31, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.B.; Urban, J.P. Evidence for a negative Pasteur effect in articular cartilage. Biochem. J. 1997, 321, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cui, Z.; Urban, J.P.G. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: A modeling study. Arthritis Rheum. 2004, 50, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Silver, I.A. Measurement of pH and ionic composition of pericellular sites. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1975, 271, 261–272. [Google Scholar] [CrossRef]
- Anderson, D.E.; Markway, B.D.; Bond, D.; McCarthry, H.E.; Johnstone, B. Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity. Stem Cell Res. Ther. 2016, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Gong, X.; Dou, C.; Cao, Z.; Dong, S. Redox control of chondrocyte differentiation and chondrogenesis. Free Radic. Biol. Med. 2019, 132, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Kim, H. A Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, J.; Yang, M.; Cai, J.; Fu, Q.; Ma, J.; Zhu, L. The mitochondrial unfolded protein response (UPRmt) protects against osteoarthritis. Exp. Mol. Med. 2022, 54, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Minguzzi, M.; Cetrullo, S.; D’Adamo, S.; Silvestri, Y.; Flamigni, F.; Borzi, R.M. Emerging players at the intersection of chondrocyte loss of maturational arrest, oxidative stress, senescence and low-grade inflammation in osteoarthritis. Oxid. Med. Cell Longev. 2018, 2018, 3075293. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Huang, L.E.; Arany, Z.; Livingston, D.M.; Bunn, H.F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J. Biol. Chem. 1996, 271, 32253–32259. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, M.I.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Heberstreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; Carmeliet, G. Hypoxia, hypoxia-incucible transcription factors and oxygen-sensing prolyl hydroxylases in bone development and homeostasis. Curr. Opin. Nephrol. Hypertens. 2019, 28, 328–335. [Google Scholar] [CrossRef]
- Slemc, L.; Kunej, T. Transcription factor HIF1A: Downstream targets, associated pathways, polymorphic hypoxia response element (HRE) sites, and initiative for standardization of reporting in scientific literature. Tumour Biol. 2016, 37, 14851–14861. [Google Scholar] [CrossRef]
- Khan, W.S.; Adesida, A.B.; Hardingham, T.E. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arhtritis Res. Ther. 2007, 9, R55. [Google Scholar] [CrossRef]
- Markway, B.D.; Cho, H.; Zilberman-Rudenko, J.; Holden, P.; McAlinden, A.; Johnstone, B. Hypoxia-inducible factor 3-alpha expression is associated with the stable chondrocyte phenotype. J. Orthop. Res. 2015, 33, 1561–1570. [Google Scholar] [CrossRef]
- Zhai, G. Alteration in metabolic pathways in osteoarthritis. Metabolites 2019, 9, 11. [Google Scholar] [CrossRef]
- Peansukmanee, S.; Vaughan-Thomas, A.; Carter, S.D.; Clegg, P.D.; Taylor, S.; Redmond, C.; Mobasheri, A. Effects of hypoxia on glucose transport in primary equine chondrocytes in vitro and evidence of reduced GLUT1 gene expression in pathologic cartilage in vivo. J. Orthop. Res. 2009, 27, 529–535. [Google Scholar] [CrossRef]
- Murphy, C.L.; Sambanis, A. Effect of oxygen tension and alginate encapsulation on restoration of the differentiated phenotype of passaged chondrocytes. Tissue Eng. 2001, 7, 791–803. [Google Scholar] [CrossRef]
- Murphy, C.L.; Polak, J.M. Control of human articular chondrocyte differentiation by reduced oxygen tension. J. Cell. Physiol. 2004, 199, 451–459. [Google Scholar] [CrossRef]
- Pfander, D.; Cramer, T.; Schipani, E.; Johnson, R.S. HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J. Cell Sci. 2003, 116, 1819–1826. [Google Scholar] [CrossRef]
