Optimization of the Ex Situ Biomethanation of Hydrogen and Carbon Dioxide in a Novel Meandering Plug Flow Reactor: Start-Up Phase and Flexible Operation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor Setup
2.2. Inoculum
2.3. Minimal Medium
2.4. Initial Phase
2.5. Flexible Phase
2.6. Analytical Methods
2.7. Microbial Community Analysis
3. Results and Discussion
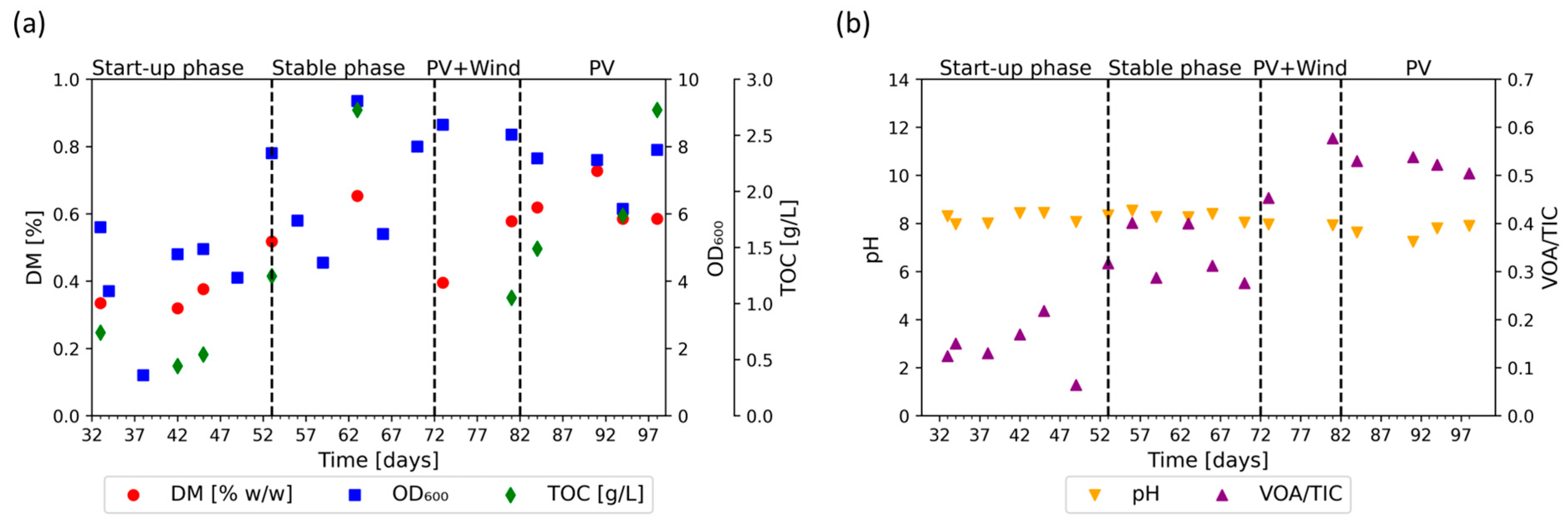
3.1. Initial Phase
3.2. Flexible Phase: Wind + PV Regime
3.3. Flexible Phase: PV Regime
3.4. Growth and Process Stability
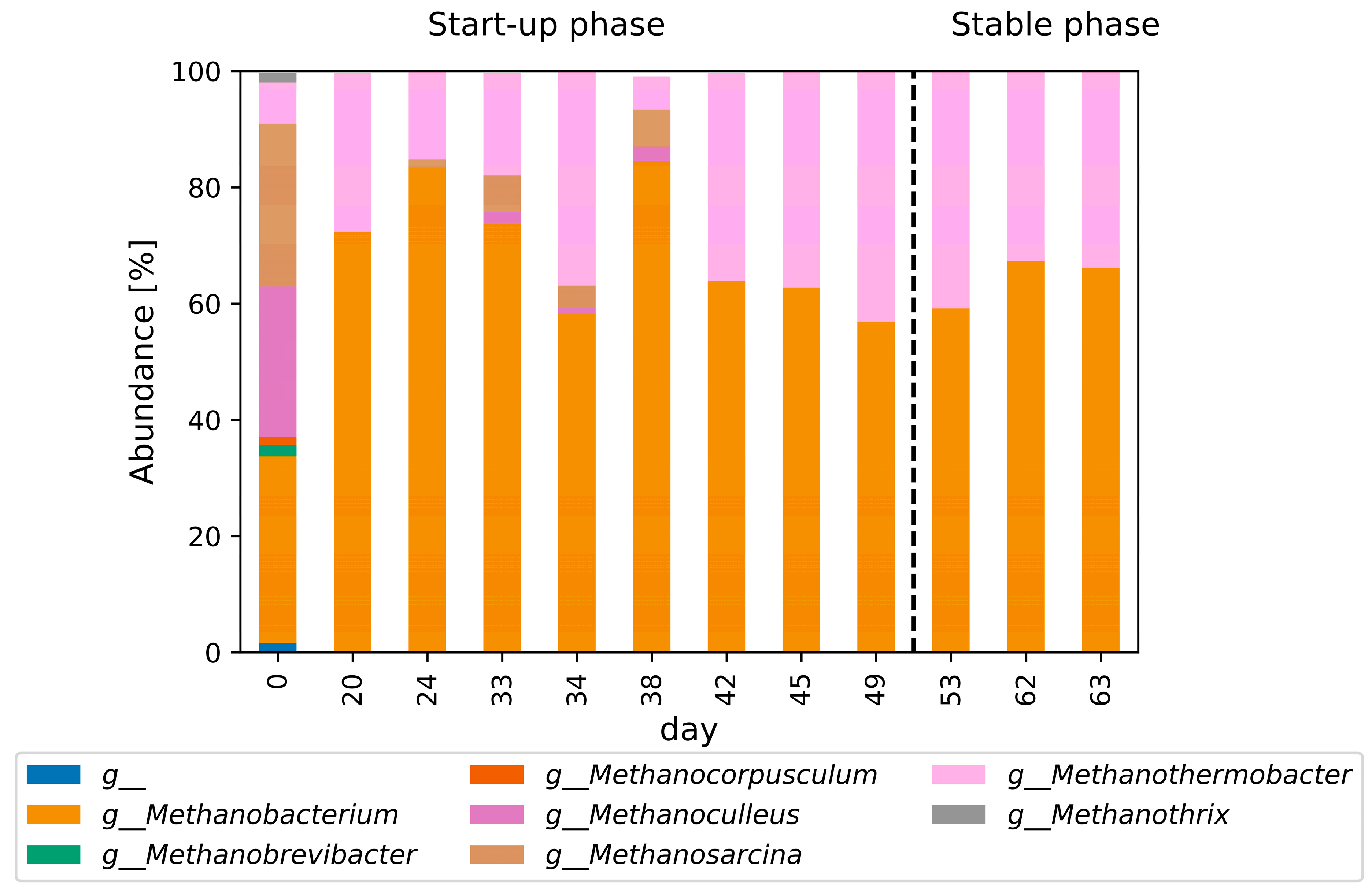
3.5. Microbial Community Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC Summary for Policymakers. Climate Change 2022: Impacts, Adaptation, and Vulnerability; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change 2022; IPCC: Geneva, Switzerland, 2022; in press.
- Electricity Market Report—July 2021. 2021. Available online: https://www.iea.org/reports/electricity-market-report-july-2021 (accessed on 26 June 2023).
- Johnson, S.C.; Rhodes, J.D.; Webber, M.E. Understanding the impact of non-synchronous wind and solar generation on grid stability and identifying mitigation pathways. Appl. Energy 2020, 262, 114492. [Google Scholar] [CrossRef]
- Maurer, F.; Rieke, C.; Schemm, R.; Stollenwerk, D. Analysis of an Urban Grid with High Photovoltaic and E-Mobility Penetration. Energies 2023, 16, 3380. [Google Scholar] [CrossRef]
- Zhang, M.; Millar, M.-A.; Yu, Z.; Yu, J. An Assessment of the Impacts of Heat Electrification on the Electric Grid in the UK. Energy Rep. 2022, 8, 14934–14946. [Google Scholar] [CrossRef]
- Rekioua, D. Energy Storage Systems for Photovoltaic and Wind Systems: A Review. Energies 2023, 16, 3893. [Google Scholar] [CrossRef]
- Hossain, E.; Faruque, H.; Sunny, M.; Mohammad, N.; Nawar, N. A Comprehensive Review on Energy Storage Systems: Types, Comparison, Current Scenario, Applications, Barriers, and Potential Solutions, Policies, and Future Prospects. Energies 2020, 13, 3651. [Google Scholar] [CrossRef]
