Hydrogel-Enhanced Autologous Chondrocyte Implantation for Cartilage Regeneration—An Update on Preclinical Studies
Abstract
1. Introduction
2. Articular Cartilage
2.1. Articular Cartilage Composition and Phases
2.2. Chondrocyte Function and Zonal Classification
2.3. Matrix Classification
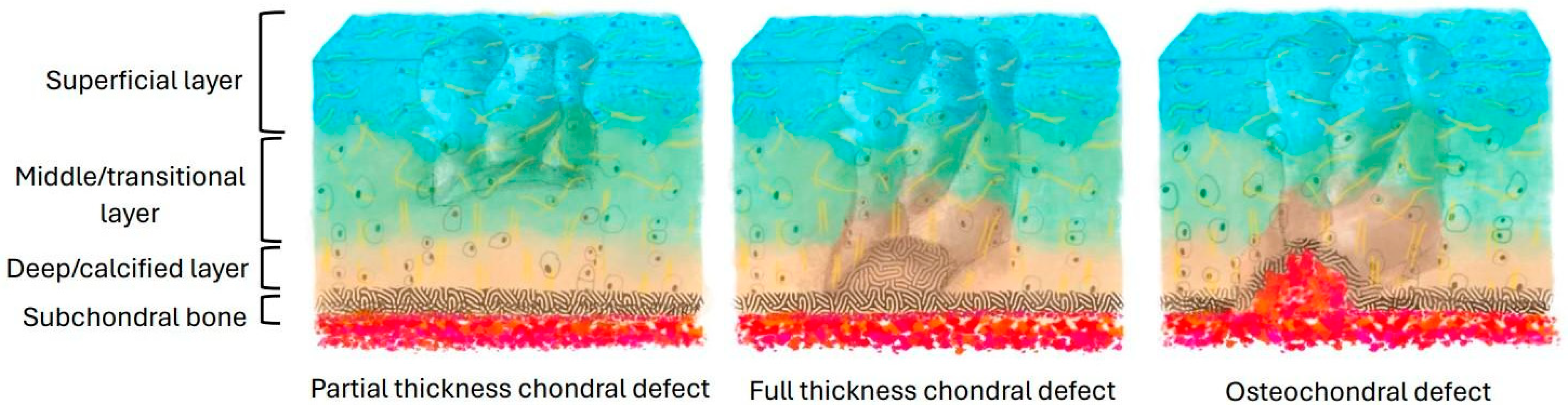
3. Cartilage Injury
4. Current Treatments of Cartilage Defects
4.1. Initial Assessment
4.2. Non-Surgical Interventions
4.3. Surgical Interventions
5. ACI-Based Therapy
6. Hydrogels in ACI
6.1. Natural-Polymer-Based Hydrogels
6.2. Synthetic-Polymer-Based Hydrogels
7. Fixation and Integration of Implants with Native Cartilage
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of Osteoarthritis. Osteoarthritis Cartilage 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Jeuken, R.M.; van Hugten, P.P.W.; Roth, A.K.; Timur, U.T.; Boymans, T.A.E.J.; van Rhijn, L.W.; Bugbee, W.D.; Emans, P.J. A Systematic Review of Focal Cartilage Defect Treatments in Middle-Aged Versus Younger Patients. Orthop. J. Sports Med. 2021, 9, 23259671211031244. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Godwin, M.; Dawes, M. Intra-Articular Steroid Injections for Painful Knees. Systematic Review with Meta-Analysis. Can. Fam. Physician Med. Fam. Can. 2004, 50, 241–248. [Google Scholar]
- Guo, X.; Xi, L.; Yu, M.; Fan, Z.; Wang, W.; Ju, A.; Liang, Z.; Zhou, G.; Ren, W. Regeneration of Articular Cartilage Defects: Therapeutic Strategies and Perspectives. J. Tissue Eng. 2023, 14, 20417314231164765. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the Repair of Articular Cartilage Defects. Tissue Eng. Part B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Muthu, S.; Korpershoek, J.V.; Novais, E.J.; Tawy, G.F.; Hollander, A.P.; Martin, I. Failure of Cartilage Regeneration: Emerging Hypotheses and Related Therapeutic Strategies. Nat. Rev. Rheumatol. 2023, 19, 403–416. [Google Scholar] [CrossRef]
- Ulrich-Vinther, M.; Maloney, M.D.; Schwarz, E.M.; Rosier, R.; O’Keefe, R.J. Articular Cartilage Biology. J. Am. Acad. Orthop. Surg. 2003, 11, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.P. Articular Cartilage Repair. Am. J. Sports Med. 1998, 26, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Carter, D.R. Articular Cartilage Functional Histomorphology and Mechanobiology: A Research Perspective. Bone 2003, 33, 1–13. [Google Scholar] [CrossRef]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage-An Overview. Life Basel Switz. 2021, 11, 302. [Google Scholar] [CrossRef]
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. Int. J. Mol. Sci. 2021, 22, 3329. [Google Scholar] [CrossRef]
- Lewis, P.B.; McCarty, L.P., 3rd; Kang, R.W.; Cole, B.J. Basic Science and Treatment Options for Articular Cartilage Injuries. J. Orthop. Sports Phys. Ther. 2006, 36, 717–727. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Articular Cartilage: Injuries and Potential for Healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202. [Google Scholar] [CrossRef]
- Correa, D.; Lietman, S.A. Articular Cartilage Repair: Current Needs, Methods and Research Directions. Semin. Cell Dev. Biol. 2017, 62, 67–77. [Google Scholar] [CrossRef]
- Falah, M.; Nierenberg, G.; Soudry, M.; Hayden, M.; Volpin, G. Treatment of Articular Cartilage Lesions of the Knee. Int. Orthop. 2010, 34, 621–630. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite Design of Injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Gilbert, S.J.; Singhrao, S.K.; Duance, V.C.; Archer, C.W. Cartilage Integration: Evaluation of the Reasons for Failure of Integration during Cartilage Repair. A Review. Eur. Cell. Mater. 2008, 16, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Biant, L.C.; McNicholas, M.J.; Sprowson, A.P.; Spalding, T. The Surgical Management of Symptomatic Articular Cartilage Defects of the Knee: Consensus Statements from United Kingdom Knee Surgeons. The Knee 2015, 22, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Mall, N.A.; Harris, J.D.; Cole, B.J. Clinical Evaluation and Preoperative Planning of Articular Cartilage Lesions of the Knee. J. Am. Acad. Orthop. Surg. 2015, 23, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Strickland, C.D.; Ho, C.K.; Merkle, A.N.; Vidal, A.F. MR Imaging of Knee Cartilage Injury and Repair Surgeries. Magn. Reson. Imaging Clin. N. Am. 2022, 30, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.S.; Bajaj, S.; Ghodadra, N.S. Basic Science and Surgical Treatment Options for Articular Cartilage Injuries of the Knee. J. Orthop. Sports Phys. Ther. 2012, 42, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Slattery, C.; Kweon, C.Y. Classifications in Brief: Outerbridge Classification of Chondral Lesions. Clin. Orthop. 2018, 476, 2101–2104. [Google Scholar] [CrossRef]
- Shah, A.J.; Patel, D. Imaging Update on Cartilage. J. Clin. Orthop. Trauma 2021, 22, 101610. [Google Scholar] [CrossRef]
- Simon, T.M.; Jackson, D.W. Articular Cartilage: Injury Pathways and Treatment Options. Sports Med. Arthrosc. Rev. 2018, 26, 31–39. [Google Scholar] [CrossRef]
- Liu, W.; Madry, H.; Cucchiarini, M. Application of Alginate Hydrogels for Next-Generation Articular Cartilage Regeneration. Int. J. Mol. Sci. 2022, 23, 1147. [Google Scholar] [CrossRef]
- Barisón, M.J.; Nogoceke, R.; Josino, R.; Horinouchi, C.D. da S.; Marcon, B.H.; Correa, A.; Stimamiglio, M.A.; Robert, A.W. Functionalized Hydrogels for Cartilage Repair: The Value of Secretome-Instructive Signaling. Int. J. Mol. Sci. 2022, 23, 6010. [Google Scholar] [CrossRef] [PubMed]