- Henderson, J.H.; Ginley, N.; Caplan, A.I.; Niyibizi, C.; Dennis, J.E. Low oxygen tnsion during incubation periods of chondrocyte expansion is sufficient to enhance postexpansion chondrogenesis. Tissue Eng. A 2010, 16, 1585–1593. [Google Scholar] [CrossRef]
- Koh, S.; Purser, M.; Wysk, R.; Piedrahita, J.A. Improved chondrogenic potential and proteomic phenotype of porcine chondrocytes grown in optimized culture conditions. Cell. Reprogram. 2017, 19, 232–244. [Google Scholar] [CrossRef]
- Piltti, J.; Bygdell, J.; Qu, C.; Lammi, M.J. Effects of long-term low oxygen tension in human chondrosarcoma cells. J. Cell. Biochem. 2018, 119, 2320–2332. [Google Scholar] [CrossRef]
- Piltti, J.; Bygdell, J.; Fernadez-Ecchevarria, C.; Marcellino, D.; Lammi, M.J. Rho-kinase inhibitor Y-27632 and hypoxia synergistically enhance chondrocytic phenotype and modify S100 protein profiles in human chondrosarcoma cells. Sci. Rep. 2017, 7, 3708. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Kisiel, L.; Rintala-Dempsey, A.C.; Shaw, G.S. Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 2006, 396, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Yammani, R.R. S100 proteins in cartilage: Role in arthritis. Biochim. Biophys. Acta 2012, 1822, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.A.; Stevenson, S.; Goldberg, V.M. S-100 protein immunostaining identifies cells expressing a chondrocytic phenotype during articular cartilage repair. J. Orthop. Res. 1992, 10, 49–57. [Google Scholar] [CrossRef]
- Saito, T.; Ikeda, T.; Nakamura, K.; Chung, U.; Kawaguchi, H. S100A1 and S100B, transcriptional targets of SOX trio, inhibit terminal differentiation of chondrocytes. EMBO Rep. 2007, 8, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Romero, J.; Quintin, A.; Schoenholzer, E.; Pauli, C.; Despont, A.; Zumstein, M.A.; Kohl, S.; Nesic, D. S100A1 and S100B expression patterns identify differentiation status of human articular chondrocytes. J. Cell. Physiol. 2014, 229, 1106–1117. [Google Scholar] [CrossRef]
- Diaz-Romero, J.; Nesic, D. S100A1 and S100B: Calcium sensors at the cross-roads of multiple chondrogenic pathways. J. Cell. Physiol. 2017, 232, 1979–1987. [Google Scholar] [CrossRef]
- Gelse, K.; Ekici, A.B.; Cipa, F.; Swoboda, B.; Carl, H.D.; Olk, A.; Hennig, F.F.; Klinger, P. Molecular differentiation between osteophytic and articular cartilage—Clues for a transient and permanent chondrocyte phenotype. Osteoarthr. Cartil. 2012, 20, 162–171. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Lennon, D.P.; Edmison, J.M.; Caplan, A.I. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: Effects on in vitro and in vivo osteochondrogenesis. J. Cell. Physiol. 2001, 187, 345–355. [Google Scholar] [CrossRef]
- Malladi, P.; Xu, Y.; Chiou, M.; Giaccia, A.J.; Longaker, M.T. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am. J. Physiol. Cell. Physiol. 2006, 290, C1139–C1146. [Google Scholar] [CrossRef]
- Kanichai, M.; Ferguson, D.; Prendergast, P.J.; Campbell, V.A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: A role for AKT and hypoxia-inducible factor (HIF)-1alpha. J. Cell. Physiol. 2008, 216, 708–715. [Google Scholar] [CrossRef]
- Khan, W.S.; Adesida, A.B.; Tew, S.R.; Lowe, E.T.; Hardingham, T.E. Bone marrow-derived mesenchymal stem cells express the pericyte marker 3G5 in culture and show enhanced chondrogenesis in hypoxic conditions. J. Orthop. Res. 2010, 28, 834–840. [Google Scholar] [CrossRef]
- Wang, D.W.; Fermor, B.; Gimble, J.M.; Awad, H.A.; Guilak, F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J. Cell. Physiol. 2005, 204, 184–191. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Majore, I.; Kasper, C.; Hass, R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun. Signal. 2010, 8, 18. [Google Scholar] [CrossRef]
- Rosova, I.; Dao, M.; Capoccia, B.; Link, D.; Nolta, J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008, 26, 2173–2182. [Google Scholar] [CrossRef]