- Zakeri, B.; Gissey, G.C.; Dodds, P.E.; Subkhankulova, D. Centralized vs. Distributed Energy Storage—Benefits for Residential Users. Energy 2021, 236, 121443. [Google Scholar] [CrossRef]
- Ogden, J.; Jaffe, A.M.; Scheitrum, D.; McDonald, Z.; Miller, M. Natural Gas as a Bridge to Hydrogen Transportation Fuel: Insights from the Literature. Energy Policy 2018, 115, 317–329. [Google Scholar] [CrossRef]
- You, Y.; Kim, S.; Lee, J.C. Comparative Study on Ammonia and Liquid Hydrogen Transportation Costs in Comparison to LNG. Int. J. Nav. Archit. Ocean. Eng. 2023, 15, 100523. [Google Scholar] [CrossRef]
- Evans, P.N.; Boyd, J.A.; Leu, A.O.; Woodcroft, B.J.; Parks, D.H.; Hugenholtz, P.; Tyson, G.W. An Evolving View of Methane Metabolism in the Archaea. Nat. Rev. Microbiol. 2019, 17, 219–232. [Google Scholar] [CrossRef]
- Van Hecke, W.; Bockrath, R.; De Wever, H. Effects of Moderately Elevated Pressure on Gas Fermentation Processes. Bioresour. Technol. 2019, 293, 122129. [Google Scholar] [CrossRef]
- Ale Enriquez, F.; Ahring, B.K. Strategies to Overcome Mass Transfer Limitations of Hydrogen during Anaerobic Gaseous Fermentations: A Comprehensive Review. Bioresour. Technol. 2023, 377, 128948. [Google Scholar] [CrossRef] [PubMed]
- Savvas, S.; Donnelly, J.; Patterson, T.; Chong, Z.S.; Esteves, S.R. Biological Methanation of CO2 in a Novel Biofilm Plug-Flow Reactor: A High Rate and Low Parasitic Energy Process. Appl. Energy 2017, 202, 238–247. [Google Scholar] [CrossRef]
- Logroño, W.; Popp, D.; Nikolausz, M.; Kluge, P.; Harms, H.; Kleinsteuber, S. Microbial Communities in Flexible Biomethanation of Hydrogen Are Functionally Resilient Upon Starvation. Front. Microbiol. 2021, 12, 619632. [Google Scholar] [CrossRef] [PubMed]
- Laguillaumie, L.; Rafrafi, Y.; Moya-Leclair, E.; Delagnes, D.; Dubos, S.; Spérandio, M.; Paul, E.; Dumas, C. Stability of Ex Situ Biological Methanation of H2/CO2 with a Mixed Microbial Culture in a Pilot Scale Bubble Column Reactor. Bioresour. Technol. 2022, 354, 127180. [Google Scholar] [CrossRef] [PubMed]
