Influence of Dextran Solution and Corneal Collagen Crosslinking on Corneal Biomechanical Parameters Evaluated by Corvis ST In Vitro
Abstract
:1. Introduction
2. Materials and Methods
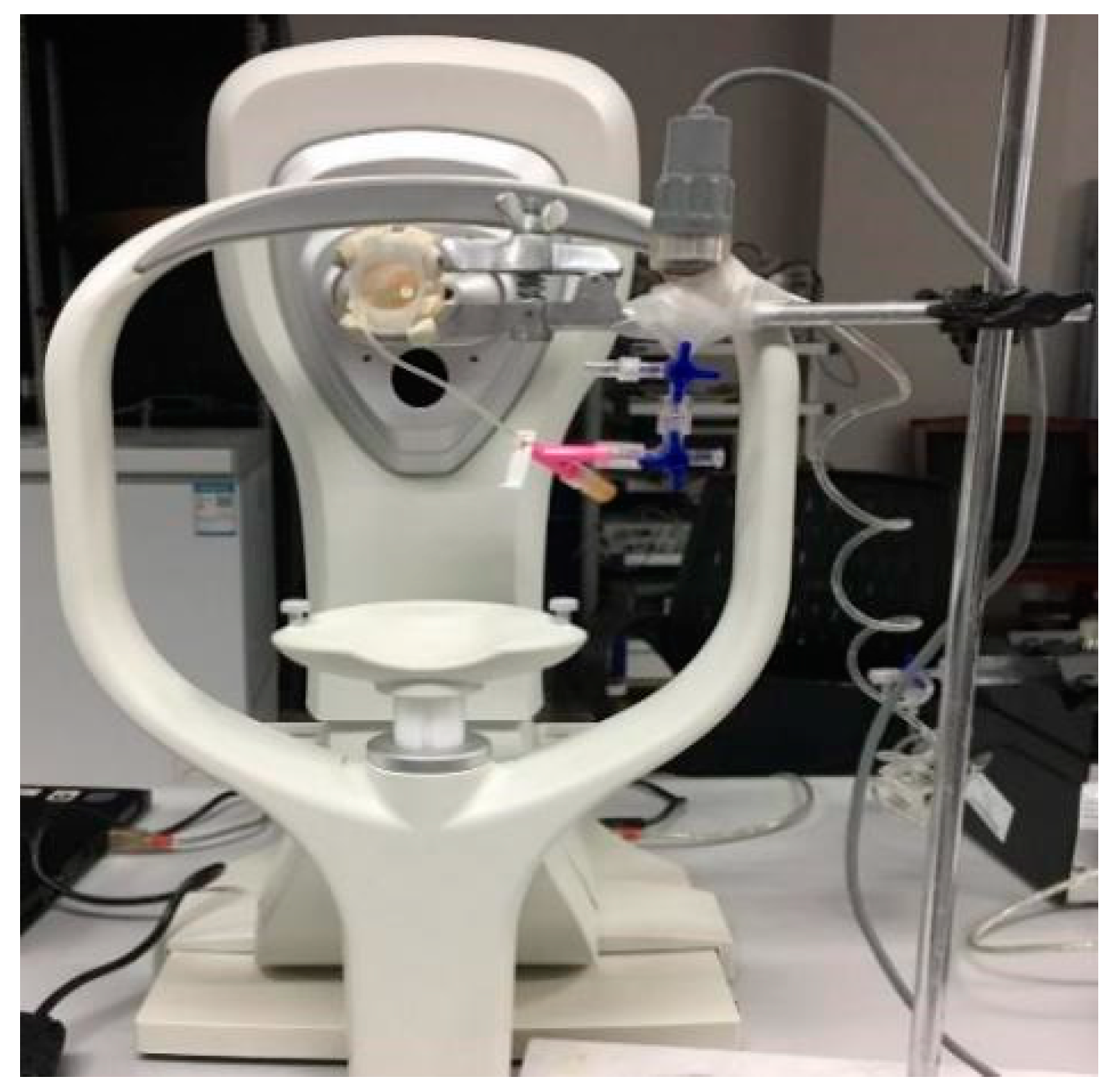
2.1. Materials and Measurements
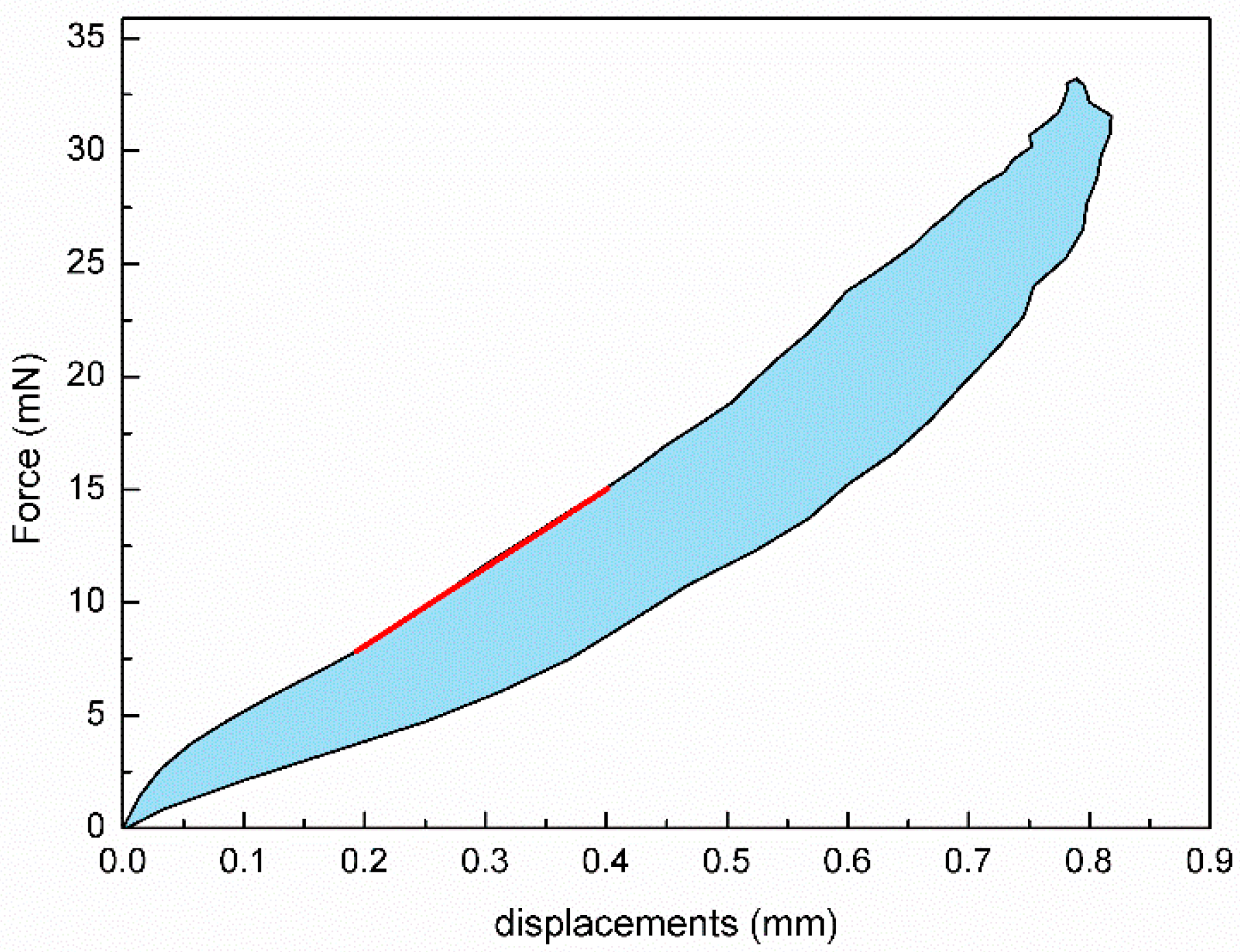
2.2. Determining Corneal Biomechanical Parameters
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Tian, L.; Huang, Y.-F. In Vivo Corneal Biomechanical Properties with Corneal Visualization Scheimpflug Technology in Chinese Population. Biomed. Res. Int. 2016, 2016, 7840284. [Google Scholar]
- Nemeth, G.; Szalai, E.; Hassan, Z.; Lipecz, A.; Flasko, Z.; Modis, L. Corneal Biomechanical Data and Biometric Parameters Measured with Scheimpflug-Based Devices on Normal Corneas. Int. J. Ophthalmol. 2017, 10, 217–222. [Google Scholar] [PubMed]
- Wang, W.; He, M.; He, H.; Zhang, C.; Jin, H.; Zhong, X. Corneal Biomechanical Metrics of Healthy Chinese Adults Using Corvis St. Cont. Lens Anterior Eye 2017, 40, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Pang, C.; Liu, C.; Cheng, W.; Ming, S.; Du, W.; Feng, X. Correlations among Corneal Biomechanical Parameters, Stiffness, and Thickness Measured Using Corvis St and Pentacam in Patients with Ocular Hypertension. J. Ophthalmol. 2022, 2022, 7387581. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, L.; Guo, L.L.; Hao, Y.; Jie, Y. In Vivo Corneal Biomechanical Properties in a Selected Chinese Population, Measured Using the Corneal Visualization Scheimpflug Technology. Front. Bioeng. Biotechnol. 2022, 10, 863240. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.N.; Jhanji, V.; Wang, X.; Li, D.; Du, X. Parental Corneal Tomographic and Biomechanical Characteristics of Patients with Keratoconus. Am. J. Ophthalmol. 2023, 256, 146–155. [Google Scholar] [CrossRef]
- Lu, W.; Ding, W.; Ji, R.; Tian, Y.; Zhao, C.; Li, H.; Jiao, M.; Guo, Z.; Leng, L. Repeatability and Correlation of Corneal Biomechanical Measurements Obtained by Corvis St in Orthokeratology Patients. Cont. Lens Anterior Eye 2023, 46, 101793. [Google Scholar] [CrossRef]
- Wang, L.K.; Tian, L.; Zheng, Y.P. Determining in vivo elasticity and viscosity with dynamic Scheimpflug imaging analysis in keratoconic and healthy eyes. J. Biophotonics 2016, 9, 454–463. [Google Scholar] [CrossRef]
- Qin, X.; Tian, L.; Zhang, H.; Chen, X.; Li, L. Evaluation of corneal elastic modulus based on Corneal Visualization Scheimpflug Technology. Biomed. Eng. Online 2019, 18, 42. [Google Scholar] [CrossRef]