- Detterline, A.J.; Goldberg, S.; Bach, B.R.J.; Cole, B.J. Treatment Options for Articular Cartilage Defects of the Knee. Orthop. Nurs. 2005, 24, 361–366, quiz 367–368. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.J.; Aman, Z.S.; DePhillipo, N.N.; Dickens, J.F.; Anz, A.W.; LaPrade, R.F. Chondral Lesions of the Knee: An Evidence-Based Approach. J. Bone Joint Surg. Am. 2021, 103, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.; Liao, Q.; Gee, C.W. Surgical Management of Osteochondral Defects of the Knee: An Educational Review. Curr. Rev. Musculoskelet. Med. 2021, 14, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, M.; Szomor, A.; Chen, D.B.; MacDessi, S.J. A Retrospective Study Assessing Safety and Efficacy of Bipolar Radiofrequency Ablation for Knee Chondral Lesions. Cartilage 2018, 9, 241–247. [Google Scholar] [CrossRef]
- Anderson, S.R.; Faucett, S.C.; Flanigan, D.C.; Gmabardella, R.A.; Amin, N.H. The History of Radiofrequency Energy and Coblation in Arthroscopy: A Current Concepts Review of Its Application in Chondroplasty of the Knee. J. Exp. Orthop. 2019, 6, 1. [Google Scholar] [CrossRef]
- Liu, Y.W.; Tran, M.D.; Skalski, M.R.; Patel, D.B.; White, E.A.; Tomasian, A.; Gross, J.S.; Vangsness, C.T.; Matcuk, G.R.J. MR Imaging of Cartilage Repair Surgery of the Knee. Clin. Imaging 2019, 58, 129–139. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Minas, T. The Quality of Healing: Articular Cartilage. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2014, 22 Suppl 1, 30–38. [Google Scholar] [CrossRef]
- Redondo, M.L.; Naveen, N.B.; Liu, J.N.; Tauro, T.M.; Southworth, T.M.; Cole, B.J. Preservation of Knee Articular Cartilage. Sports Med. Arthrosc. Rev. 2018, 26, e23–e30. [Google Scholar] [CrossRef]
- Krych, A.J.; Harnly, H.W.; Rodeo, S.A.; Williams, R.J. 3rd Activity Levels Are Higher after Osteochondral Autograft Transfer Mosaicplasty than after Microfracture for Articular Cartilage Defects of the Knee: A Retrospective Comparative Study. J. Bone Joint Surg. Am. 2012, 94, 971–978. [Google Scholar] [CrossRef]
- Gudas, R.; Kalesinskas, R.J.; Kimtys, V.; Stankevicius, E.; Toliusis, V.; Bernotavicius, G.; Smailys, A. A Prospective Randomized Clinical Study of Mosaic Osteochondral Autologous Transplantation versus Microfracture for the Treatment of Osteochondral Defects in the Knee Joint in Young Athletes. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2005, 21, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Chui, K.; Jeys, L.; Snow, M. Knee Salvage Procedures: The Indications, Techniques and Outcomes of Large Osteochondral Allografts. World J. Orthop. 2015, 6, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Makarczyk, M.J. Cell Therapy Approaches for Articular Cartilage Regeneration. Organogenesis 2023, 19, 2278235. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous Chondrocyte Implantation in the Knee: Systematic Review and Economic Evaluation. Health Technol. Assess. Winch. Engl. 2017, 21, 1–294. [Google Scholar] [CrossRef]
- Welch, T.; Mandelbaum, B.; Tom, M. Autologous Chondrocyte Implantation: Past, Present, and Future. Sports Med. Arthrosc. Rev. 2016, 24, 85–91. [Google Scholar] [CrossRef]
- Krill, M.; Early, N.; Everhart, J.S.; Flanigan, D.C. Autologous Chondrocyte Implantation (ACI) for Knee Cartilage Defects: A Review of Indications, Technique, and Outcomes. JBJS Rev. 2018, 6, e5. [Google Scholar] [CrossRef]
- Minas, T.; Von Keudell, A.; Bryant, T.; Gomoll, A.H. The John Insall Award: A Minimum 10-Year Outcome Study of Autologous Chondrocyte Implantation. Clin. Orthop. 2014, 472, 41–51. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Brophy, R.H.; Lattermann, C.; Carey, J.L.; Flanigan, D.C. Failures, Re-Operations, and Complications after Autologous Chondrocyte Implantation—A Systematic Review. Osteoarthritis Cartilage 2011, 19, 779–791. [Google Scholar] [CrossRef]
- Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications. Polymers 2022, 14, 839. [Google Scholar] [CrossRef]
- Foster, N.C.; Henstock, J.R.; Reinwald, Y.; El Haj, A.J. Dynamic 3D Culture: Models of Chondrogenesis and Endochondral Ossification. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Householder, N.A.; Raghuram, A.; Agyare, K.; Thipaphay, S.; Zumwalt, M. A Review of Recent Innovations in Cartilage Regeneration Strategies for the Treatment of Primary Osteoarthritis of the Knee: Intra-Articular Injections. Orthop. J. Sports Med. 2023, 11, 23259671231155950. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-G.; Kang, K.S.; Hong, J.M.; Jang, J.; Park, M.N.; Jeong, Y.H.; Cho, D.-W. Effects of Electromagnetic Field Frequencies on Chondrocytes in 3D Cell-Printed Composite Constructs. J. Biomed. Mater. Res. A 2016, 104, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and Tissue Engineering Strategies for Articular Cartilage and Meniscus Repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef]
- Stefani, R.M.; Barbosa, S.; Tan, A.R.; Setti, S.; Stoker, A.M.; Ateshian, G.A.; Cadossi, R.; Vunjak-Novakovic, G.; Aaron, R.K.; Cook, J.L.; et al. Pulsed Electromagnetic Fields Promote Repair of Focal Articular Cartilage Defects with Engineered Osteochondral Constructs. Biotechnol. Bioeng. 2020, 117, 1584–1596. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Liu, X.; Zhu, D.; Dang, J.; Xue, Y.; Fan, H. Investigating the Protective Effect of Tanshinone IIA against Chondrocyte Dedifferentiation: A Combined Molecular Biology and Network Pharmacology Approach. Ann. Transl. Med. 2021, 9, 249. [Google Scholar] [CrossRef]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; de Llano, J.J.M.; Carda, C. In Vivo Articular Cartilage Regeneration Using Human Dental Pulp Stem Cells Cultured in an Alginate Scaffold: A Preliminary Study. Stem Cells Int. 2017, 2017, 8309256. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, Z.; Liu, K.; Wan, Y.; Li, X.; Luo, X.; Bai, Y.; Yang, Z.; Feng, G. Repair of Articular Cartilage Defects in Rabbits through Tissue-Engineered Cartilage Constructed with Chitosan Hydrogel and Chondrocytes. J. Zhejiang Univ. Sci. B 2015, 16, 914–923. [Google Scholar] [CrossRef]