- Jønson, B.D.; Tsapekos, P.; Tahir Ashraf, M.; Jeppesen, M.; Ejbye Schmidt, J.; Bastidas-Oyanedel, J.-R. Pilot-Scale Study of Biomethanation in Biological Trickle Bed Reactors Converting Impure CO2 from a Full-Scale Biogas Plant. Bioresour. Technol. 2022, 365, 128160. [Google Scholar] [CrossRef] [PubMed]
- Strübing, D.; Moeller, A.B.; Mößnang, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. Anaerobic Thermophilic Trickle Bed Reactor as a Promising Technology for Flexible and Demand-Oriented H2/CO2 Biomethanation. Appl. Energy 2018, 232, 543–554. [Google Scholar] [CrossRef]
- Khesali Aghtaei, H.; Püttker, S.; Maus, I.; Heyer, R.; Huang, L.; Sczyrba, A.; Reichl, U.; Benndorf, D. Adaptation of a Microbial Community to Demand-Oriented Biological Methanation. Biotechnol. Biofuels 2022, 15, 125. [Google Scholar] [CrossRef]
- Hoffstadt, K.; Cheenakula, D.; Nikolausz, M.; Krafft, S.; Harms, H.; Kuperjans, I. Design and Construction of a New Reactor for Flexible Biomethanation of Hydrogen. Fermentation 2023, 9, 774. [Google Scholar] [CrossRef]
- Ashraf, M.T.; Yde, L.; Triolo, J.M.; Wenzel, H. Optimizing the Dosing and Trickling of Nutrient Media for Thermophilic Biomethanation in a Biotrickling Filter. Biochem. Eng. J. 2021, 176, 108220. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A Critical Review on Inhibition of Anaerobic Digestion Process by Excess Ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Liu, Y.; Beer, L.L.; Whitman, W.B. Methanogens: A Window into Ancient Sulfur Metabolism. Trends Microbiol. 2012, 20, 251–258. [Google Scholar] [CrossRef]
- Logroño, W.; Popp, D.; Kleinsteuber, S.; Sträuber, H.; Harms, H.; Nikolausz, M. Microbial Resource Management for Ex Situ Biomethanation of Hydrogen at Alkaline pH. Microorganisms 2020, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- SMARD|SMARD—Strommarktdaten, Stromhandel Und Stromerzeugung in Deutschland. Available online: https://www.smard.de/home (accessed on 11 April 2023).
- FLUIDAT® on the Net, Mass Flow and Physical Properties Calculations. Available online: https://fluidat.com/default.asp (accessed on 4 April 2023).