- Tian, L.; Qin, X.; Zhang, H.; Zhang, D.; Guo, L.L.; Zhang, H.X.; Wu, Y.; Jie, Y.; Li, L. A Potential Screening Index of Corneal Biomechanics in Healthy Subjects, Forme Fruste Keratoconus Patients and Clinical Keratoconus Patients. Front. Bioeng. Biotechnol. 2021, 9, 766605. [Google Scholar] [CrossRef]
- Sinjab, M.M.; Cummings, A.B. Corneal Cross-linking in Children. Corneal Collagen Cross Linking. 2017, 8, 229–268. [Google Scholar]
- Subasinghe, S.K.; Ogbuehi, K.C.; Dias, G.J. Current perspectives on corneal collagen crosslinking (CXL). Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1363–1384. [Google Scholar] [CrossRef] [PubMed]
- Rapuano, P.B.; Mathews, P.M.; Florakis, G.J.; Trokel, S.L.; Suh, L.H. Corneal collagen crosslinking in patients treated with dextran versus isotonic hydroxypropyl methylcellulose (HPMC) riboflavin solution: A retrospective analysis. Eye Vis. 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, J.A.; Matlov Kormas, R.; Malyugin, B.E.; Boyko, M.; Tuuminen, R.; Knyazer, B. Ethnicity, Progressive Keratoconus, and Outcomes after Corneal Cross-Linking in Southern Israel. Life 2023, 13, 2294. [Google Scholar] [CrossRef]
- Maskill, D.; Okonkwo, A.; Onsiong, C.; Hristova, S.; Dodd, A.; Anand, S. Repeat corneal collagen cross-linking after failure of primary cross-linking in keratoconus. Br. J. Ophthalmol. 2024, 108, 662–666. [Google Scholar] [CrossRef]
- Sarac, O.; Caglayan, M.; Uysal, B.S.; Uzel, A.G.T.; Tanriverdi, B.; Cagil, N. Accelerated versus standard corneal collagen cross-linking in pediatric keratoconus patients: 24 months follow-up results. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2018, 41, 442–447. [Google Scholar] [CrossRef]
- Iqbal, M.; Gad, A.; Kotb, A.; Abdelhalim, M. Analysis of the outcomes of three different cross-linking protocols for treatment of paediatric keratoconus: A multicentre randomized controlled trial. Acta Ophthalmol. 2024, 102, e105–e116. [Google Scholar] [CrossRef]
- Lombardo, G.; Micali, N.L.; Villari, V.; Leone, N.; Serrao, S.; Rusciano, D.; Lombardo, M. Assessment of stromal riboflavin concentration-depth profile in nanotechnology-based transepithelial corneal crosslinking. J. Cataract Refract. Surg. 2017, 43, 680–686. [Google Scholar] [CrossRef]
- Jiang, L.Z.; Jiang, W.; Qiu, S.Y. Conventional vs. pulsed-light accelerated corneal collagen cross-linking for the treatment of progressive keratoconus: 12-month results from a prospective study. Exp. Ther. Med. 2017, 14, 4238–4244. [Google Scholar] [CrossRef]
