Long-Term Histological Evaluation of a Novel Dermal Template in the Treatment of Pediatric Burns
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Material
2.3. Histological Assessment
2.4. Hematoxylin and Eosin
2.5. Elastica Van Gieson
2.6. Staining Protocol for Immunofluorescence
Antibodies
2.7. CD68
3. Results
3.1. Patients
3.2. Epidermal Compartment and Dermo-Epidermal Junction
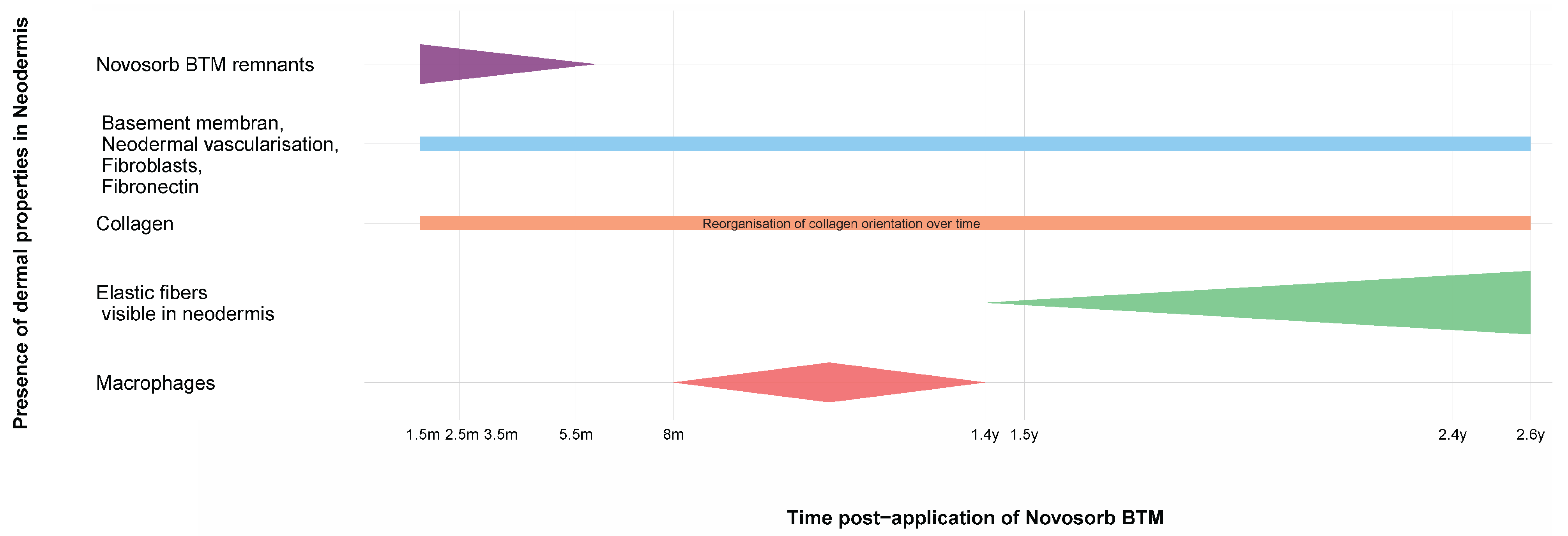
3.3. Dermal Compartment
- -
- NovoSorb® BTM remnants: Visible at early time points (up to 3.5 months) but completely resorbed by 5.5 months;
- -
- Basement membrane, dermal vascularization, migrated fibroblasts, and fibronectin: The basement membrane becomes continuous across the dermo-epidermal junction (DEJ), with consistent vascularization observed at all time points. Migrated fibroblasts were evenly distributed within the scaffold structure, and fibronectin was prominently detected along the DEJ;
- -
- Collagen fibers: First observed at 1.5 months, becoming increasingly visible up to 2.6 years with a dynamic change in organization pattern over time;
- -
- Elastic fibers: Increasingly visible within the neodermis at 1.4 years to 2.6 years;
- -
- Macrophages: Active in early phases around NovoSorb® BTM remnants, with activity diminishing as the dermis matures.
4. Discussion
4.1. Clinical Aspect
4.2. Histological Analysis
5. Conclusions
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, S.; Krishnamurthy, K. Histology, Skin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK537325/ (accessed on 1 November 2022).