- Steinberg, L.M.; Regan, J.M. Phylogenetic Comparison of the Methanogenic Communities from an Acidic, Oligotrophic Fen and an Anaerobic Digester Treating Municipal Wastewater Sludge. Appl. Environ. Microbiol. 2008, 74, 6663–6671. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor Revision to V4 Region SSU rRNA 806R Gene Primer Greatly Increases Detection of SAR11 Bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Gerhard, E.; Butsch, B.M.; Marison, I.W.; Von Stockar, U. Improved Growth and Methane Production Conditions for Methanobacterium Thermoautotrophicum. Appl. Microbiol. Biotechnol. 1993, 40, 432–437. [Google Scholar] [CrossRef]
- Chen, H.; Gan, Q.; Fan, C. Methyl-Coenzyme M Reductase and Its Post-Translational Modifications. Front. Microbiol. 2020, 11, 578356. [Google Scholar] [CrossRef]
- Wintsche, B.; Jehmlich, N.; Popp, D.; Harms, H.; Kleinsteuber, S. Metabolic Adaptation of Methanogens in Anaerobic Digesters Upon Trace Element Limitation. Front. Microbiol. 2018, 9, 405. [Google Scholar] [CrossRef]
- Abdel Azim, A.; Pruckner, C.; Kolar, P.; Taubner, R.-S.; Fino, D.; Saracco, G.; Sousa, F.L.; Rittmann, S.K.-M.R. The Physiology of Trace Elements in Biological Methane Production. Bioresour. Technol. 2017, 241, 775–786. [Google Scholar] [CrossRef]
- Maharaj, B.C.; Mattei, M.R.; Frunzo, L.; Van Hullebusch, E.D.; Esposito, G. A General Framework to Model the Fate of Trace Elements in Anaerobic Digestion Environments. Sci. Rep. 2021, 11, 7476. [Google Scholar] [CrossRef]
- Dupnock, T.L.; Deshusses, M.A. Detailed Investigations of Dissolved Hydrogen and Hydrogen Mass Transfer in a Biotrickling Filter for Upgrading Biogas. Bioresour. Technol. 2019, 290, 121780. [Google Scholar] [CrossRef] [PubMed]
- Strübing, D.; Huber, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. High Performance Biological Methanation in a Thermophilic Anaerobic Trickle Bed Reactor. Bioresour. Technol. 2017, 245, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I.; Pérez, C.; Alfaro, N.; Fdz-Polanco, F. A Feasibility Study on the Bioconversion of CO2 and H2 to Biomethane by Gas Sparging through Polymeric Membranes. Bioresour. Technol. 2015, 185, 246–253. [Google Scholar] [CrossRef]