- Heirani-Tabasi, A.; Hosseinzadeh, S.; Rabbani, S.; Ahmadi Tafti, S.H.; Jamshidi, K.; Soufizomorrod, M.; Soleimani, M. Cartilage Tissue Engineering by Co-Transplantation of Chondrocyte Extracellular Vesicles and Mesenchymal Stem Cells, Entrapped in Chitosan-Hyaluronic Acid Hydrogel. Biomed. Mater. Bristol Engl. 2021, 16, 055003. [Google Scholar] [CrossRef]
- Chen, J.; An, P.; Zhang, H.; Zhang, Y.; Wei, H.; Zhou, Y.; Zhu, Y. Hydrogels with Tunable Modulus Regulate Chondrocyte Microaggregates Growth for Cartilage Repair. Biomed. Mater. Bristol Engl. 2021, 17, 014106. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Liu, Q.; Lu, Z.; Zheng, L.; Zhao, J.; Zhang, X. Correction: Therapy for Cartilage Defects: Functional Ectopic Cartilage Constructed by Cartilage-Simulating Collagen, Chondroitin Sulfate and Hyaluronic Acid (CCH) Hybrid Hydrogel with Allogeneic Chondrocytes. Biomater. Sci. 2018, 6, 2270. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.H.; Jeun, J.H.; Kim, D.H.; Park, S.H.; Kim, S.-J.; Lee, W.S.; Hwang, S.H.; Lim, J.Y.; Kim, S.W. Evaluation of Collagen Gel-Associated Human Nasal Septum-Derived Chondrocytes As a Clinically Applicable Injectable Therapeutic Agent for Cartilage Repair. Tissue Eng. Regen. Med. 2020, 17, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, W.; Zhao, R.; Lu, W.; Chen, L.; Su, W.; Zeng, M.; Hu, Y. Fabrication and Characterization of Microstructure-Controllable COL-HA-PVA Hydrogels for Cartilage Repair. J. Mater. Sci. Mater. Med. 2021, 32, 100. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, J.; Lu, G.; Xie, Y.; Sun, Y.; Wang, Q.; Liang, J.; Fan, Y.; Zhang, X. Repair of Osteochondral Defects in a Rabbit Model with Artificial Cartilage Particulates Derived from Cultured Collagen-Chondrocyte Microspheres. J. Mater. Chem. B 2018, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Lafont, J.E.; Buffier, M.; Verset, M.; Cohendet, A.; Contamin, H.; Confais, J.; Sankar, S.; Rioult, M.; Perrier-Groult, E.; et al. Repair of Full-Thickness Articular Cartilage Defects Using IEIK13 Self-Assembling Peptide Hydrogel in a Non-Human Primate Model. Sci. Rep. 2021, 11, 4560. [Google Scholar] [CrossRef]
- Tee, C.A.; Yang, Z.; Wu, Y.; Ren, X.; Baranski, M.; Lin, D.J.; Hassan, A.; Han, J.; Lee, E.H. A Pre-Clinical Animal Study for Zonal Articular Cartilage Regeneration Using Stratified Implantation of Microcarrier Expanded Zonal Chondrocytes. Cartilage 2022, 13, 19476035221093063. [Google Scholar] [CrossRef]
- Wang, L.-S.; Du, C.; Toh, W.S.; Wan, A.C.A.; Gao, S.J.; Kurisawa, M. Modulation of Chondrocyte Functions and Stiffness-Dependent Cartilage Repair Using an Injectable Enzymatically Crosslinked Hydrogel with Tunable Mechanical Properties. Biomaterials 2014, 35, 2207–2217. [Google Scholar] [CrossRef]
- Wang, C.-C.; Yang, K.-C.; Lin, K.-H.; Liu, Y.-L.; Yang, Y.-T.; Kuo, T.-F.; Chen, I.-H. Expandable Scaffold Improves Integration of Tissue-Engineered Cartilage: An In Vivo Study in a Rabbit Model. Tissue Eng. Part A 2016, 22, 873–884. [Google Scholar] [CrossRef]
- Niemietz, T.; Zass, G.; Hagmann, S.; Diederichs, S.; Gotterbarm, T.; Richter, W. Xenogeneic Transplantation of Articular Chondrocytes into Full-Thickness Articular Cartilage Defects in Minipigs: Fate of Cells and the Role of Macrophages. Cell Tissue Res. 2014, 358, 749–761. [Google Scholar] [CrossRef]
- Hua, Y.; Xia, H.; Jia, L.; Zhao, J.; Zhao, D.; Yan, X.; Zhang, Y.; Tang, S.; Zhou, G.; Zhu, L.; et al. Ultrafast, Tough, and Adhesive Hydrogel Based on Hybrid Photocrosslinking for Articular Cartilage Repair in Water-Filled Arthroscopy. Sci. Adv. 2021, 7, eabg0628. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Rui, B.; Lin, J.; Shen, J.; Xiao, H.; Liu, X.; Chai, Y.; Xu, J.; Yang, Y. A Photoannealed Granular Hydrogel Facilitating Hyaline Cartilage Regeneration via Improving Chondrogenic Phenotype. ACS Appl. Mater. Interfaces 2022, 14, 40674–40687. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Chopra, V.; Rajput, S.; Guha, R.; Chattopadhyay, N.; Ghosh, D. Post-Implantation Stiffening by a Bioinspired, Double-Network, Self-Healing Hydrogel Facilitates Minimally Invasive Cell Delivery for Cartilage Regeneration. Biomacromolecules 2023, 24, 3313–3326. [Google Scholar] [CrossRef]
- Bothe, F.; Deubel, A.-K.; Hesse, E.; Lotz, B.; Groll, J.; Werner, C.; Richter, W.; Hagmann, S. Treatment of Focal Cartilage Defects in Minipigs with Zonal Chondrocyte/Mesenchymal Progenitor Cell Constructs. Int. J. Mol. Sci. 2019, 20, 653. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, F.; Tsang, W.P.; Wan, C.; Wu, C. Fabrication of Injectable High Strength Hydrogel Based on 4-Arm Star PEG for Cartilage Tissue Engineering. Biomaterials 2017, 120, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xiao, M.; Almaqrami, B.S.; Kang, H.; Shao, Z.; Chen, X.; Zhang, Y. Regenerated Silk Fibroin Based on Small Aperture Scaffolds and Marginal Sealing Hydrogel for Osteochondral Defect Repair. Biomater. Res. 2023, 27, 50. [Google Scholar] [CrossRef] [PubMed]
- Critchley, S.; Sheehy, E.J.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S.F.; Jeon, O.; Alsberg, E.; Brama, P.A.J.; Kelly, D.J. 3D Printing of Fibre-Reinforced Cartilaginous Templates for the Regeneration of Osteochondral Defects. Acta Biomater. 2020, 113, 130–143. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Fan, L.; Gao, C.; Liu, X.; Jing, X.; Zhang, H.; Huang, Y.; Guo, R.; Long, C.; et al. Cartilage Injury Repair by Human Umbilical Cord Wharton’s Jelly/Hydrogel Combined with Chondrocyte. Tissue Eng. Part C Methods 2023, 29, 110–120. [Google Scholar] [CrossRef]