- Pattappa, G.; Johnstone, B.; Zellner, J.; Docheva, D.; Angele, P. The importance of physioxia in mesenchymal stem cell chondrogenesis and the mechanisms controlling its response. Int. J. Mol. Sci. 2019, 20, 484. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Benz, K.; Ahlers, M.; Gaissmaier, C.; Mollenhauer, J. Hypoxic condition durin expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transplant. 2011, 20, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Abesida, A.B.; Mulet-Sierra, A.; Jomha, N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012, 3, 9. [Google Scholar] [CrossRef]
- Ranera, B.; Remacha, A.R.; Álvarez-Arguedas, S.; Castiella, T.; Vásquez, F.J.; Romero, A.; Zaragoza, P.; Martín-Burriel, I.; Rodellar, C. Expansion under hypoxic conditions enhances the chondrogenic potential of equine bone marrow-derived mesenchymal stem cells. Vet. J. 2013, 195, 248–251. [Google Scholar] [CrossRef]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar] [CrossRef]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef]
- Song, L.; Tuan, R.S. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004, 18, 980–982. [Google Scholar] [CrossRef]
- Hellingham, C.A.; Koevoet, W.; van Osch, G.J.V.M. Can one generate stable hyaline cartilage from adult mesenchymal stem cells? A developmental approach. J. Tissue Eng. Regen. Med. 2012, 6, e1–e11. [Google Scholar] [CrossRef]
- Sheehy, E.J.; Buckley, C.T.; Kelly, D.J. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 417, 305–310. [Google Scholar] [CrossRef]
- Gawlitta, D.; van Rijen, M.H.P.; Schrijver, E.J.M.; Alblas, J.; Dhert, W.J.A. Hypoxia impedes hypertophic chondrogenesis of human multipotent stromal cells. Tissue Eng. Part A 2012, 18, 1957–1966. [Google Scholar] [CrossRef]
- Betre, H.; Ong, S.R.; Guilak, F.; Chilkoti, A.; Fermor, B.; Setton, L.A. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials 2006, 27, 91–99. [Google Scholar] [CrossRef]
- Koay, E.J.; Athanasiou, K.A. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthr. Cartil. 2008, 16, 1450–1456. [Google Scholar] [CrossRef]
- Boreström, C.; Simonsson, S.; Enochson, L.; Bigdeli, N.; Brantsing, C.; Ellerström, C.; Hyllner, J.; Lindahl, A. Footprint-free human induced pluripotent stem cells from articular cartilage with redifferentiation capacity: A first step toward a clinical-grade cell source. Stem Cells Transl. Med. 2014, 3, 433–447. [Google Scholar] [CrossRef]
- Liu, H.; Wu, C.; Zhao, H.; Zhang, F.; Zhao, G.; Lin, X.; Wang, S.; Wang, X.; Yu, F.; Ning, Y.; et al. The first human induced pluripotent stem cell line of Kashin-Beck disease reveals involvement of heparan sulfate proteoglycan biosynthesis and PPAR pathway. FEBS J. 2022, 289, 279–293. [Google Scholar] [CrossRef]
- Shinomura, S.; Inoue, H.; Arai, Y.; Nakagawa, S.; Fujii, Y.; Kishida, T.; Shin-Ya, M.; Ichimaru, S.; Tsuchida, S.; Mazda, O.; et al. Hypoxia promotes differentiation of pure cartilage from human induced pluripotent stem cells. Mol. Med. Rep. 2022, 26, 229. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Hangody, L.; Kish, G.; Kárpáti, Z.; Udvarhelyi, I.; Szigeti, I.; Bély, M. Mosaicplasty for the treatment of articular cartilage defects: Application in clinical practice. Orthopedics 1998, 21, 751–756. [Google Scholar] [CrossRef]
- Zelinka, A.; Roelofs, A.J.; Kandel, R.A.; de Bari, C. Cellular therapy and tissue engineering for cartilage repair. Osteoarthr. Cartil. 2022, 30, 1547–1560. [Google Scholar] [CrossRef]
- Carroll, S.F.; Buckley, C.T.; Kelly, D.J. Measuring and modeling oxygen transport and consumption in 3D hydrogels containing chondrocytes and stem cells of different origins. Front. Bioeng. Biotechnol. 2021, 9, 591126. [Google Scholar] [CrossRef]
- Malda, J.; Rouwkema, J.; Martens, D.E.; le Comte, E.P.; Kooy, F.K.; Tramper, J.; van Blitterswijk, C.A.; Riesle, J. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: Measurement and modeling. Biotechnol. Bioeng. 2004, 86, 9–18. [Google Scholar] [CrossRef]