- Alfaro, N.; Fdz-Polanco, M.; Fdz-Polanco, F.; Díaz, I. Evaluation of Process Performance, Energy Consumption and Microbiota Characterization in a Ceramic Membrane Bioreactor for Ex-Situ Biomethanation of H2 and CO2. Bioresour. Technol. 2018, 258, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, A.; Niskanen, M.; Aura, E. Biocatalytic Methanation of Hydrogen and Carbon Dioxide in a Fixed Bed Bioreactor. Bioresour. Technol. 2015, 196, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, A.; Orellana, E.; Gaspari, M.; Kontogiannopoulos, K.; Treu, L.; Zouboulis, A.; Kougias, P.G. Comparative Study on Packing Materials for Improved Biological Methanation in Trickle Bed Reactors. Bioresour. Technol. 2023, 385, 129456. [Google Scholar] [CrossRef] [PubMed]
- Kamravamanesh, D.; Rinta Kanto, J.M.; Ali-Loytty, H.; Myllärinen, A.; Saalasti, M.; Rintala, J.; Kokko, M. Ex-Situ Biological Hydrogen Methanation in Trickle Bed Reactors: Integration into Biogas Production Facilities. Chem. Eng. Sci. 2023, 269, 118498. [Google Scholar] [CrossRef]
- Voelklein, M.A.; Rusmanis, D.; Murphy, J.D. Biological Methanation: Strategies for in-Situ and Ex-Situ Upgrading in Anaerobic Digestion. Appl. Energy 2019, 235, 1061–1071. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Tsapekos, P.; Peprah, M.; Kougias, P.; Zhu, X.; Kovalovszki, A.; Zervas, A.; Zha, X.; Jacobsen, C.S.; Angelidaki, I. Ex-Situ Biogas Upgrading in Thermophilic up-Flow Reactors: The Effect of Different Gas Diffusers and Gas Retention Times. Bioresour. Technol. 2021, 340, 125694. [Google Scholar] [CrossRef] [PubMed]
- European Commission; Joint Research Centre. Blending Hydrogen from Electrolysis into the European Gas Grid; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Mira, P.; Yeh, P.; Hall, B.G. Estimating Microbial Population Data from Optical Density. PLoS ONE 2022, 17, e0276040. [Google Scholar] [CrossRef] [PubMed]
- Bisutti, I.; Hilke, I.; Raessler, M. Determination of Total Organic Carbon—An Overview of Current Methods. TrAC Trends Anal. Chem. 2004, 23, 716–726. [Google Scholar] [CrossRef]
- Polag, D.; May, T.; Müller, L.; König, H.; Jacobi, F.; Laukenmann, S.; Keppler, F. Online Monitoring of Stable Carbon Isotopes of Methane in Anaerobic Digestion as a New Tool for Early Warning of Process Instability. Bioresour. Technol. 2015, 197, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the Effect of High Concentrations of Volatile Fatty Acids in Anaerobic Digestion on Methanogenic Communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Figeac, N.; Trably, E.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Temperature and Inoculum Origin Influence the Performance of Ex-Situ Biological Hydrogen Methanation. Molecules 2020, 25, 5665. [Google Scholar] [CrossRef] [PubMed]