- Godugu, C.; Patel, A.R.; Desai, U.; Andey, T.; Sams, A.; Singh, M. AlgiMatrixTM Based 3D Cell Culture System as an In-Vitro Tumor Model for Anticancer Studies. PloS One 2013, 8, e53708. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-Laden Hydrogels for Osteochondral and Cartilage Tissue Engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering. Front. Chem. 2020, 8, 53. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate Hydrogels for Bone Tissue Engineering, from Injectables to Bioprinting: A Review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef] [PubMed]
- Cigan, A.D.; Roach, B.L.; Nims, R.J.; Tan, A.R.; Albro, M.B.; Stoker, A.M.; Cook, J.L.; Vunjak-Novakovic, G.; Hung, C.T.; Ateshian, G.A. High Seeding Density of Human Chondrocytes in Agarose Produces Tissue-Engineered Cartilage Approaching Native Mechanical and Biochemical Properties. J. Biomech. 2016, 49, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.A.; Nair, L.S. Injectable Hydrogels for Bone and Cartilage Repair. Biomed. Mater. Bristol Engl. 2012, 7, 024105. [Google Scholar] [CrossRef]
- Comblain, F.; Rocasalbas, G.; Gauthier, S.; Henrotin, Y. Chitosan: A Promising Polymer for Cartilage Repair and Viscosupplementation. Biomed. Mater. Eng. 2017, 28, S209–S215. [Google Scholar] [CrossRef]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules 2020, 11, 29. [Google Scholar] [CrossRef]
- Kowalski, M.A.; Fernandes, L.M.; Hammond, K.E.; Labib, S.; Drissi, H.; Patel, J.M. Cartilage-Penetrating Hyaluronic Acid Hydrogel Preserves Tissue Content and Reduces Chondrocyte Catabolism. J. Tissue Eng. Regen. Med. 2022, 16, 1138–1148. [Google Scholar] [CrossRef]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Shakibaei, M.; Csaki, C.; Mobasheri, A. Diverse Roles of Integrin Receptors in Articular Cartilage. Adv. Anat. Embryol. Cell Biol. 2008, 197, 1–60. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L.; et al. Natural Hydrogels for Cartilage Regeneration: Modification, Preparation and Application. J. Orthop. Transl. 2019, 17, 26–41. [Google Scholar] [CrossRef]
- Jia, X.; Fan, X.; Chen, C.; Lu, Q.; Zhou, H.; Zhao, Y.; Wang, X.; Han, S.; Ouyang, L.; Yan, H.; et al. Chemical and Structural Engineering of Gelatin-Based Delivery Systems for Therapeutic Applications: A Review. Biomacromolecules 2024, 25, 564–589. [Google Scholar] [CrossRef] [PubMed]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, Preparation and Use of Cell-Laden Gelatin Methacryloyl-Based Hydrogels as Modular Tissue Culture Platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Bayer, I.S. Advances in Fibrin-Based Materials in Wound Repair: A Review. Mol. Basel Switz. 2022, 27, 4504. [Google Scholar] [CrossRef]
- Sanz-Horta, R.; Matesanz, A.; Gallardo, A.; Reinecke, H.; Jorcano, J.L.; Acedo, P.; Velasco, D.; Elvira, C. Technological Advances in Fibrin for Tissue Engineering. J. Tissue Eng. 2023, 14, 20417314231190288. [Google Scholar] [CrossRef]
- Rojas-Murillo, J.A.; Simental-Mendía, M.A.; Moncada-Saucedo, N.K.; Delgado-Gonzalez, P.; Islas, J.F.; Roacho-Pérez, J.A.; Garza-Treviño, E.N. Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair. Int. J. Mol. Sci. 2022, 23, 9879. [Google Scholar] [CrossRef]
- Barsotti, M.C.; Magera, A.; Armani, C.; Chiellini, F.; Felice, F.; Dinucci, D.; Piras, A.M.; Minnocci, A.; Solaro, R.; Soldani, G.; et al. Fibrin Acts as Biomimetic Niche Inducing Both Differentiation and Stem Cell Marker Expression of Early Human Endothelial Progenitor Cells. Cell Prolif. 2011, 44, 33–48. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. ScientificWorldJournal 2015, 2015, 685690. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable Natural Polymer Hydrogels for Articular Cartilage Tissue Engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Almany, L.; Seliktar, D. Biosynthetic Hydrogel Scaffolds Made from Fibrinogen and Polyethylene Glycol for 3D Cell Cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Belda Marín, C.; Fitzpatrick, V.; Kaplan, D.L.; Landoulsi, J.; Guénin, E.; Egles, C. Silk Polymers and Nanoparticles: A Powerful Combination for the Design of Versatile Biomaterials. Front. Chem. 2020, 8, 604398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Cui, J.; Wu, S.; Geng, Z.; Su, J. Silk Fibroin-Based Biomaterials for Cartilage/Osteochondral Repair. Theranostics 2022, 12, 5103–5124. [Google Scholar] [CrossRef] [PubMed]
- Madappura, A.P.; Madduri, S. A Comprehensive Review of Silk-Fibroin Hydrogels for Cell and Drug Delivery Applications in Tissue Engineering and Regenerative Medicine. Comput. Struct. Biotechnol. J. 2023, 21, 4868–4886. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Hwang, Y.; Chen, A.C.; Varghese, S.; Sah, R.L. Cartilage-like Mechanical Properties of Poly (Ethylene Glycol)-Diacrylate Hydrogels. Biomaterials 2012, 33, 6682–6690. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S. The Effects of Scaffold Thickness on Tissue Engineered Cartilage in Photocrosslinked Poly(Ethylene Oxide) Hydrogels. Biomaterials 2001, 22, 619–626. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S.; Lee, D.A.; Bader, D.L. Crosslinking Density Influences the Morphology of Chondrocytes Photoencapsulated in PEG Hydrogels during the Application of Compressive Strain. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2004, 22, 1143–1149. [Google Scholar] [CrossRef]