- Raiskup, F.; Herber, R.; Lenk, J.; Pillunat, L.E.; Spoerl, E. Crosslinking with UV-A and riboflavin in progressive keratoconus: From laboratory to clinical practice—Developments over 25 years. Prog. Retin. Eye Res. 2024, 102, 101276. [Google Scholar] [CrossRef]
- Akhtar, S.; Smedowski, A.; Khan, A.A.; Debasi, H.; Mofty, H.; Samivel, R.; Almubrad, T. Glycosaminoglycans and collagen fibril distribution at various depths of the corneal stroma of normal and CXL treated rats. Exp. Eye Res. 2024, 239, 109780. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xin, Y.; Wang, C.; Fan, Y.; Yang, P.; Li, L.; Yin, D.; Zhang, E.; Hong, Y.; Bao, H.; et al. Use of Nanoindentation in Determination of Regional Biomechanical Properties of Rabbit Cornea After UVA Cross-Linking. Investig. Ophthalmol. Vis. Sci. 2023, 64, 26. [Google Scholar] [CrossRef] [PubMed]
- Rechichi, M.; Mazzotta, C.; Daya, S.; Mencucci, R.; Lanza, M.; Meduri, A. Intraoperative OCT Pachymetry in Patients Undergoing Dextran-Free Riboflavin UVA Accelerated Corneal Collagen Crosslinking. Curr. Eye Res. 2016, 41, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luo, S.; Dong, N.; Lin, Z.; Liu, Z.; Shang, X. [The clinical study of corneal cross-linking with hypo-osmolar riboflavin solution in thin keratoconic corneas]. [Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2014, 50, 681–686. [Google Scholar]
- Rosenblat, E.; Hersh, P.S. Intraoperative corneal thickness change and clinical outcomes after corneal collagen crosslinking: Standard crosslinking versus hypotonic riboflavin. J. Cataract Refract. Surg. 2016, 42, 596–605. [Google Scholar] [CrossRef]
- Nicula, C.A.; Rednik, A.M.; Bulboacă, A.E.; Nicula, D. Comparative Results Between "Epi-Off" Conventional and Accelerated Corneal Collagen Crosslinking for Progressive Keratoconus in Pediatric Patients. Ther. Clin. Risk Manag. 2019, 15, 1483–1490. [Google Scholar] [CrossRef]
- Akkaya, S.; Ulusoy, D.M.; Duru, Z.; Demirtaş, A.A. Long-term Outcomes of Accelerated Corneal Cross-linking in the Treatment of Keratoconus: Comparison of Hypotonic Riboflavin Solution With Standard Riboflavin Solution. J. Refract. Surg. 2020, 36, 110–117. [Google Scholar] [CrossRef]
- Celik Buyuktepe, T.; Ucakhan, O.O. Long-term visual, refractive, tomographic and aberrometric outcomes of corneal collagen crosslinking (CXL) with or without hypoosmolar riboflavin solution in the treatment of progressive keratoconus patients with thin corneas. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 1225–1235. [Google Scholar] [CrossRef]