- Breznak, J.A. The Genus Sporomusa. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer US: New York, NY, USA, 2006; pp. 991–1001. ISBN 978-0-387-25494-4. [Google Scholar]
- Dehning, I.; Stieb, M.; Schink, B. Sporomusa Malonica Sp. Nov., a Homoacetogenic Bacterium Growing by Decarboxylation of Malonate or Succinate. Arch. Microbiol. 1989, 151, 421–426. [Google Scholar] [CrossRef]
- Fortina, M.G.; Pukall, R.; Schumann, P.; Mora, D.; Parini, C.; Manachini, P.L.; Stackebrandt, E. Ureibacillus gen. nov., a New Genus to Accommodate Bacillus thermosphaericus (Andersson et al. 1995), Emendation of Ureibacillus thermosphaericus and Description of Ureibacillus terrenus sp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 1258. [Google Scholar] [CrossRef]
- Sträuber, H.; Bühligen, F.; Kleinsteuber, S.; Dittrich-Zechendorf, M. Carboxylic Acid Production from Ensiled Crops in Anaerobic Solid-State Fermentation—Trace Elements as pH Controlling Agents Support Microbial Chain Elongation with Lactic Acid. Eng. Life Sci. 2018, 18, 447–458. [Google Scholar] [CrossRef]
- Beppu, T.; Ueda, K. Symbiobacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–4. ISBN 978-1-118-96060-8. [Google Scholar]
- Oshima, K.; Ueda, K.; Beppu, T.; Nishida, H. Unique Evolution of Symbiobacterium Thermophilum Suggested from Gene Content and Orthologous Protein Sequence Comparisons. Int. J. Evol. Biol. 2011, 2011, 376831. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.B.; Kjeldsen, K.U.; Ingvorsen, K. Desulfitibacter Alkalitolerans Gen. Nov., Sp. Nov., an Anaerobic, Alkalitolerant, Sulfite-Reducing Bacterium Isolated from a District Heating Plant. Int. J. Syst. Evol. Microbiol. 2006, 56, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M.E.; Pavan, E.E.; Kämpfer, P.; Pettinari, M.J.; López, N.I. Coprothermobacteria. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; Wiley: Hoboken, NJ, USA, 2019; p. 1. ISBN 978-1-118-96060-8. [Google Scholar]
- Xu, J.; Bu, F.; Zhu, W.; Luo, G.; Xie, L. Microbial Consortiums of Hydrogenotrophic Methanogenic Mixed Cultures in Lab-Scale Ex-Situ Biogas Upgrading Systems under Different Conditions of Temperature, pH and CO. Microorganisms 2020, 8, 772. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wu, M.; Liu, H.; Wang, Z.; Guo, C.; Wang, S. Study on Enhancing Sludge Methanogenesis by Adding Acetylene Black and Effect on the Characteristics & Microbial Community of Anaerobic Granular Sludge. RSC Adv. 2019, 9, 23086–23095. [Google Scholar] [CrossRef] [PubMed]
