Around-Body Versus On-Body Motion Sensing: A Comparison of Efficacy Across a Range of Body Movements and Scales
Abstract
:1. Introduction
2. Materials and Methods
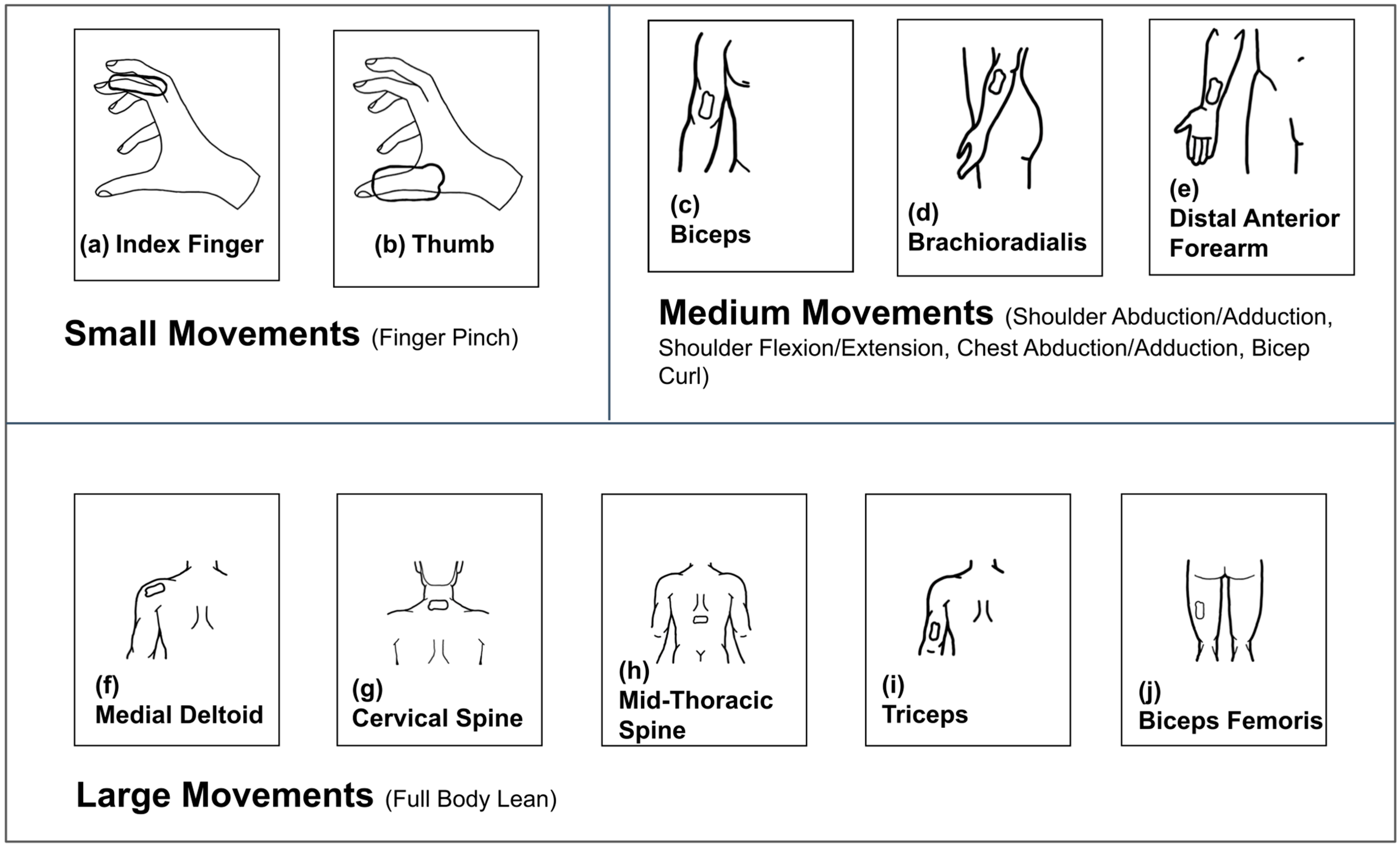
2.1. Materials
2.2. Experimental Methods
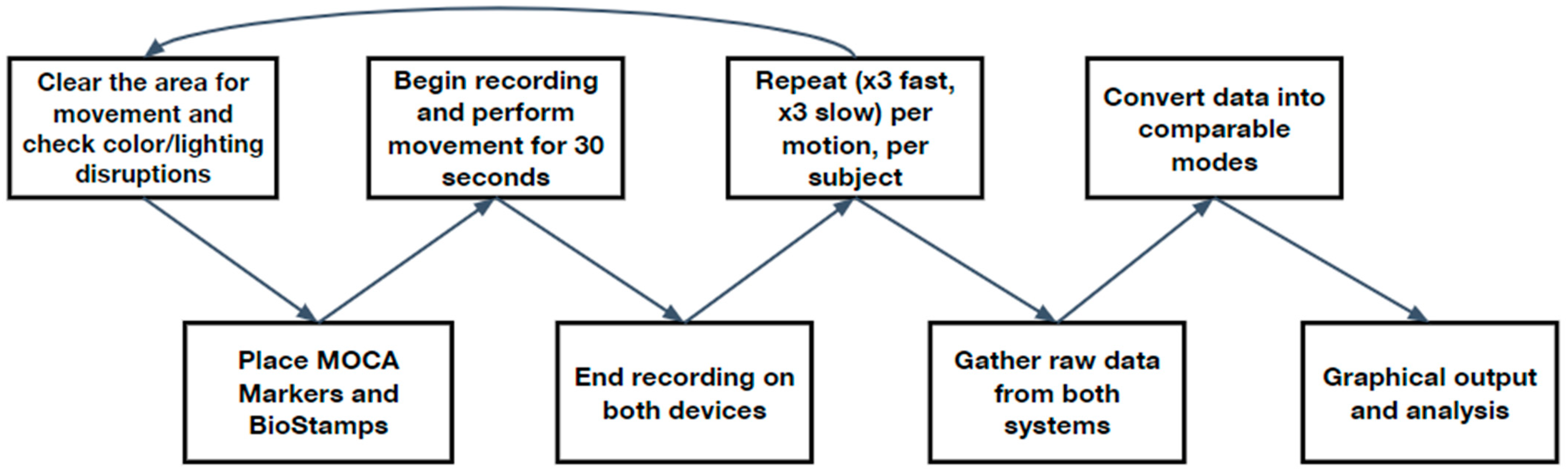
2.3. Generic Protocol
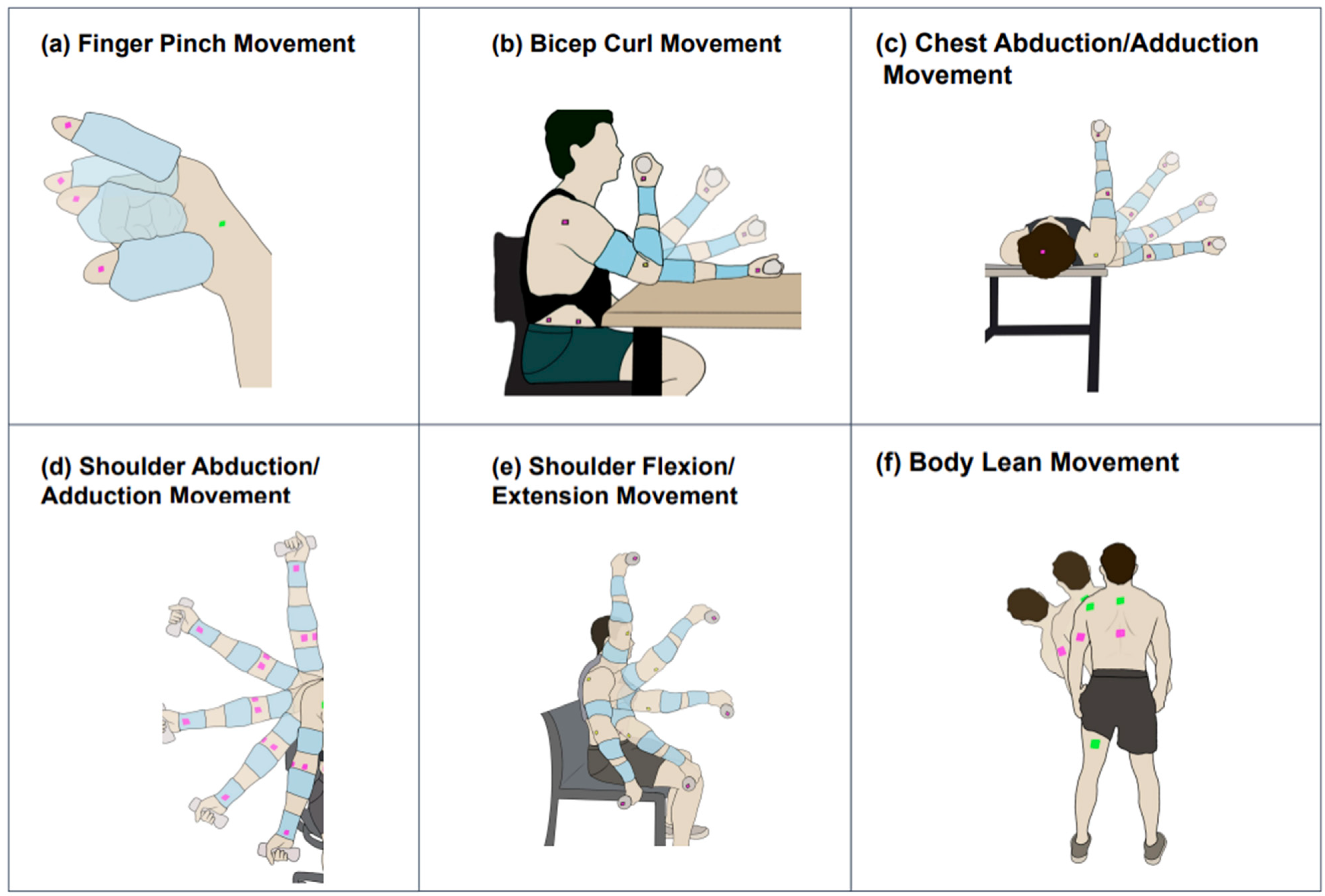
2.3.1. Finger Pinch (FingerP) Movement Protocol
2.3.2. Bicep Curl (BicepC) Movement Protocol
2.3.3. Chest Abduction/Adduction (ChestAA) Movement Protocol
2.3.4. Shoulder Abduction/Adduction (ShoulderAA) Movement Protocol
2.3.5. Shoulder Flexion/Extension (ShoulderFE) Movement Protocol
2.3.6. Body Lean (BodyL) Movement Protocol
2.4. MoCa Statistical Methods
2.5. BioStamp Statistical Methods
2.6. Comparison Methods
2.6.1. Maximum Angular Displacement Comparison
2.6.2. Alignment of Trials
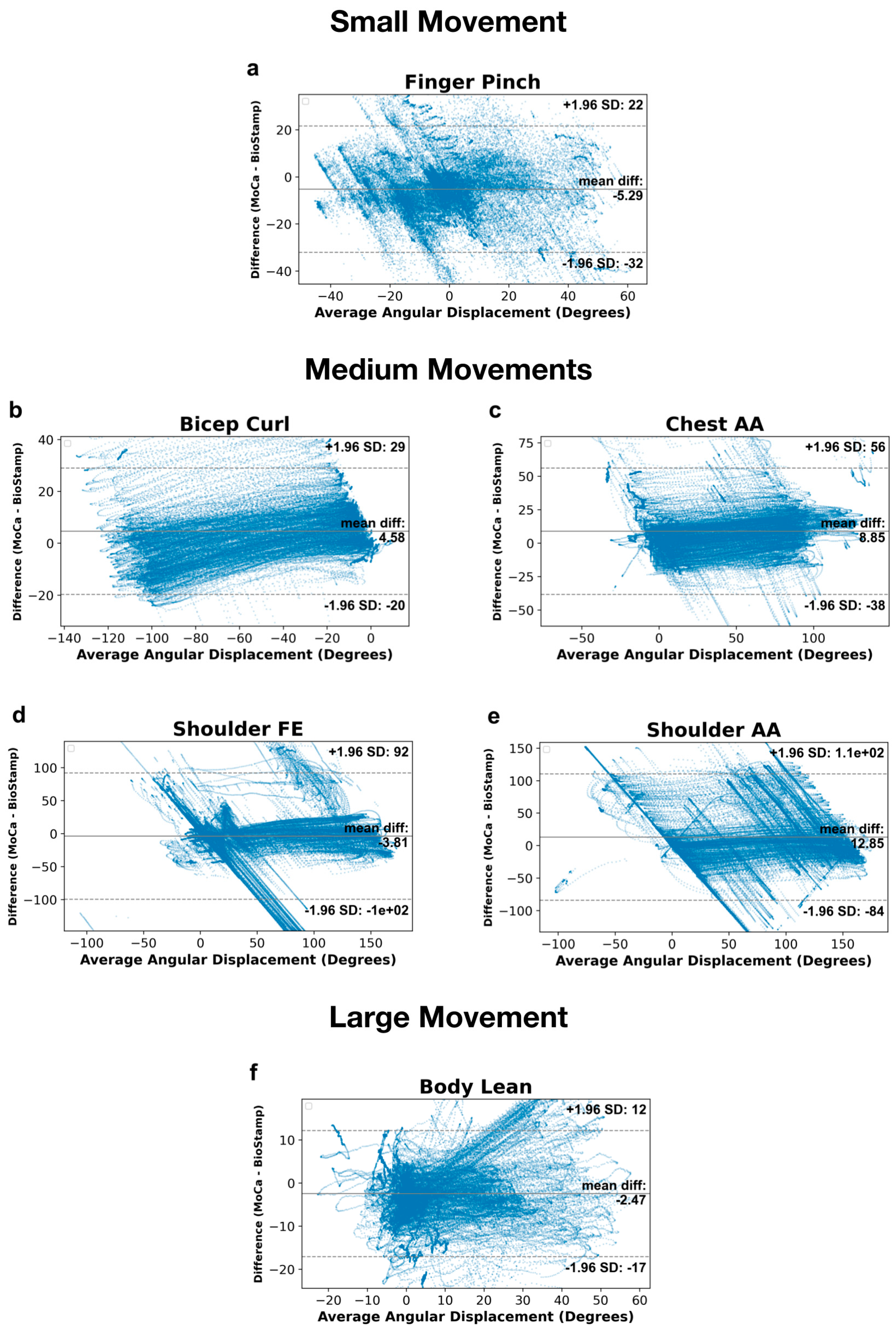
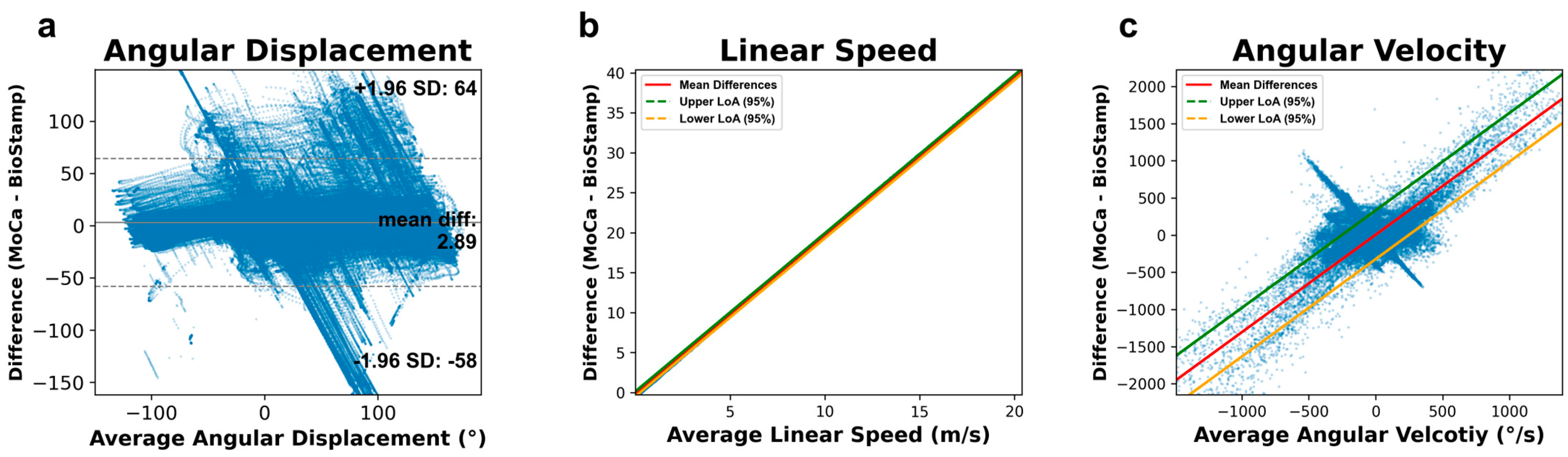
2.6.3. Bland–Altman Analysis
2.6.4. Relative Limits of Agreements
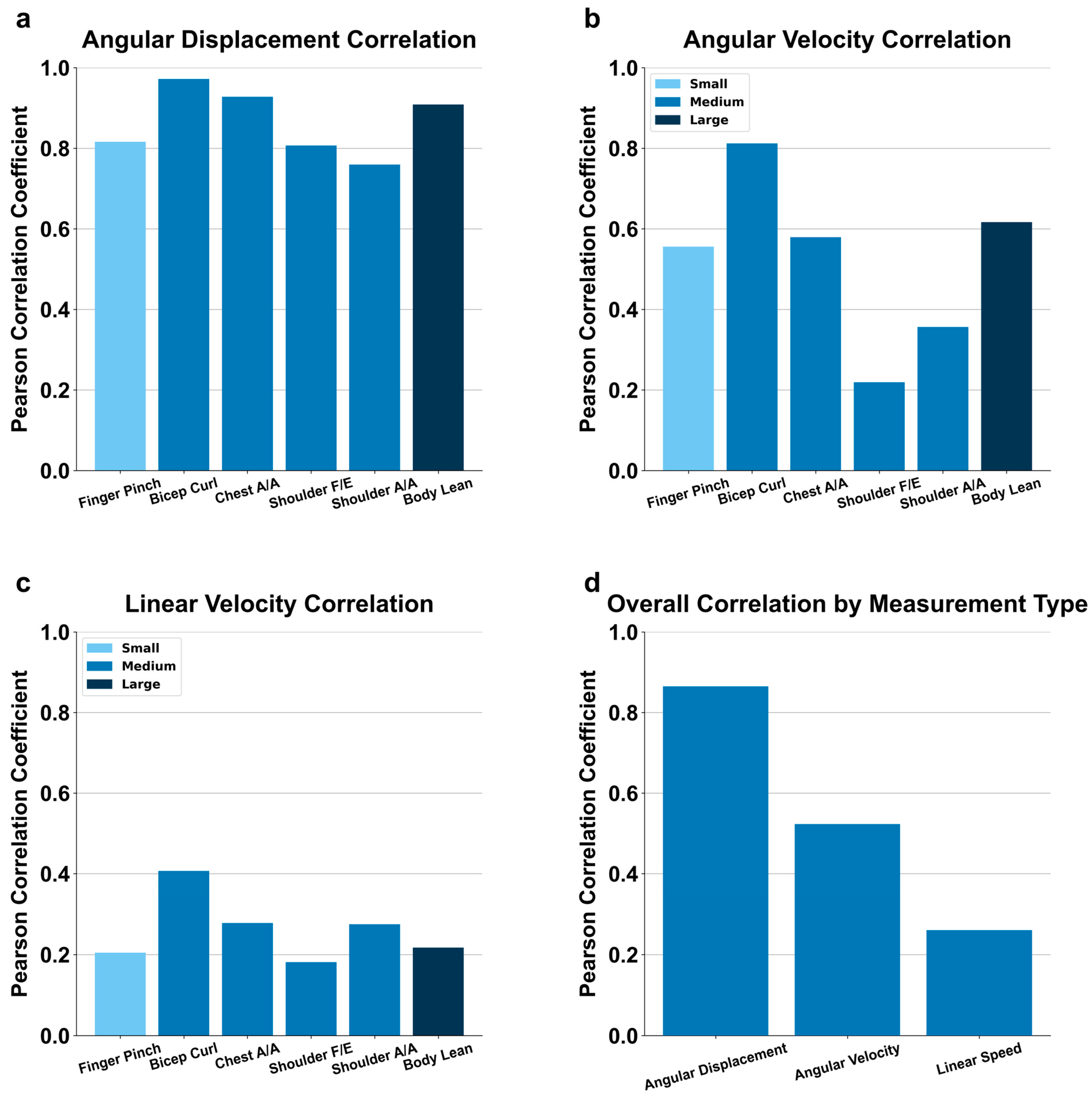
2.6.5. 1-to-1 Correlations
2.6.6. Aggregate Bar Charts
3. Results
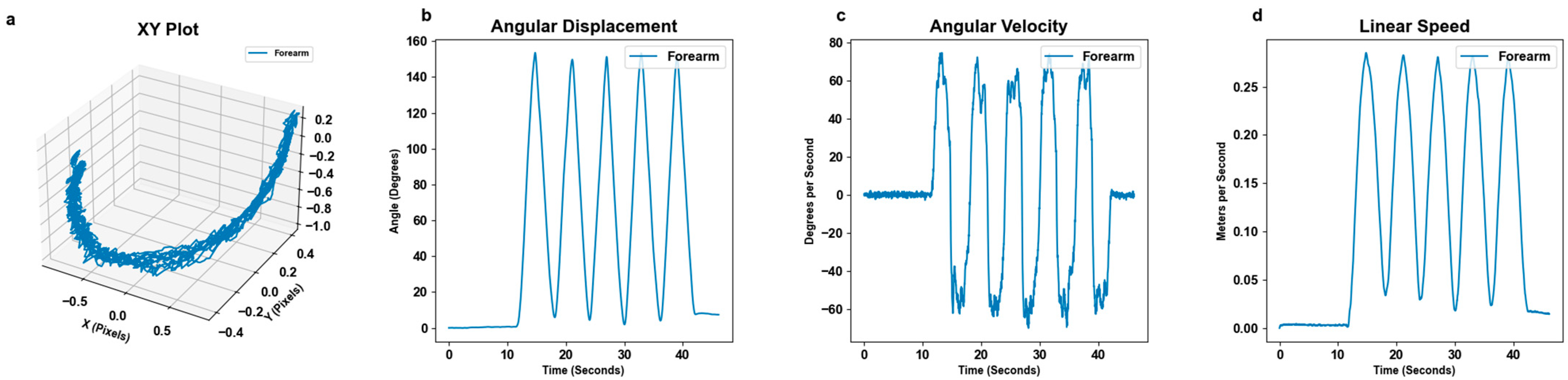
3.1. Motion Tracking
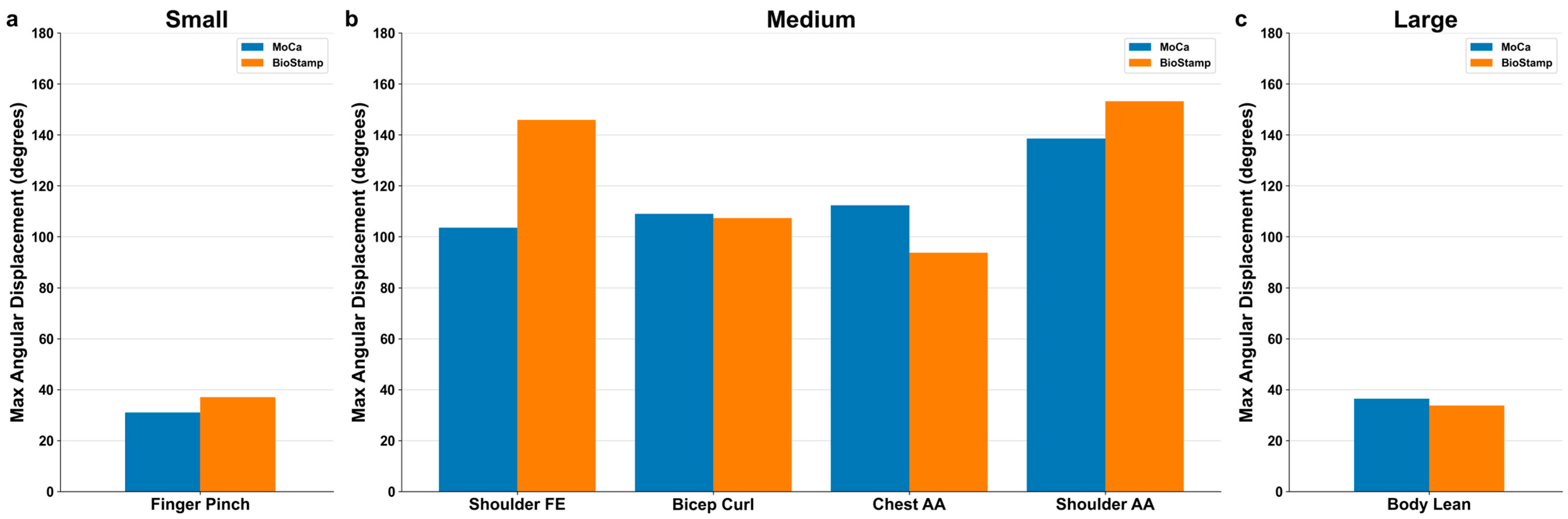
3.2. Motion Detection by Excursion Size
3.3. Motion Detection Endpoint Variables
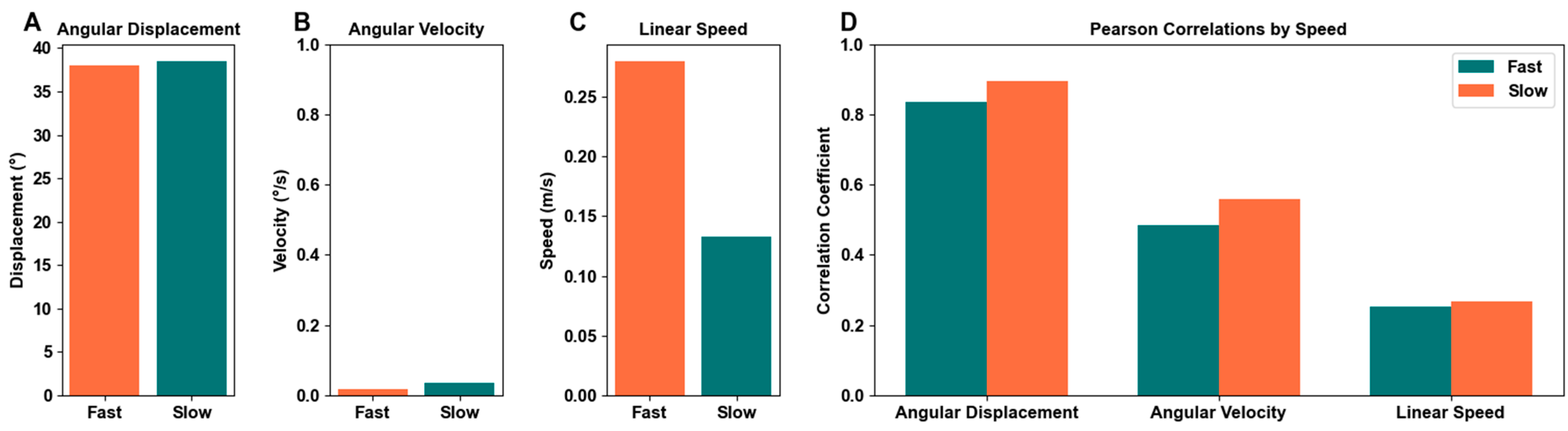
3.4. Motion Detection by Speed
4. Discussion
4.1. Trackability
4.2. Motion Size Comparison
4.2.1. Small Motion
4.2.2. Medium Motions
4.2.3. Large Motion
4.2.4. Size Definitions
4.3. Endpoint Variables
4.4. Speed Comparison
4.5. System Comparison
4.6. Human Activity Recognition Implications
4.7. Study Limitations
4.8. Current Technology
4.9. Future Improvements
4.10. Clinical Utility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tintignac, L.A.; Brenner, H.-R.; Rüegg, M.A. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol. Rev. 2015, 95, 809–852. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B.; Health ABC Study. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J. Gerontol. Ser. A 2005, 60, 324–333. [Google Scholar] [CrossRef]