- Sridhar, B.V.; Doyle, N.R.; Randolph, M.A.; Anseth, K.S. Covalently Tethered TGF-Β1 with Encapsulated Chondrocytes in a PEG Hydrogel System Enhances Extracellular Matrix Production. J. Biomed. Mater. Res. A 2014, 102, 4464–4472. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced Hydrogels for the Repair of Cartilage Defects and Regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, J.; Wang, S.; Liu, W. PVA-Based Hydrogels: Promising Candidates for Articular Cartilage Repair. Macromol. Biosci. 2021, 21, e2100147. [Google Scholar] [CrossRef]
- Branco, A.C.; Oliveira, A.S.; Monteiro, I.; Nolasco, P.; Silva, D.C.; Figueiredo-Pina, C.G.; Colaço, R.; Serro, A.P. PVA-Based Hydrogels Loaded with Diclofenac for Cartilage Replacement. Gels Basel Switz. 2022, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a Novel Polyvinyl Alcohol/Chitosan Porous Hydrogel Combined with Bone Marrow Mesenchymal Stem Cells and Its Application in Articular Cartilage Repair. BMC Musculoskelet. Disord. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, Y.; Li, X.; Liu, W.; Yu, X.; Yan, F.; Sun, J. Dynamic Hydrophobic Domains Enable the Fabrication of Mechanically Robust and Highly Elastic Poly(Vinyl Alcohol)-Based Hydrogels with Excellent Self-Healing Ability. ACS Mater. Lett. 2020, 2, 764–770. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, Q.; Yu, H.; Shao, L.; Cui, X.; Pang, Q.; Zhu, Y.; Hou, R. Construction Methods and Biomedical Applications of PVA-Based Hydrogels. Front. Chem. 2024, 12, 1376799. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, W.; Badv, M.; Moshaverinia, A.; Weiss, P.S. Modified Poly(ε-Caprolactone) with Tunable Degradability and Improved Biofunctionality for Regenerative Medicine. ACS Mater. Au 2023, 3, 540–547. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent Advances in 3D-Printed Polylactide and Polycaprolactone-Based Biomaterials for Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Properties and Medical Applications of Polylactic Acid: A Review. EXPRESS Polym. Lett. 2015, 9, 435–455. [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(Lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties-From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Vaid, R.; Yildirim, E.; Pasquinelli, M.A.; King, M.W. Hydrolytic Degradation of Polylactic Acid Fibers as a Function of pH and Exposure Time. Mol. Basel Switz. 2021, 26, 7554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, S.; Chen, H.; Wang, S.; Nie, K.; Li, Z. Effects of Poly(Ethylene Glycol) Grafted Silica Nanoparticles on Crystallization Behavior of Poly(d-Lactide). Polym. Int. 2015, 64, 1066–1071. [Google Scholar] [CrossRef]
- Xie, D.; Zhao, Y.; Li, Y.; LaChance, A.M.; Lai, J.; Sun, L.; Chen, J. Rheological, Thermal, and Degradation Properties of PLA/PPG Blends. Mater. Basel Switz. 2019, 12, 3519. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, J. Poly(Lactide-Co-Glycolide) Porous Scaffolds for Tissue Engineering and Regenerative Medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef]
- Trengove, A.; Di Bella, C.; O’Connor, A.J. The Challenge of Cartilage Integration: Understanding a Major Barrier to Chondral Repair. Tissue Eng. Part B Rev. 2022, 28, 114–128. [Google Scholar] [CrossRef]
- Jelodari, S.; Ebrahimi Sadrabadi, A.; Zarei, F.; Jahangir, S.; Azami, M.; Sheykhhasan, M.; Hosseini, S. New Insights into Cartilage Tissue Engineering: Improvement of Tissue-Scaffold Integration to Enhance Cartilage Regeneration. BioMed Res. Int. 2022, 2022, 7638245. [Google Scholar] [CrossRef]
- Lotz, B.; Bothe, F.; Deubel, A.-K.; Hesse, E.; Renz, Y.; Werner, C.; Schäfer, S.; Böck, T.; Groll, J.; von Rechenberg, B.; et al. Preclinical Testing of New Hydrogel Materials for Cartilage Repair: Overcoming Fixation Issues in a Large Animal Model. Int. J. Biomater. 2021, 2021, 5583815. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Quinn, T.M. Surgical Removal of Articular Cartilage Leads to Loss of Chondrocytes from Cartilage Bordering the Wound Edge. J. Bone Joint Surg. Am. 2003, 85 (Suppl. 2), 85–92. [Google Scholar] [CrossRef]
- Patel, J.M.; Sennett, M.L.; Martin, A.R.; Saleh, K.S.; Eby, M.R.; Ashley, B.S.; Miller, L.M.; Dodge, G.R.; Burdick, J.A.; Carey, J.L.; et al. Resorbable Pins to Enhance Scaffold Retention in a Porcine Chondral Defect Model. Cartilage 2021, 13, 1676S–1687S. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, J.H.; Zlotnick, H.M.; Locke, R.C.; Gupta, S.; Fogarty, N.L.; Masada, K.M.; Stoeckl, B.D.; Laforest, L.; Castilho, M.; Malda, J.; et al. Evaluation of Surgical Fixation Methods for the Implantation of Melt Electrowriting-Reinforced Hyaluronic Acid Hydrogel Composites in Porcine Cartilage Defects. Int. J. Bioprinting 2023, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Schuurmans, C.C.L.; Mihajlovic, M.; Hiemstra, C.; Ito, K.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid and Chondroitin Sulfate (Meth)Acrylate-Based Hydrogels for Tissue Engineering: Synthesis, Characteristics and Pre-Clinical Evaluation. Biomaterials 2021, 268, 120602. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional Chondroitin Sulphate for Cartilage Tissue-Biomaterial Integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Fermanian, S.; Gibson, M.; Unterman, S.; Herzka, D.A.; Cascio, B.; Coburn, J.; Hui, A.Y.; Marcus, N.; Gold, G.E.; et al. Human Cartilage Repair with a Photoreactive Adhesive-Hydrogel Composite. Sci. Transl. Med. 2013, 5, 167ra6. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R.; et al. Catechol-Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Singla, S.; Amarpuri, G.; Dhopatkar, N.; Blackledge, T.A.; Dhinojwala, A. Hygroscopic Compounds in Spider Aggregate Glue Remove Interfacial Water to Maintain Adhesion in Humid Conditions. Nat. Commun. 2018, 9, 1890. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry Double-Sided Tape for Adhesion of Wet Tissues and Devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Kuang, B.; Yang, Y.; Lin, H. Infiltration and In-Tissue Polymerization of Photocross-Linked Hydrogel for Effective Fixation of Implants into Cartilage-An In Vitro Study. ACS Omega 2019, 4, 18540–18544. [Google Scholar] [CrossRef]