- Syed, Z.A.; Yu, J.; Crespo, M.A.; Daher, N.D.; Chang, C.Y. Dynamics of Corneal Swelling With Hypoosmolar Riboflavin After Induction During Corneal Collagen Crosslinking in Patients With Progressive Keratoconus. Cornea 2024, 43, 1348. [Google Scholar] [CrossRef]
- Vantipalli, S.; Li, J.; Singh, M.; Aglyamov, S.R.; Larin, K.V.; Twa, M.D. Effects of Thickness on Corneal Biomechanical Properties Using Optical Coherence Elastography. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2018, 95, 299–308. [Google Scholar] [CrossRef]
- Höllhumer, R.; Watson, S.; Beckingsale, P. Persistent Epithelial Defects and Corneal Opacity After Collagen Cross-Linking With Substitution of Dextran (T-500) With Dextran Sulfate in Compounded Topical Riboflavin. Cornea 2017, 36, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Diakonis, V.F.; Kankariya, V.P.; Yoo, S.H.; Ziebarth, N.M. Anterior and posterior corneal stroma elasticity after corneal collagen crosslinking treatment. Exp. Eye Res. 2013, 116, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Mita, M.; Huseynova, T. Accelerated versus conventional corneal collagen crosslinking. J. Cataract Refract. Surg. 2014, 40, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, S.; Virga, A.; Paladini, I.; Mencucci, R.; Corvi, A. Investigation into the elastic properties of ex vivo porcine corneas subjected to inflation test after cross-linking treatment. J. Appl. Biomater. Funct. Mater. 2016, 14, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, L.; Shen, Y.; Tian, M.; Zhao, J.; Zhao, Y.; Li, M.; Zhou, X. Biomechanical and Histopathologic Effects of Pulsed-Light Accelerated Epithelium-On/-Off Corneal Collagen Cross-Linking. Cornea 2017, 36, 854–859. [Google Scholar] [CrossRef]
- Li, H.; Liu, T.; Mu, B.; Zhao, X.; Xue, C.; Shen, M.; Jhanji, V.; Wang, Y. Biomechanical effect of ultraviolet-A-riboflavin cross-linking on simulated human corneal stroma model and its correlation with changes in corneal stromal microstructure. Exp. Eye Res. 2020, 197, 108109. [Google Scholar] [CrossRef]
- Steinberg, J.; Katz, T.; Mousli, A.; Frings, A.; Casagrande, M.K.; Druchkiv, V.; Richard, G.; Linke, S.J. Corneal biomechanical changes after crosslinking for progressive keratoconus with the corneal visualization scheimpflug technology. J. Ophthalmol. 2014, 2014, 579190. [Google Scholar] [CrossRef]