- Greenwood, J. The evolution of acute burn care—Retiring the split skin graft. Ann. R. Coll. Surg. Engl. 2017, 99, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, C.; Neuhaus, K.; Meuli, M.; Farkas, M.; Hartmann-Fritsch, F.; Elrod, J.; Bressan, J.; Reichmann, E.; Böttcher-Haberzeth, S. Long-term outcomes of a cultured autologous dermo-epidermal skin substitute in children: 5 year results of a phase I clinical trial. J. Burn Care Res. 2024, irae150. [Google Scholar] [CrossRef]
- Meuli, M.; Hartmann-Fritsch, F.; Hüging, M.; Marino, D.; Saglini, M.; Hynes, S.; Neuhaus, K.; Manuel, E.; Middelkoop, E.; Reichmann, E.; et al. A Cultured Autologous Dermo-epidermal Skin Substitute for Full-Thickness Skin Defects: A Phase I, Open, Prospective Clinical Trial in Children. Plast. Reconstr. Surg. 2019, 144, 188–198. [Google Scholar] [CrossRef]
- Cheshire, P.A.; Herson, M.R.; Cleland, H.; Akbarzadeh, S. Artificial dermal templates: A comparative study of NovoSorbTM Biodegradable Temporising Matrix (BTM) and Integra® Dermal Regeneration Template (DRT). Burns 2016, 42, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Obed, D.; Bingöl, A.S.; März, V.; Vogt, P.M.; Krezdorn, N. Treatment of Complex Wounds with NovoSorb® Biodegradable Temporising Matrix (BTM)—A Retrospective Analysis of Clinical Outcomes. J. Pers. Med. 2022, 12, 2002. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Pham, T.N. Use of Dermal Regenerative Templates for Burns. J. Burn Care Res. 2023, 44 (Suppl. 1), S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Wu-Fienberg, Y.; Wu, S.S.; Gatherwright, J.; Chepla, K.J. An Alternative Dermal Template for Reconstruction of Complex Upper Extremity Wounds. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3674. [Google Scholar] [CrossRef] [PubMed]
- Tapking, C.; Thomas, B.F.; Hundeshagen, G.; Haug, V.F.M.; Gazyakan, E.; Bliesener, B.; Bigdeli, A.K.; Kneser, U.; Vollbach, F.H. NovoSorb® Biodegradable Temporising Matrix (BTM): What we learned from the first 300 consecutive cases. J. Plast. Reconstr. Aesthet. Surg. 2024, 92, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Wells, M.; Ascha, M.; Gatherwright, J.; Chepla, K.J. Upper Extremity Wounds Treated with Biodegradable Temporizing Matrix versus Collagen-Chondroitin Silicone Bilayer. J. Hand Microsurg. 2022, 15, 340–350. [Google Scholar] [CrossRef]
- Reconstruction of Complex Upper Extremity Wounds with Novosorb Biodegradable Temporizing Matrix Versus Integra Collagen-Chondroitin Silicone: A Cost Analysis. ePlasty 2024, 24, e38. Available online: https://www.hmpgloballearningnetwork.com/site/eplasty/original-research/reconstruction-complex-upper-extremity-wounds-novosorb-biodegradable (accessed on 9 October 2024).
- Wagstaff, M.J.D.; Schmitt, B.J.; Caplash, Y.; Greenwood, J.E. Free Flap Donor Site Reconstruction: A Prospective Case Series Using an Optimized Polyurethane Biodegradable Temporizing Matrix. Eplasty 2015, 15, e27. [Google Scholar] [PubMed]
- Damkat-Thomas, L.; Greenwood, J.E.; Wagstaff, M.J.D. A Synthetic Biodegradable Temporising Matrix in Degloving Lower Extremity Trauma Reconstruction: A Case Report. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2110. [Google Scholar] [CrossRef] [PubMed]
- Saha, S. Minimalistic reconstruction of exposed skull in a complex craniovertebral polytrauma. Surg. Neurol. Int. 2021, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lim, P.; Stanley, E.; Lee, G.; Lin, S.; Neoh, D.; Liew, J.; Ng, S.K. Experience with NovoSorb® Biodegradable Temporising Matrix in reconstruction of complex wounds. ANZ J. Surg. 2021, 91, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, M.J.; Caplash, Y.; Greenwood, J.E. Reconstruction of an Anterior Cervical Necrotizing Fasciitis Defect Using a Biodegradable Polyurethane Dermal Substitute. Eplasty 2017, 17, e3. [Google Scholar] [PubMed]
- Concannon, E.; Coghlan, P.; DamKat Thomas, L.; Solanki, N.S.; Greenwood, J.E. Biodegradable Temporizing Matrix Reconstruction of Complex Perineal Burn Wound: A Case Report. J. Burn Care Res. 2021, 42, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.S.; York, B.; Gao, Y.; Baker, P.; Wong She, R.B. A consecutive case series of defects reconstructed using NovoSorbⓇ Biodegradable Temporising Matrix: Initial experience and early results. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.R.; Deodhar, A.; Offer, G.J. A novel use for the biodegradable temporizing matrix. Eur. J. Plast. Surg. 2022, 45, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Banakh, I.; Cheshire, P.; Rahman, M.; Carmichael, I.; Jagadeesan, P.; Cameron, N.R.; Cleland, H.; Akbarzadeh, S. A Comparative Study of Engineered Dermal Templates for Skin Wound Repair in a Mouse Model. Int. J. Mol. Sci. 2020, 21, 4508. [Google Scholar] [CrossRef]
- Dearman, B.L.; Greenwood, J.E. Long-term follow-up of a major burn treated using composite cultured skin. Burns Open 2022, 6, 156–163. [Google Scholar] [CrossRef]
- Schiestl, C.; Meuli, M.; Vojvodic, M.; Pontiggia, L.; Neuhaus, D.; Brotschi, B.; Reichmann, E.; Böttcher-Haberzeth, S.; Neuhaus, K. Expanding into the future: Combining a novel dermal template with distinct variants of autologous cultured skin substitutes in massive burns. Burns Open 2021, 5, 145–153. [Google Scholar] [CrossRef]
- United States. NovoSorb® BTM|Synthetic Polymer for Traumatic Wound Treatment. Available online: https://polynovo.com/novosorb-btm/ (accessed on 12 June 2023).