- Brognara, L.; Luna, O.C.; Traina, F.; Cauli, O. Inflammatory Biomarkers and Gait Impairment in Older Adults: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1368. [Google Scholar] [CrossRef]
- Anker, S.D.; Chua, T.P.; Ponikowski, P.; Harrington, D.; Swan, J.W.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Hormonal Changes and Catabolic/Anabolic Imbalance in Chronic Heart Failure and Their Importance for Cardiac Cachexia. Circulation 1997, 96, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal Muscle Performance and Ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, H.W.; Van Den Engel-Hoek, L.; Steenks, M.H.; Bronkhorst, E.M.; Creugers, N.H.J.; de Groot, I.J.M.; Kalaykova, S.I. Reduced Mandibular Range of Motion in Duchenne Muscular Dystrophy: Predictive Factors. J. Oral Rehabil. 2015, 42, 430–438. [Google Scholar] [CrossRef]
- Hill, S.; Mong, S.; Vo, Q. Three-Dimensional Motion Analysis for Occupational Therapy Upper Extremity Assessment and Rehabilitation: A Scoping Review. Open J. Occup. Ther. 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Ferreira Dos Santos, L.; Christ, O.; Mate, K.; Schmidt, H.; Krüger, J.; Dohle, C. Movement Visualisation in Virtual Reality Rehabilitation of the Lower Limb: A Systematic Review. Biomed. Eng. OnLine 2016, 15, 144. [Google Scholar] [CrossRef]
- Debnath, B.; O’Brien, M.; Yamaguchi, M.; Behera, A. A Review of Computer Vision-Based Approaches for Physical Rehabilitation and Assessment. Multimed. Syst. 2022, 28, 209–239. [Google Scholar] [CrossRef]
- Colyer, S.L.; Evans, M.; Cosker, D.P.; Salo, A.I.T. A Review of the Evolution of Vision-Based Motion Analysis and the Integration of Advanced Computer Vision Methods Towards Developing a Markerless System. Sports Med.-Open 2018, 4, 24. [Google Scholar] [CrossRef]
- Chander, H.; Burch, R.F.; Talegaonkar, P.; Saucier, D.; Luczak, T.; Ball, J.E.; Turner, A.; Kodithuwakku Arachchige, S.N.K.; Carroll, W.; Smith, B.K.; et al. Wearable Stretch Sensors for Human Movement Monitoring and Fall Detection in Ergonomics. Int. J. Environ. Res. Public Health 2020, 17, 3554. [Google Scholar] [CrossRef] [PubMed]