- Litti, Y.V.; Kovalev, D.A.; Kovalev, A.A.; Merkel, A.Y.; Vishnyakova, A.V.; Russkova, Y.I.; Nozhevnikova, A.N. Auto-Selection of Microorganisms of Sewage Sludge Used as an Inoculum for Fermentative Hydrogen Production from Different Substrates. Int. J. Hydrogen Energy 2021, 46, 29834–29845. [Google Scholar] [CrossRef]
- Bedoya, K.; Niño, J.; Acero, J.; Jaimes-Prada, R.; Cabarcas, F.; Alzate, J.F. Metagenomic Analysis of Biocide-Treated Neotropical Oil Reservoir Water Unveils Microdiversity of Thermophile Tepidiphilus. Front. Microbiol. 2021, 12, 741555. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R. Methanothermobacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–8. ISBN 978-1-118-96060-8. [Google Scholar]
- Hendriksen, H.V.; Ahring, B.K. Effects of Ammonia on Growth and Morphology of Thermophilic Hydrogen-Oxidizing Methanogenic Bacteria. FEMS Microbiol. Ecol. 1991, 8, 241–245. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic Archaea: Ecologically Relevant Differences in Energy Conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef]
- Rosenberg, E.; DeLong, E.F.; Lory, S.; Stackebrandt, E.; Thompson, F. (Eds.) The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-38953-5. [Google Scholar]
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.-M.R. Archaea Biotechnology. Biotechnol. Adv. 2021, 47, 107668. [Google Scholar] [CrossRef]
- Peillex, J.-P.; Fardeau, M.-L.; Belaich, J.-P. Growth of Methanobacterium Thermoautotrophicum on H2-CO2: High CH4 Productivities in Continuous Culture. Biomass 1990, 21, 315–321. [Google Scholar] [CrossRef]
- Sinóros-Szabó, B.; Zavarkó, M.; Popp, F.; Grima, P.; Csedő, Z. Biomethane Production Monitoring and Data Analysis Based on the Practical Operation Experiences of an Innovative Power-to-Gas Benchscale Prototype. Acta Agrar. Debr. 2018, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R. Methanobacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–8. ISBN 978-1-118-96060-8. [Google Scholar]
- Porté, H.; Kougias, P.G.; Alfaro, N.; Treu, L.; Campanaro, S.; Angelidaki, I. Process Performance and Microbial Community Structure in Thermophilic Trickling Biofilter Reactors for Biogas Upgrading. Sci. Total Environ. 2019, 655, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Chellapandi, P.; Bharathi, M.; Sangavai, C.; Prathiviraj, R. Methanobacterium Formicicum as a Target Rumen Methanogen for the Development of New Methane Mitigation Interventions: A Review. Vet. Anim. Sci. 2018, 6, 86–94. [Google Scholar] [CrossRef]
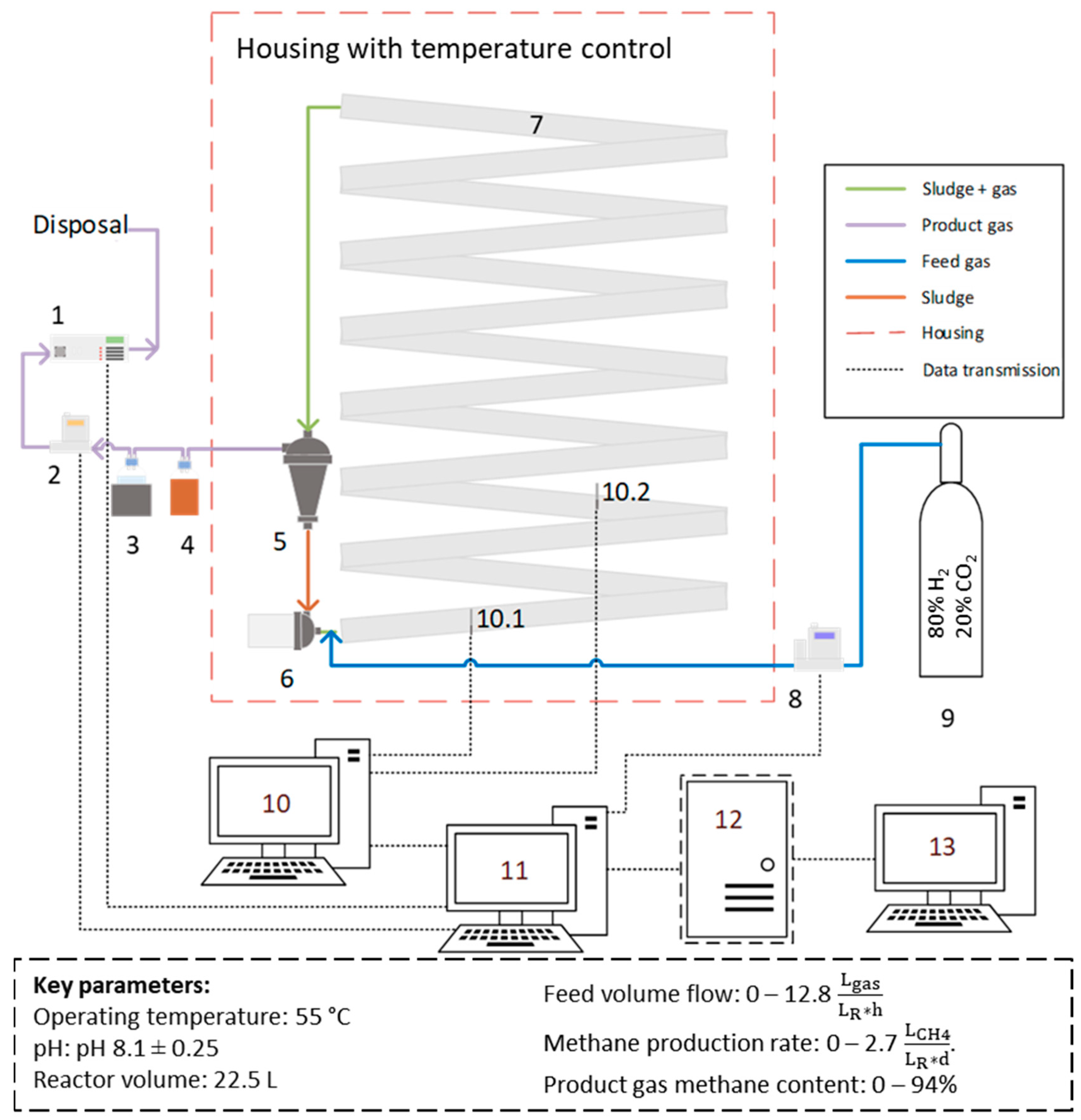
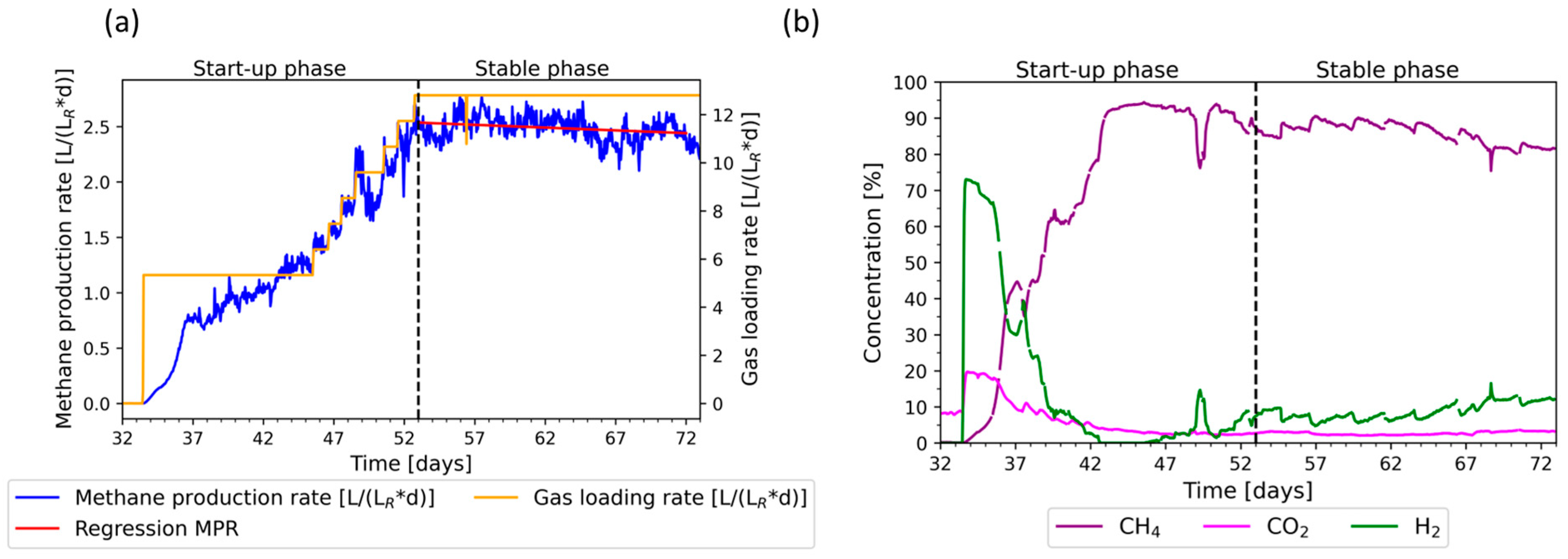