- First Product Order from PMI and First Patient Treated in Germany|BioMelbourne Network. 2020. Available online: https://biomelbourne.org/first-product-order-from-pmi-and-first-patient-treated-in-germany/ (accessed on 4 March 2024).
- Chemali, M.; Laurent, A.; Scaletta, C.; Waselle, L.; Simon, J.-P.; Michetti, M.; Brunet, J.-F.; Flahaut, M.; Hirt-Burri, N.; Raffoul, W.; et al. Burn Center Organization and Cellular Therapy Integration: Managing Risks and Costs. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2021, 42, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Still, J.M.; Orlet, H.K.; Law, E.J. Use of cultured epidermal autografts in the treatment of large burns. Burns 1994, 20, 539–541. [Google Scholar] [CrossRef]
- Moiemen, N.; Schiestl, C.; Hartmann-Fritsch, F.; Neuhaus, K.; Reichmann, E.; Löw, A.; Stenger, C.; Böttcher-Haberzeth, S.; Meuli, M. First time compassionate use of laboratory engineered autologous Zurich skin in a massively burned child. Burns Open 2021, 5, 113–117. [Google Scholar] [CrossRef]
- Wagstaff, M.J.D.; Schmitt, B.J.; Coghlan, P.; Finkemeyer, J.P.; Caplash, Y.; Greenwood, J.E. A Biodegradable Polyurethane Dermal Matrix in Reconstruction of Free Flap Donor Sites: A Pilot Study. Eplasty 2015, 15, e13. [Google Scholar] [PubMed]
- Je, G. Evaluation of NovoSorbTM novel biodegradable polymer for the generation of a dermal matrix. Wound Pract. Res. 2010, 18, 9. [Google Scholar]
- Aleemardani, M.; Trikić, M.Z.; Green, N.H.; Claeyssens, F. The Importance of Mimicking Dermal-Epidermal Junction for Skin Tissue Engineering: A Review. Bioengineering 2021, 8, 148. [Google Scholar]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Pushkin, D.V.; Petrova, M.M.; Petrov, A.V.; Dmitrenko, D.V.; Karpova, E.I.; Demina, O.M.; Shnayder, N.A. The Role of Extracellular Matrix in Skin Wound Healing. J. Clin. Med. 2021, 10, 5947. [Google Scholar] [CrossRef]
- Burke, J.F. Observations on the development and clinical use of artificial skin--an attempt to employ regeneration rather than scar formation in wound healing. Jpn. J. Surg. 1987, 17, 431–438. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, V.L.; Mintz, B.; Gandhi, A.; Smith, J. Design matters: A comparison of natural versus synthetic skin substitutes across benchtop and porcine wound healing metrics: An experimental study. Health Sci. Rep. 2023, 6, e1462. [Google Scholar] [CrossRef]
- Dearman, B.L.; Boyce, S.T.; Greenwood, J.E. Comparison of biopolymer scaffolds for the fabrication of skin substitutes in a porcine wound model. Wound Repair Regen. 2023, 31, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Gardeazabal, L.; Izeta, A. Elastin and collagen fibres in cutaneous wound healing. Exp. Dermatol. 2024, 33, e15052. [Google Scholar] [CrossRef] [PubMed]
- Moiemen, N.; Yarrow, J.; Hodgson, E.; Constantinides, J.; Chipp, E.; Oakley, H.; Shale, E.; Freeth, M. Long-Term Clinical and Histological Analysis of Integra Dermal Regeneration Template. Plast. Reconstr. Surg. 2011, 127, 1149. [Google Scholar] [CrossRef]
- Rajaram, R.; Zhang, M.; Premaratne, G.; Ng, S. Novosorb® BTM- history, production and application in challenging wounds. Front. Bioeng. Biotechnol. 2024, 12, 1450973. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.W.; Austin, C.L.; Thompson, S.J. Treatment of a Full-Thickness Burn Injury With NovoSorb Biodegradable Temporizing Matrix and RECELL Autologous Skin Cell Suspension: A Case Series. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2020, 41, 215–219. [Google Scholar] [CrossRef]







| Patients | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age at thermal injury | 14 yrs | 1.4 yrs | 2 yrs | 1.3 yrs |