- Benmessabih, T.; Slama, R.; Havard, V.; Baudry, D. Online Human Motion Analysis in Industrial Context: A Review. Eng. Appl. Artif. Intell. 2024, 131, 107850. [Google Scholar] [CrossRef]
- Breasail, Ó.M.; Biswas, B.; Smith, M.D.; Mazhar, M.K.A.; Tenison, E.; Cullen, A.; Lithander, F.E.; Roudaut, A.; Henderson, E.J. Wearable GPS and Accelerometer Technologies for Monitoring Mobility and Physical Activity in Neurodegenerative Disorders: A Systematic Review. Sensors 2021, 21, 8261. [Google Scholar] [CrossRef]
- Mündermann, L.; Corazza, S.; Andriacchi, T.P. The Evolution of Methods for the Capture of Human Movement Leading to Markerless Motion Capture for Biomechanical Applications. J. Neuroeng. Rehabil. 2006, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Chinmilli, P.; Redkar, S.; Zhang, W.; Sugar, T. A Review on Wearable Inertial Tracking Based Human Gait Analysis and Control Strategies of Lower-Limb Exoskeletons. Int. Robot. Autom. J. 2017, 3, 398–415. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ghaffari, R.; Lu, N.; Rogers, J.A. Flexible and Stretchable Electronics for Biointegrated Devices. Annu. Rev. Biomed. Eng. 2012, 14, 113–128. [Google Scholar] [CrossRef]
- Vincent, A.C.; Furman, H.; Slepian, R.C.; Ammann, K.R.; Di Maria, C.; Chien, J.H.; Siu, K.-C.; Slepian, M.J. Smart Phone-Based Motion Capture and Analysis: Importance of Operating Envelope Definition and Application to Clinical Use. Appl. Sci. 2022, 12, 6173. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Tan, T. Recent Developments in Human Motion Analysis. Pattern Recognit. 2003, 36, 585–601. [Google Scholar] [CrossRef]
- Pediaditis, M.; Tsiknakis, M.; Leitgeb, N. Vision-Based Motion Detection, Analysis and Recognition of Epileptic Seizures—A Systematic Review. Comput. Methods Programs Biomed. 2012, 108, 1133–1148. [Google Scholar] [CrossRef]