- Arvayo, A.L.; Wong, I.J.; Dragoo, J.L.; Levenston, M.E. Enhancing Integration of Articular Cartilage Grafts via Photochemical Bonding. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 2406–2415. [Google Scholar] [CrossRef]
- Zhao, J.; Kirillova, A.; Kelly, C.N.; Xu, H.; Koshut, W.J.; Yang, F.; Gall, K.; Wiley, B.J. High-Strength Hydrogel Attachment through Nanofibrous Reinforcement. Adv. Healthc. Mater. 2021, 10, e2001119. [Google Scholar] [CrossRef]
- Janssen, L.M.; In der Maur, C.D.; Bos, P.K.; Hardillo, J.A.; van Osch, G.J.V.M. Short-Duration Enzymatic Treatment Promotes Integration of a Cartilage Graft in a Defect. Ann. Otol. Rhinol. Laryngol. 2006, 115, 461–468. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Singhrao, S.K.; Khan, I.M.; Gonzalez, L.G.; Thomson, B.M.; Burdon, D.; Duance, V.C.; Archer, C.W. Enhanced Tissue Integration during Cartilage Repair in Vitro Can Be Achieved by Inhibiting Chondrocyte Death at the Wound Edge. Tissue Eng. Part A 2009, 15, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, T.; Lottman, L.M.; Harwood, F.; Amiel, D.; Sah, R.L. Integrative Cartilage Repair: Inhibition by Beta-Aminopropionitrile. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1999, 17, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Athens, A.A.; Makris, E.A.; Hu, J.C. Induced Collagen Cross-Links Enhance Cartilage Integration. PloS One 2013, 8, e60719. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.J.; Levenston, M.E. Maturation and Integration of Tissue-Engineered Cartilages within an in Vitro Defect Repair Model. Tissue Eng. 2004, 10, 736–746. [Google Scholar] [CrossRef]
- Lutzweiler, G.; Ndreu Halili, A.; Engin Vrana, N. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Kim, H.; Kumbar, S.G.; Nukavarapu, S.P. Biomaterial-Directed Cell Behavior for Tissue Engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100260. [Google Scholar] [CrossRef]
- Wu, M.; Wu, S.; Chen, W.; Li, Y.-P. The Roles and Regulatory Mechanisms of TGF-β and BMP Signaling in Bone and Cartilage Development, Homeostasis and Disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef]
- Peng, Y.; Zhuang, Y.; Liu, Y.; Le, H.; Li, D.; Zhang, M.; Liu, K.; Zhang, Y.; Zuo, J.; Ding, J. Bioinspired Gradient Scaffolds for Osteochondral Tissue Engineering. Explor. Beijing China 2023, 3, 20210043. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Faurholm, B.; Dell’Accio, F.; Manzo, A.; Seed, M.; Eltawil, N.; Marrelli, A.; Gould, D.; Subang, C.; Al-Kashi, A.; et al. Human Single-Chain Variable Fragment That Specifically Targets Arthritic Cartilage. Arthritis Rheum. 2010, 62, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent Advances in Hydrogels for Cartilage Tissue Engineering. Eur. Cell. Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Boushell, M.K.; Hung, C.T.; Hunziker, E.B.; Strauss, E.J.; Lu, H.H. Current Strategies for Integrative Cartilage Repair. Connect. Tissue Res. 2017, 58, 393–406. [Google Scholar] [CrossRef] [PubMed]

| Grade 1 | Soft indentations and/or superficial cracks |
| Grade 2 | Small cracks or lesions that extend to less than half of the cartilage depth |
| Grade 3 | Deep cracks or gaps exceeding 50% of the cartilage depth |
| Grade 4 | Cracks extending the entire thickness of the cartilage to the underlying bone |
| Grade 5 | Defects encompassing full thickness of cartilage, involving the subchondral bone |
| Material | Cell Source | Animal Model | Defect Size (⌀ × Depth) | Seeding Density (Cells/mL) | Weeks | Summary + Outcome Measures (ICRS/OARSI/Young’s Modulus) | * Ref. | |
|---|---|---|---|---|---|---|---|---|
| Agarose | dogs | dogs | 6 mm × 1 mm | 30 × 106 | 13 | Application of pulsed electromagnetic fields to tissue-engineered scaffolds resulted in increased outcome scores and enhanced repair outcomes, irrespective of the implantation or microfracture procedures utilized. | OARSI Scores at 13 weeks: TE + PEMF = 19, TE-PEMF = 18.5 KEY: TE = tissue engineered scaffolds, PEMF = pulsed electromagnetic fields, +/− = with/without PEMF | 1 |
| Alginate, sodium hyaluronate (Alg-HA) | rats | rats | 0.8 mm × 1.2–1.5 mm | 4 × 105 | 3 | The administration of TIIA through scaffolds in pharmacological applications demonstrated protective effects against chondrocyte dedifferentiation. Furthermore, they pinpointed several regulatory pathways, with SOX6 emerging as a primary candidate believed to directly regulate the chondrocyte dedifferentiation process. | N/A—histology and immunohistochemistry | 2 |
| Alginate | rabbits | rabbits | 3 mm × 1 mm | 2 × 106 | 13 | In contrast to blank scaffolds and primary chondrocytes, hDPSCs exhibited superior chondrogenic outcomes. These results could be attributed to the potential anti-inflammatory properties of hDPSCs, as well as the duration of incubation in a chondrogenic medium. | N/A—histology and immunohistochemistry | 3 |
| Chitosan | rabbits | rabbits | 4 mm × 3 mm | 1 × 107 | 4, 8, 12 | The experimental group exhibited notably higher average values in both gross and histological assessments (p < 0.05). The authors observed that repair occurred independently with pure chitosan hydrogels. | ICRS Morphology Scores at 12 weeks: Control = ~6, Blank = 7, Experimental = ~10 Histology Scores at 12 weeks: Control = 10, Blank = 5, Experimental = 16 | 4 |