- Li, S.; Oreffo, R.O.C.; Sengers, B.G.; Tare, R.S. The effect of oxygen tension on human articular chondrocytes matrix synthesis: Integration of experimental and computational approaches. Biotechnol. Bioeng. 2014, 111, 1876–1885. [Google Scholar] [CrossRef]
- Qu, C.; Lindeberg, H.; Ylärinne, J.H.; Lammi, M.J. Five percent oxygen tension is not beneficial for neocartilage formation in scaffold-free cell cultures. Cell Tissue Res. 2012, 348, 109–117. [Google Scholar] [CrossRef]
- Mauck, R.L.; Yuan, X.; Tuan, R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr. Cartil. 2006, 14, 179–189. [Google Scholar] [CrossRef]
- Thorpe, S.D.; Nagel, T.; Carroll, S.F.; Kelly, D.J. Modulating gradients in regulatory signals within mesenchymal stem cell seeded hydrogels: A novel strategy to engineer zonal articular cartilage. PLoS ONE 2013, 8, e60764. [Google Scholar] [CrossRef]
- Buckley, C.T.; Vinardell, T.; Kelly, D.J. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 1345–1354. [Google Scholar] [CrossRef]
- Sanchez-Adams, J.; Athanasiou, K. Dermis isolated stem cells for cartilage tissue engineering. Biomaterials 2012, 33, 109–119. [Google Scholar] [CrossRef]
- Mizuno, S.; Glowacki, J. Low oxygen tension enhances chondroinduction by demineralized bone matrix in human dermal fibroblasts in vitro. Cells Tissues Organs 2005, 180, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kalpakci, K.N.; Brown, W.E.; Hu, J.C.; Athanasiou, K. Cartilage tissue engineering using dermis isolated adult stem cells: The use of hypoxia during expansion versus chondrogenic differentiation. PLoS ONE 2014, 9, e98570. [Google Scholar] [CrossRef] [PubMed]
- vam de Wakker, S.I.; Meijers, F.M.; Sluijter, J.P.G.; Vader, P. Extracellular vesicle heterogeneity and its impact for regenerative medicine applications. Pharmacol. Rev. 2023, 75, 1043–1061. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-coding RNAs and their integrated networks. J. Integr. Bioinfom. 2019, 16, 20190027. [Google Scholar] [CrossRef]
- Lehmann, T.P.; Golik, M.; Olejnik, J.; Łukaszewska, M.; Markowska, D.; Drożdżyńska, M.; Kotecki, A.; Glowacki, M.; Jagodziński, P.P. Potential applications of using tissue-specific EVs in targeted therapy and vaccinology. Biomed. Pharmacother. 2023, 166, 115308. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res. Ther. 2018, 9, 320. [Google Scholar] [CrossRef]
- Gorgun, C.; Ceresa, D.; Lesage, R.; Villa, F.; Reverberi, D.; Balbi, C.; Santamaria, S.; Cortese, K.; Malatesta, P.; Geris, L.; et al. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials 2021, 269, 120633. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Translat. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Zhang, H.; Mei, Z.; Hongyi, Z.; Wu, Y.; Xu, W.; Ma, Y.; Yang, W.; Liang, Y.; Gu, T.; et al. An inducible long noncoding RNA, LncZFHX2, facilitates DNA repair to mediate osteoarthritis pathology. Redox Biol. 2023, 66, 102858. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lammi, M.J.; Qu, C. Regulation of Oxygen Tension as a Strategy to Control Chondrocytic Phenotype for Cartilage Tissue Engineering and Regeneration. Bioengineering 2024, 11, 211. https://doi.org/10.3390/bioengineering11030211
Lammi MJ, Qu C. Regulation of Oxygen Tension as a Strategy to Control Chondrocytic Phenotype for Cartilage Tissue Engineering and Regeneration. Bioengineering. 2024; 11(3):211. https://doi.org/10.3390/bioengineering11030211
Chicago/Turabian StyleLammi, Mikko J., and Chengjuan Qu. 2024. "Regulation of Oxygen Tension as a Strategy to Control Chondrocytic Phenotype for Cartilage Tissue Engineering and Regeneration" Bioengineering 11, no. 3: 211. https://doi.org/10.3390/bioengineering11030211
APA StyleLammi, M. J., & Qu, C. (2024). Regulation of Oxygen Tension as a Strategy to Control Chondrocytic Phenotype for Cartilage Tissue Engineering and Regeneration. Bioengineering, 11(3), 211. https://doi.org/10.3390/bioengineering11030211