- Ristić-Durrant, D.; Franke, M.; Michels, K. A Review of Vision-Based On-Board Obstacle Detection and Distance Estimation in Railways. Sensors 2021, 21, 3452. [Google Scholar] [CrossRef]
- Randers, M.B.; Mujika, I.; Hewitt, A.; Santisteban, J.; Bischoff, R.; Solano, R.; Zubillaga, A.; Peltola, E.; Krustrup, P.; Mohr, M. Application of Four Different Football Match Analysis Systems: A Comparative Study. J. Sports Sci. 2010, 28, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ammann, K.R.; Ahamed, T.; Sweedo, A.L.; Ghaffari, R.; Weiner, Y.E.; Slepian, R.C.; Jo, H.; Slepian, M.J. Human Motion Component and Envelope Characterization via Wireless Wearable Sensors. BMC Biomed. Eng. 2020, 2, 3. [Google Scholar] [CrossRef]
- Garlant, J.A.; Ammann, K.R.; Slepian, M.J. Stretchable Electronic Wearable Motion Sensors Delineate Signatures of Human Motion Tasks. ASAIO J. 2018, 64, 351. [Google Scholar] [CrossRef] [PubMed]
- Parks, M.; Chien, J.H.; Siu, K.-C. Development of a Mobile Motion Capture (MO2CA) System for Future Military Application. Mil. Med. 2019, 184 (Suppl. 1), 65–71. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Lei, S.; Cui, L.; Lu, C.-T. Med-MMHL: A Multi-Modal Dataset for Detecting Human- and LLM-Generated Misinformation in the Medical Domain. arXiv 2023, arXiv:2306.08871. [Google Scholar] [CrossRef]
- Bourke, A.K.; O’Donovan, K.; Clifford, A.; Ólaighin, G.; Nelson, J. Optimum Gravity Vector and Vertical Acceleration Estimation Using a Tri-Axial Accelerometer for Falls and Normal Activities. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 7896–7899. [Google Scholar] [CrossRef]
- Yeng, S.; Lin, T.; Lin, Y.-F. The N-Dimensional Pythagorean Theorem. Linear Multilinear Algebra 1990, 26, 9–13. [Google Scholar] [CrossRef]
- Blu, T.; Thévenaz, P.; Unser, M. Linear Interpolation Revitalized. IEEE J. Mag. 2004, 13, 710–719. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 1, 307–310. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring Agreement in Method Comparison Studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Sun, R.; Moon, Y.; McGinnis, R.S.; Seagers, K.; Motl, R.W.; Sheth, N.; Wright, J.A.; Ghaffari, R.; Patel, S.; Sosnoff, J.J. Assessment of Postural Sway in Individuals with Multiple Sclerosis Using a Novel Wearable Inertial Sensor. Digit. Biomark. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Mahmood, O.A.; Aliraqi, M.G.; Ali, A.A. Movement Disorders in Chronic Kidney Disease Patients on Hemodialysis in Mosul City. Open Access Maced. J. Med. Sci. 2023, 11, 145–149. [Google Scholar] [CrossRef]
- do Vilas-Boas, M.C.; Cunha, J.P.S. Movement Quantification in Neurological Diseases: Methods and Applications. IEEE Rev. Biomed. Eng. 2016, 9, 15–31. [Google Scholar] [CrossRef]
- Obukhov, A.; Volkov, A.; Pchelintsev, A.; Nazarova, A.; Teselkin, D.; Surkova, E.; Fedorchuk, I. Examination of the Accuracy of Movement Tracking Systems for Monitoring Exercise for Musculoskeletal Rehabilitation. Sensors 2023, 23, 8058. [Google Scholar] [CrossRef] [PubMed]
- Kempe, V. Inertial MEMS: Principles and Practice; Cambridge University Press: Cambridge, UK, 2011; ISBN 978-0521766586. [Google Scholar]
- Du, J.; Gerdtman, C.; Lindén, M. Signal Quality Improvement Algorithms for MEMS Gyroscope-Based Human Motion Analysis Systems: A Systematic Review. Sensors 2018, 18, 1123. [Google Scholar] [CrossRef]
- Fusagawa, H.; Sato, T.; Yamada, T.; Ashida, Y.; Ichise, N.; Ogon, I.; Teramoto, A.; Yamashita, T.; Tohse, N. Different Metabolic Changes between Fast- and Slow-Twitch Skeletal Muscle Underlie Impaired Muscle Fatigue Resistance in Chronic Kidney Disease. Biophys. J. 2022, 121, 514a. [Google Scholar] [CrossRef]
- Safarpour, Y.; Vaziri, N.D.; Jabbari, B. Movement Disorders in Chronic Kidney Disease—A Descriptive Review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105408. [Google Scholar] [CrossRef]
- Artom, M.; Moss-Morris, R.; Caskey, F.; Chilcot, J. Fatigue in Advanced Kidney Disease. Kidney Int. 2014, 86, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Parker Gregg, L.; Bossola, M.; Ostrosky-Frid, M.S.; Hedayati, S. Fatigue in CKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 1445–1455. [Google Scholar] [CrossRef]