| Gender | Male | Female | Female | Female |
| Cause of thermal injury | Flame injury | Flame injury | Scald injury | Scald injury |
| TBSA affected (%) | 95 | 37 | 37 | 25 |
| Grade of thermal injury | 3 | 2–3 | 2–3 | 3 |
| Time between step 1 and 2 (days) | 31–41 | 37 | 28 | 27 |
| Autograft | CEAs/CDEAs/dS | STSGs | MEEK | STSG |
| Time of biopsy after BTM application | 1.5 m–2.4 yrs | 8 m | 1.4 yrs | 2.6 yrs |
| Paper | Preclinical | Animal-Based | Human-Based | Case Report | Histological Analysis |
|---|---|---|---|---|---|
| Schiestl et al. (2024) (“Long-term outcomes of a cultured autologous dermo-epidermal skin substitute in children: 5 year results of a phase I clinical trial”) [3] | X | X | X | ||
| Meuli et al. (2019) (“A Cultured Autologous Dermo-epidermal Skin Substitute for Full-Thickness Skin Defects: A Phase I, Open, Prospective Clinical Trial in Children”) [4] | X | X | X | ||
| Cheshire et al. (2016) (“Artificial dermal templates: A comparative study of NovoSorbTM Biodegradable Temporising Matrix (BTM) and Integra® Dermal Regeneration Template (DRT)”) [5] | X | X | X | ||
| Wagstaff et al. (2015) (“Free Flap Donor Site Reconstruction: A Prospective Case Series Using an Optimized Polyurethane Biodegradable Temporizing Matrix”) [12] | X | X | X | ||
| Banakh et al. (2020) (“A Comparative Study of Engineered Dermal Templates for Skin Wound Repair in a Mouse Model”) [20] | X | X | X | ||
| Dearman and Greenwood (2022) (“Long-term follow-up of a major burn treated using composite cultured skin”) [21] | X | X | X | ||
| Schiestl et al. (2021) (“Expanding into the future: Combining a novel dermal template with distinct variants of autologous cultured skin substitutes in massive burns”) [22] | X | X | X | ||
| Greenwood et al. (“Evaluation of NovoSorb™ novel biodegradable polymer for the generation of a dermal matrix Part 2: In-vivo Studies”) [29] | X | X | X | ||
| Stefanelli et al. (2023) (“Design matters: A comparison of natural versus synthetic skin substitutes across benchtop and porcine wound healing metrics: An experimental study”) [34] | X | X | X | ||
| Dearman et al. (2023) (“Comparison of biopolymer scaffolds for the fabrication of skin substitutes in a porcine wound model”) [35] | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerster-Barzanji, Z.; Woodtli, V.; Klix, M.; Biedermann, T.; Schiestl, C.; Neuhaus, K.; Farkas, M.; Kamarachev, J.; Rittirsch, D.; Böttcher-Haberzeth, S. Long-Term Histological Evaluation of a Novel Dermal Template in the Treatment of Pediatric Burns. Bioengineering 2024, 11, 1270. https://doi.org/10.3390/bioengineering11121270
Gerster-Barzanji Z, Woodtli V, Klix M, Biedermann T, Schiestl C, Neuhaus K, Farkas M, Kamarachev J, Rittirsch D, Böttcher-Haberzeth S. Long-Term Histological Evaluation of a Novel Dermal Template in the Treatment of Pediatric Burns. Bioengineering. 2024; 11(12):1270. https://doi.org/10.3390/bioengineering11121270
Chicago/Turabian StyleGerster-Barzanji, Zeena, Vivienne Woodtli, Mira Klix, Thomas Biedermann, Clemens Schiestl, Kathrin Neuhaus, Melinda Farkas, Jivko Kamarachev, Daniel Rittirsch, and Sophie Böttcher-Haberzeth. 2024. "Long-Term Histological Evaluation of a Novel Dermal Template in the Treatment of Pediatric Burns" Bioengineering 11, no. 12: 1270. https://doi.org/10.3390/bioengineering11121270
APA StyleGerster-Barzanji, Z., Woodtli, V., Klix, M., Biedermann, T., Schiestl, C., Neuhaus, K., Farkas, M., Kamarachev, J., Rittirsch, D., & Böttcher-Haberzeth, S. (2024). Long-Term Histological Evaluation of a Novel Dermal Template in the Treatment of Pediatric Burns. Bioengineering, 11(12), 1270. https://doi.org/10.3390/bioengineering11121270