| Chitosan, hyaluronic acid (CS-HA) | TKA human patients | rabbits | 4 mm × 3 mm | 7 × 105 | 4, 24 | Among the various scaffolds examined, the CS-HA/EV/MSC group displayed moderately encouraging ICRS (MORPH SCORE = 2.2) after 4 weeks. However, after 24 weeks, it demonstrated scores most akin to “normal” unimpaired cartilage (MORPH SCORE = 4) in comparison to other experimental scaffold combinations. | ICRS Gross Morphology Scores at 24 weeks via MRI evaluation (p < 0.01 and p< 0.0001): EV/MSC = 2.65, MSC = 1.85, CS-HA = 0.65, Control = 0.55 Histology Scores at 24 weeks: Normal = 3, Control = 0, CS-HA = 0, MSC = 1.1, EV/MSC = 2.15, CS-HA/MSC = 2.9, CS-HA/EV/MSC = 3 | 5 |
| Chitosan methacrylate, PVA (CHMA-PVA) | rabbits | rabbits | 4 mm × 4 mm | 1 × 107 | 8 | Initial findings indicated cartilage regeneration when compared to the control group. The CHMA-PVA hydrogels exhibited promising features including complete closure, effective integration of superficial neocartilage, and manageable inflammation, suggesting their potential as biocompatible scaffolds for accelerated regeneration. | N/A—histology and immunohistochemistry, rheological testing | 6 |
| Chondroitin sulfate/collagen/hyaluronic acid (CCH) | rabbits | rabbits | 3 mm × 2 mm | 5 × 107 | 4, 8, 12 | Consistently, the CCH scaffold maintained significantly higher scores compared to both the collagen group and the control group (p < 0.05). Notably, while collagen showed clear margins, the edges of the CCH group were indistinguishable at multiple points. | ICRS Gross Morphology Scores at 12 weeks: Control = 11, CCH = 13, C = ~11 KEY: C = cell-collagen group, CCH = cell-collagen chondroitin sulfate/HA group (conducted at 1, 2, and 3 months by blinded scorers) | 7 |
| Collagen | human patient donors | rats | 2 mm × 2 mm | 1 × 106 | 8 | In animal defect models, hNC collagen hydrogels demonstrated enhanced proteoglycan production and glycosaminoglycan (GAG) synthesis compared to the collagen control group. | N/A—histology and immunohistofluorescence | 8 |
| Collagen, hydroxyapatite, PVA (COL-HA-PVA) | goats | goats | 8 mm × 8 mm | 1 × 106 | 4 | The COL-HA-PVA hydrogel exhibited good biocompatibility and integration compared to the cell-free hydrogel and the empty control. In addition, it was noted that the goat model may have some self-regenerative properties by exhibiting some regeneration in the empty control group based on histology results. | N/A—histology and spectrophotometry | 9 |
| Collagen hydrogel microspheres, artificial cartilage particulates (ACPs) | rabbits | rabbits | 4 mm × 2.5 mm | 1 × 106 | 4, 13 | Artificial cartilage particulates (ACPs) within collagen hydrogels demonstrated comparable repair outcomes to native tissue. Notably, there was significantly improved cell migration observed after 7 days compared to 14 days of in vitro culture. Acknowledging limitations, the authors aim to explore alternative materials to enhance outcomes. | Young’s modulus Collagen hydrogel = 22 kPa ACP 7 days = 81 kPa ACP 14 days = 115 kPa | 10 |
| Fibrin and IEIK13 | monkeys | monkeys | 3.5 mm | 1 × 106 | 12 | The incorporation of IEIK13 into scaffolds that were empty or cell-laden with chondrocytes produced similar and comparable results. This outcome supports the incorporation of EIK13 in regenerative efforts of osteochondral defects. | ICRS Only the quality of repair was assessed in the three animal subjects. Model 1 and Model 2 defects were classified as grade II (nearly normal), while Model 3 was graded as grade III (abnormal repair). The outcomes comparing seeded versus acellular scaffolds were depicted through images rather than numerical scores. | 11 |
| Fibrin | mini pigs | mini pigs | 3 mm × 1–2 mm 6 mm × 1 mm | 1 × 107 | 26 | Encouraging outcomes regarding zonal chondrocyte architecture and the formation of healthy subchondral bone support the effectiveness of a stratified zonal chondrocyte implantation approach. | Young’s modulus Full thickness: WB = < 0.1 MPa, NWB = ~0.3 MPa Medium/large chondrocytes: WB = ~0.25 MPa, NWB = ~0.25 MPa | 12 |
| Gelatin-hydroxyphenyl propionic acid (Gtn-HPA) | rabbits | rabbits | 2 mm × 1 mm | 1 × 107 | 4, 12 | In comparing hydrogels with different stiffness levels, discovered that medium-stiffness hydrogels yielded favorable regenerative results in defect models compared to low- and high-stiffness ones. Additionally, the cellular processes of chondrocytes were found to be influenced by the stiffness of the hydrogel. | N/A—Wakitani, histological grading scale, rheological assessment | 13 |
| Gelatin, microbubbles | rabbits | rabbits | 3 mm × 3 mm | 1 × 106 | 8, 17, 26 | The expandable hydrogel model demonstrated encouraging outcomes, achieving an 87% integration at the interfaces over a period of up to 6 months. Moreover, the hydrogel effectively absorbed high compressive forces comparable to those experienced by normal cartilage in the defect models. | Young’s modulus at 8 weeks: S = 0.22 C + S = 0.41 Control = N/A at 17 weeks: S = 0.27 C + S = 0.59 Control = 0.89 at 26 weeks: S = 0.32 C + S = 0.83 Control = N/A KEY: S = scaffold only (blank), C + S = cells + scaffold | 14 |
| Hyaluronic acid–albumin Novocart Inject and fibrin glue | human patient donors | mini pigs | 6 mm diameter | 1 × 106 | 2, 4 | Hydrogels containing human articular cartilage experienced rapid degeneration within 2 weeks post-implantation, accompanied by the infiltration of surrounding macrophages. Consequently, these grafts were rejected in the animal models, leading to a lack of cartilage regeneration. | N/A—blinded histomorphometry evaluation and modified O’Driscoll scores | 15 |