- Dang, L.M.; Min, K.; Wang, H.; Piran, M.J.; Lee, C.H.; Moon, H. Sensor-Based and Vision-Based Human Activity Recognition: A Comprehensive Survey. Pattern Recognit. 2020, 108, 107561. [Google Scholar] [CrossRef]
- Hegde, N.; Bries, M.; Swibas, T.; Melanson, E.; Sazonov, E. Automatic Recognition of Activities of Daily Living Utilizing Insole-Based and Wrist-Worn Wearable Sensors. IEEE J. Biomed. Health Inform. 2018, 22, 979–988. [Google Scholar] [CrossRef]
- Cao, Z.; Simon, T.; Wei, S.-E.; Sheikh, Y. Realtime Multi-Person 2D Pose Estimation Using Part Affinity Fields. arXiv 2017, arXiv:1611.08050. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Camps, O.; Sznaier, M. Key Frame Proposal Network for Efficient Pose Estimation in Videos. In Computer Vision—ECCV 2020; Vedaldi, A., Bischof, H., Brox, T., Frahm, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 609–625. [Google Scholar] [CrossRef]
- Osokin, D. Real-Time 2D Multi-Person Pose Estimation on CPU: Lightweight OpenPose. arXiv 2018, arXiv:1811.12004. [Google Scholar] [CrossRef]
- Wootton, R. Telemedicine. BMJ 2001, 323, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- George, U.Z.; Moon, K.S.; Lee, S.Q. Extraction and Analysis of Respiratory Motion Using a Comprehensive Wearable Health Monitoring System. Sensors 2021, 21, 1393. [Google Scholar] [CrossRef]

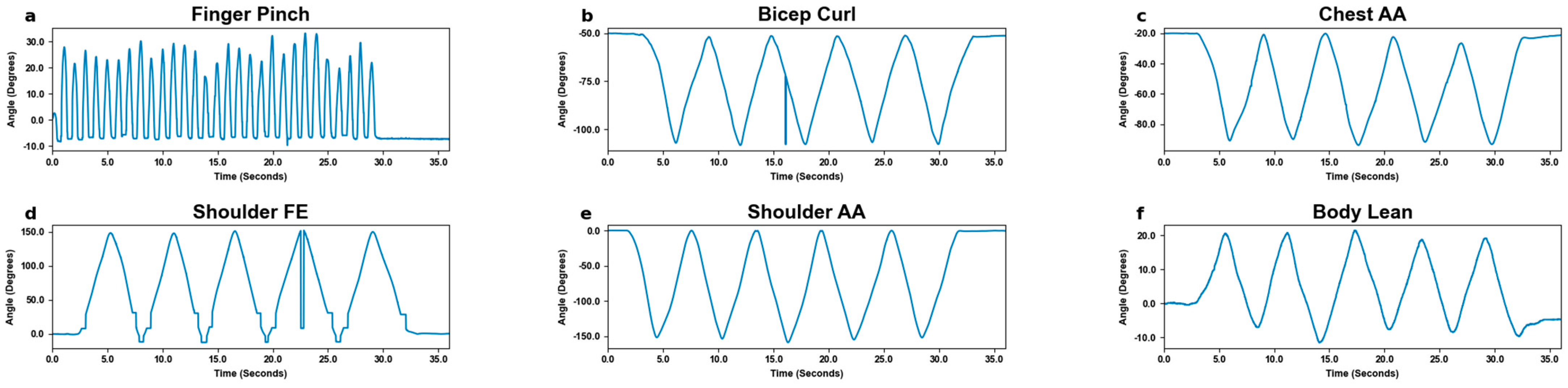
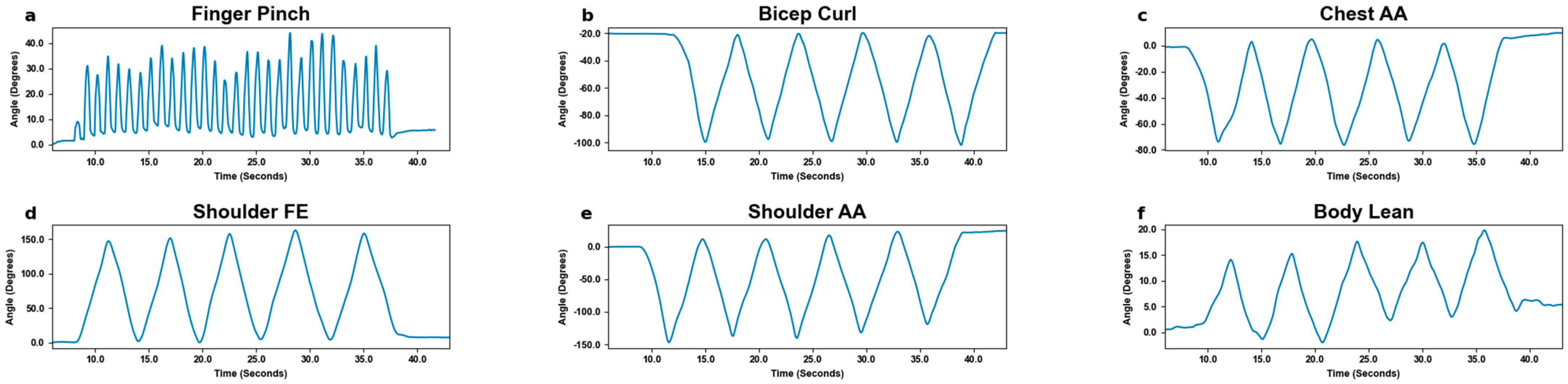



| Movement Size | Slow Pace (×3) | Fast Pace (×3) | Movement Area Requirement | MoCa Stamp Size |
|---|---|---|---|---|
| Small | 1 rep/1 s | 3 reps/1 s | 1 sq ft | 2 × 2 mm |
| Medium | 1 rep/6 s | 1 rep/2 s | 5 sq ft | 10 × 10 mm |
| Large | 1 rep/6 s | 1 rep/2 s | 10 sq ft | 10 × 10 mm |
| Movement Size | Movement Area Requirement | Movements |
|---|---|---|
| Small | 1 sq ft | Finger Pinch |
| Medium | 5 sq ft | Bicep Curl Chest Abduction/Adduction Shoulder Abduction/Adduction Shoulder Flexion/Extension |
| Large | 1 rep/6 s | Body Lean |