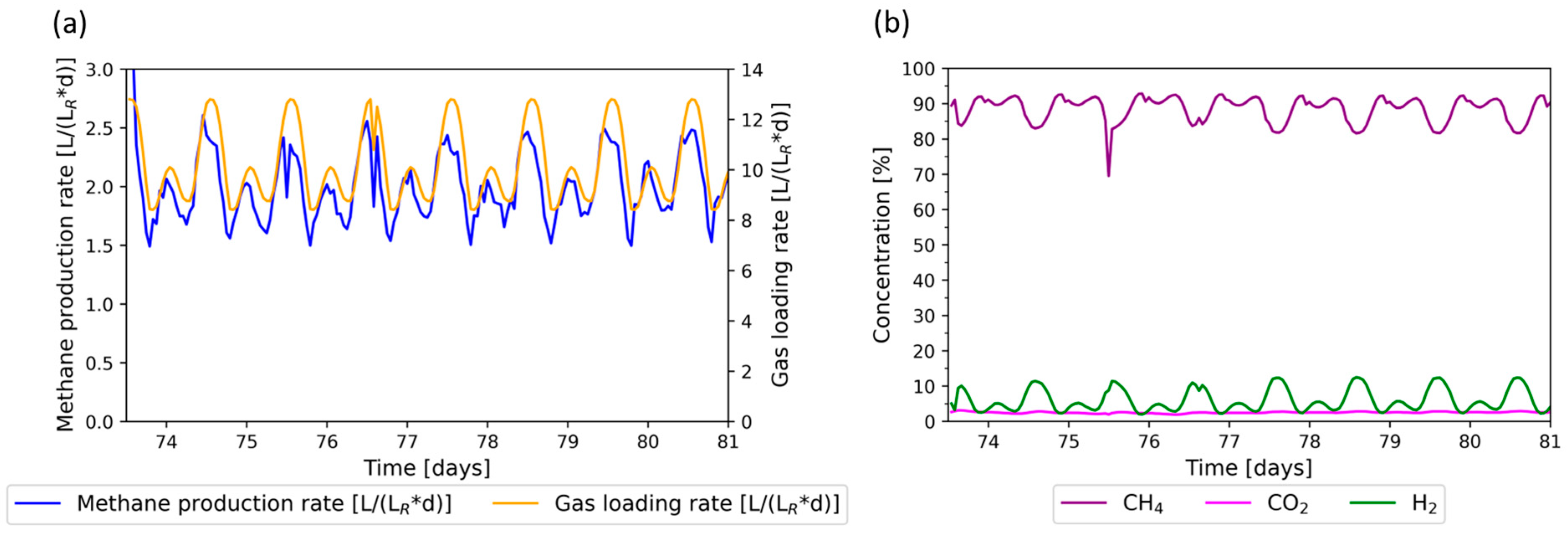
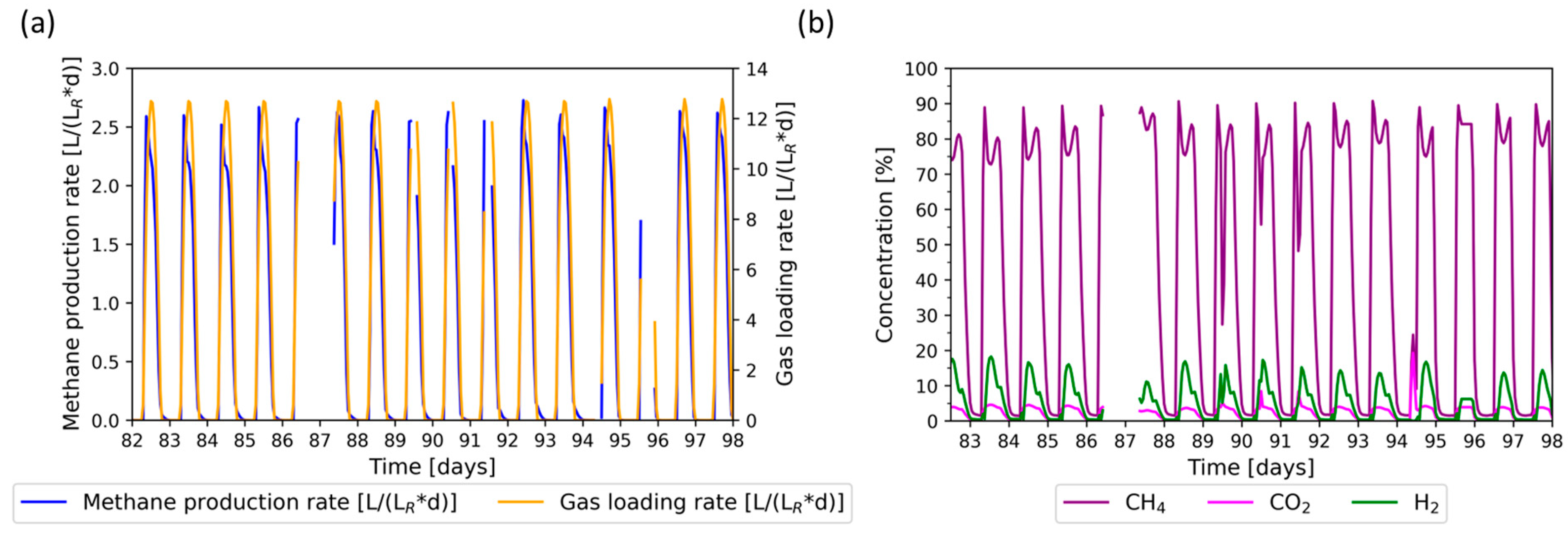

| Type | VR [L] | LRH2 [LH2/ (L × d)] | CH4 [%] | MPR [LCH4/(LR × d)] | Feed Ratio H2:CH4:CO2 | Recirculation (Liquid; Gas) | pH | Reference |
|---|---|---|---|---|---|---|---|---|
| HFM | 31 | 40.2 | 8.84 | 4:0:1 | no; 4.83 m3/d | [41] | ||
| HFM | 60 | 30 | 6.6 | 4:0:1 | 60 L/h; 17.7 m3/d | [42] | ||
| TBR 2 Stage | 2 1/2 2 | 7.2 | 90 | 1.73 | N.a. | 1 L/72 h; no | 6.9 1/6.7 2 | [43] |
| TBR | 58.1 | 62.1 | 98 | 15.4 | 4:0:1 | 10 L/h; no | ~7 | [40] |
| TBR | 1 | 12.8 | 95 | 3.13 | 4:0:1 | yes; no | 7–8 | [44] |
| TBR | 8.3 | 10.8 | 86 | 2.60 | 4:0:1 | 75 mL/min; no | 8.5–9.3 | [45] |
| TBR 2 Stage | 1000 1/1000 2 | 41 | 90 | 10 | 62:22:16 | 0.7 m3/(m3R × h); no | 8.5 | [17] |
| TBR | 58.1 | 52.5 | 97.5 | 13.1 | 3.78:0:1 | 3 L/h; no | 7 | [18] |
| BCR | 9.5 | 73.3 | 15 | 9.1 | 4:0:1 | No | 7.4 | [46] |
| BCR | 1 | 5.95 | 87 | 1.3 | 62:23:15 | no; 117 L/(LR × day) | 8.5 | [47] |
| BCR | 18 | 14.5 | 90 | 4 | 4.2:0:1 | no; 120 L/(L × d)] | 5.5–8 | [16] |
| PFR | 22.5 | 81–94 | 2.5 | 4:0:1 | 258 L/h; no | 8.1 | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffstadt, K.; Nikolausz, M.; Krafft, S.; Bonatelli, M.L.; Kumar, V.; Harms, H.; Kuperjans, I. Optimization of the Ex Situ Biomethanation of Hydrogen and Carbon Dioxide in a Novel Meandering Plug Flow Reactor: Start-Up Phase and Flexible Operation. Bioengineering 2024, 11, 165. https://doi.org/10.3390/bioengineering11020165
Hoffstadt K, Nikolausz M, Krafft S, Bonatelli ML, Kumar V, Harms H, Kuperjans I. Optimization of the Ex Situ Biomethanation of Hydrogen and Carbon Dioxide in a Novel Meandering Plug Flow Reactor: Start-Up Phase and Flexible Operation. Bioengineering. 2024; 11(2):165. https://doi.org/10.3390/bioengineering11020165
Chicago/Turabian StyleHoffstadt, Kevin, Marcell Nikolausz, Simone Krafft, Maria Letícia Bonatelli, Vivekanantha Kumar, Hauke Harms, and Isabel Kuperjans. 2024. "Optimization of the Ex Situ Biomethanation of Hydrogen and Carbon Dioxide in a Novel Meandering Plug Flow Reactor: Start-Up Phase and Flexible Operation" Bioengineering 11, no. 2: 165. https://doi.org/10.3390/bioengineering11020165
APA StyleHoffstadt, K., Nikolausz, M., Krafft, S., Bonatelli, M. L., Kumar, V., Harms, H., & Kuperjans, I. (2024). Optimization of the Ex Situ Biomethanation of Hydrogen and Carbon Dioxide in a Novel Meandering Plug Flow Reactor: Start-Up Phase and Flexible Operation. Bioengineering, 11(2), 165. https://doi.org/10.3390/bioengineering11020165