| Hyaluronic acid, MES, and NB | pigs | pigs | 7 mm diameter (full thickness) | 100 × 106 | 4, 13, 26 | Following implantation, the hydrogel exhibited indications of successful cartilage regeneration; however, excessive, and hypertrophic cartilage growth was noted. It was therefore concluded that the hydrogel was biocompatible and viable, but the use of BMSCs was proposed instead of chondrocytes due to their dual osteogenic and chondrogenic characteristics. | Young’s modulus Normal = 22.5 MPa, Control = ~14 MPa, Experimental = ~23 MPa | 16 |
| Hyaluronic acid, PEG, and gelatin | rats | rats | 2 mm × 2 mm | 1 × 107 | 6, 12 | Granular hydrogel (GH) scaffolds replicated cartilage that closely resembled healthy cartilage, whereas nongranular (nGH) scaffolds exhibited a more fibrous appearance. At 12 weeks, GH achieved higher scores compared to nGH. These scores were further analyzed across categories, including matrix staining, cell morphology, surface architecture, basal integration, tissue morphology, and chondrocyte clustering. | ICRS Gross Morphology Scores at 12 weeks: GH = 8.6 nGH = 8.1 Histology Scores at 12 weeks (p < 0.05): Control = 24.2 GH = 65 nGH = 31.9 | 17 |
| CSA-NH2, Odex, and HA-PNIPAAm | human patient donors | rabbits | 3 mm × 2 mm | 5 × 104 | 6 | The bioinspired, double network hydrogel (BDNH) degraded appropriately and facilitated the formation of neocartilage at the defect site in the animal model. Particularly noteworthy was its thermosensitive nature, which led to enhanced stiffening at physiological temperatures upon implantation. | N/A—histology and immunohistochemistry, rheological testing | 18 |
| StarPEG PCL | pigs | mini pigs | 6 mm × 1 mm | 20 × 106 | 26 | The control empty defects had the highest macroscopic ICRS scores compared to the zonal and non-zonal constructs. To this effect, no significant score differences were found between zonal and non-zonal scaffolds. | ICRS Gross Morphology Scores at 26 weeks: Control = ~10 Zonal = ~4 Non-zonal = ~5 (averaged amongst 3 blinded independent scorers) | 19 |
| StarPEG vinyl sulfone (sPEG-VS) | mice | mice | 1 mm × 1 mm | 1 × 105 | 6, 12 | Emphasis on enhancing the mechanical strength of the hydrogels facilitated an optimal environment for cell proliferation. Within the defect model, the development of new hyaline cartilage underscored the scaffold’s biocompatibility, suggesting its potential for future regenerative applications. | Young’s modulus 0 weeks = ~1 kPa 1 week = ~6 kPa 3 weeks = ~11 kPa 6 weeks = ~16 kPa 12 weeks = ~30 kPa | 20 |
| N-butanol and different concentrations of HA, COL1, B-TCP | rabbits | rabbits | 5 mm diameter (full thickness) | 5–7 × 105 | 17, 19 | Various experimental groups displayed accelerated rates of cartilage formation compared to the negative control. Among them, TCP + mixed cells + 14 wt% RSF solution and TCP + mixed cells achieved the highest scores. The former maintained a steady score of 11 at 19 weeks, while the latter saw a significant increase in score from approximately 7 to 10 between 17 and 19 weeks. | ICRS Gross Morphology Scores at 17 weeks: Blank = 2, A = ~2.5, B = 7, C = 5, D = ~7, E = ~10 Gross Morphology Scores at 19 weeks: Blank = 5, A = 5, B = 6, C = 6, D = 10, E = 11 KEY: A = all chondrocytes, B = all BMSCs, C = mixed cells, D = mixed cells + beta-TCP, E = mixed cells + beta-TCP + 14 wt% RSF soln. | 21 |
| PCL, PLA, PLGA (85:15) and PLGA (65:35) | goats | goats | 6 mm × 6 mm | 40 × 106 cells/mL of a 3:1 co-culture of FPSCs/chondrocytes | 26 | Across various categories, there were no significant differences in scores between controls and bi-phasic SA tissues. Nonetheless, in specific categories like matrix staining and cell morphology, distinct significant differences were observed, indicating instances where bi-phasic scaffolds exhibited signs of improvement in repair outcomes. | ICRS Average Histology Scores at 26 weeks: Control = ~40 Bi-phasic SA = ~60 | 22 |
| Human umbilical cord Wharton’s Jelly, GCS + DF-PEG | rabbits | rabbits | ~1.2 mm deep | 5 × 106 (hydrogel) 2 × 105 (HUCWJ) | 13, 26 | The biocompatible nature and availability of HUCWJ resulted in satisfactory outcomes post-implantation. Histological analysis revealed effective integration and notable progress in regeneration at both 3 and 6 months. The authors emphasized that extending the incubation period, particularly up to 6 months, greatly reinforces the efficacy of this approach in cartilage regeneration efforts. | N/A—Wakitani Histological Scoring | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadpoor, X.; Sun, J.; Douglas, N.; Zhu, W.; Lin, H. Hydrogel-Enhanced Autologous Chondrocyte Implantation for Cartilage Regeneration—An Update on Preclinical Studies. Bioengineering 2024, 11, 1164. https://doi.org/10.3390/bioengineering11111164
Ahmadpoor X, Sun J, Douglas N, Zhu W, Lin H. Hydrogel-Enhanced Autologous Chondrocyte Implantation for Cartilage Regeneration—An Update on Preclinical Studies. Bioengineering. 2024; 11(11):1164. https://doi.org/10.3390/bioengineering11111164
Chicago/Turabian StyleAhmadpoor, Xenab, Jessie Sun, Nerone Douglas, Weimin Zhu, and Hang Lin. 2024. "Hydrogel-Enhanced Autologous Chondrocyte Implantation for Cartilage Regeneration—An Update on Preclinical Studies" Bioengineering 11, no. 11: 1164. https://doi.org/10.3390/bioengineering11111164
APA StyleAhmadpoor, X., Sun, J., Douglas, N., Zhu, W., & Lin, H. (2024). Hydrogel-Enhanced Autologous Chondrocyte Implantation for Cartilage Regeneration—An Update on Preclinical Studies. Bioengineering, 11(11), 1164. https://doi.org/10.3390/bioengineering11111164