| MoCa Disadvantages | MoCa Advantages | BioStamp Disadvantages | BioStamp Advantages |
|---|---|---|---|
| Longer processing period post recordings | Tracks angular displacement very well | Greater stamp quantity required for large movement sizes | Tracked consistently across all motions |
| Rotation in movement causes obscurity | Tracked well on both fast and slow | Suffers from gyroscopic sensor-drift | Tracked well on both fast and slow |
| Markers easily get obscured | Flexible marker placement | Strict marker placement | Data outputted quickly |
| Body Element | Movement Disorders | Disease States | MoCa | BioStamp |
|---|---|---|---|---|
| Limited ROM | Traumatic injury arthritis | Moderate: ROM testing may require tracking over larger distances | Moderate: Should be able to track well but perhaps be wary of motions in the vertical axis | |
| Large Whole Body | Chorea | Lesions affecting the striatum Huntington disease Sydenham chorea Levodopa-induced dyskinesia | Poor: Unpredictable large motions may obscure marker placement and be poorly tracked | No issues |
| Akathisia | Psychomotor disorder | Moderate: There are multiple manifestations of akathisia. May be appropriate for larger movements, but difficulty tracking smaller motions. | Moderate: Due to BioStamp’s difficulty tracking vertical motion, may have some trouble with tracking | |
| Gait Abnormalities | Parkinson disease Osteoarthritis Traumatic injury Stroke Cerebral palsy | Poor: Due to the unpredictability of ataxic gait, there is a high likelihood of markers on extremities becoming occluded and interfering with tracking | No issues | |
| Limited ROM | Traumatic injury arthritis | No issues | No issues | |
| Myoclonus | Uremia Creutzfeldt-Jakob disease Epilepsy syndromes | No issues | No issues | |
| Restless Leg Syndrome | Low iron stores Uremia Peripheral neuropathy | No issues | No issues | |
| Medium Upper and Lower Extremities | Ballismus | Subthalamic nucleus lesion | Moderate: Subjects can present with large involuntary movements and may not be able to prevent marker occlusion | No issues |
| Resting Tremor | Parkinson disease Lewy body dementia Multiple system atrophy Progressive supranuclear palsy Wilson disease Dopamine antagonists | No issues | No issues | |
| Postural Tremor | Essential tremor Orthostatic tremor | No issues | No issues | |
| Small Wrist, Ankle, Hands, Feet, and Digits | Limited ROM | Traumatic injury arthritis | No issues | No issues |
| Asterixis | Hepatic encephalopathy Uremic encephalopathy Hypercapnia | No issues | No issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohrer, K.; De Anda, L.; Grubb, C.; Hansen, Z.; Rodriguez, J.; St Pierre, G.; Sheikhlary, S.; Omer, S.; Tran, B.; Lawendy, M.; et al. Around-Body Versus On-Body Motion Sensing: A Comparison of Efficacy Across a Range of Body Movements and Scales. Bioengineering 2024, 11, 1163. https://doi.org/10.3390/bioengineering11111163
Rohrer K, De Anda L, Grubb C, Hansen Z, Rodriguez J, St Pierre G, Sheikhlary S, Omer S, Tran B, Lawendy M, et al. Around-Body Versus On-Body Motion Sensing: A Comparison of Efficacy Across a Range of Body Movements and Scales. Bioengineering. 2024; 11(11):1163. https://doi.org/10.3390/bioengineering11111163
Chicago/Turabian StyleRohrer, Katelyn, Luis De Anda, Camila Grubb, Zachary Hansen, Jordan Rodriguez, Greyson St Pierre, Sara Sheikhlary, Suleyman Omer, Binh Tran, Mehrail Lawendy, and et al. 2024. "Around-Body Versus On-Body Motion Sensing: A Comparison of Efficacy Across a Range of Body Movements and Scales" Bioengineering 11, no. 11: 1163. https://doi.org/10.3390/bioengineering11111163
APA StyleRohrer, K., De Anda, L., Grubb, C., Hansen, Z., Rodriguez, J., St Pierre, G., Sheikhlary, S., Omer, S., Tran, B., Lawendy, M., Alqaraghuli, F., Hedgecoke, C., Abdelkeder, Y., Slepian, R. C., Ross, E., Chung, R., & Slepian, M. J. (2024). Around-Body Versus On-Body Motion Sensing: A Comparison of Efficacy Across a Range of Body Movements and Scales. Bioengineering, 11(11), 1163. https://doi.org/10.3390/bioengineering11111163