- Sedaghat, M.R.; Momeni-Moghaddam, H.; Ambrósio, R., Jr.; Roberts, C.J.; Yekta, A.A.; Danesh, Z.; Reisdorf, S.; Khabazkhoob, M.; Heidari, H.R.; Sadeghi, J. Long-term Evaluation of Corneal Biomechanical Properties After Corneal Cross-linking for Keratoconus: A 4-Year Longitudinal Study. J. Refract. Surg. 2018, 34, 849–856. [Google Scholar] [CrossRef]
- Xanthopoulou, K.; Seitz, B.; Belin, M.W.; Flockerzi, E. Reliability analysis of successive Corvis ST® measurements in keratoconus 2 years after accelerated corneal crosslinking compared to untreated keratoconus corneas. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1055–1061. [Google Scholar] [CrossRef]
- Sanchez, I.; Martin, R.; Ussa, F.; Fernandez-Bueno, I. The parameters of the porcine eyeball. Graefe’s Arch. Clin. Exp. 2011, 249, 475–482. [Google Scholar] [CrossRef]
- Khalikova, E.; Susi, P.; Korpela, T. Microbial dextran-hydrolyzing enzymes: Fundamentals and applications. Microbiol. Mol. Biol. Rev. MMBR 2005, 69, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Hatami-Marbini, H.; Etebu, E. Hydration dependent biomechanical properties of the corneal stroma. Exp. Eye Res. 2013, 116, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.; Jahadi-Hosseini, H.; Khalili, M.R.; Jahanbani-Ardakani, H. Corneal Biomechanical Changes after Corneal Cross-Linking in Patients with Keratoconus. J. Curr. Ophthalmol. 2022, 34, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Herber, R.; Francis, M.; Spoerl, E.; Pillunat, L.E.; Raiskup, F.; Roy, A.S. Evaluation of Biomechanical Changes After Accelerated Cross-Linking in Progressive Keratoconus: A Prospective Follow-Up Study. Cornea 2023, 42, 1365–1376. [Google Scholar] [CrossRef]
- Sedaghat, M.R.; Momeni-Moghaddam, H.; Kangari, H.; Moradi, A.; Akbarzadeh, R.; Naroo, S.A. Changes in corneal biomechanical parameters in keratoconus eyes with various severities after corneal cross-linking (CXL): A comparative study. Eur. J. Ophthalmol. 2023, 33, 2114–2122. [Google Scholar] [CrossRef]
- Yesilirmak, N.; Saritas, O. Comparison of Corneal Biomechanical Efficacy Between Rose Bengal-Green Light and Riboflavin-UVA Crosslinking. Curr. Eye Res. 2024, 49, 942–948. [Google Scholar] [CrossRef]
- Jabbarvand, M.; Moravvej, Z.; Shahraki, K.; Hashemian, H.; Ghasemi, H.; Berijani, S.; Amiri, Z.; Jamali, A. Corneal biomechanical outcome of collagen cross-linking in keratoconic patients evaluated by Corvis ST. Eur. J. Ophthalmol. 2021, 31, 1577–1583. [Google Scholar] [CrossRef]
- Liu, T.; Shen, M.; Li, H.; Zhang, Y.; Mu, B.; Zhao, X.; Wang, Y. Changes and quantitative characterization of hyper-viscoelastic biomechanical properties for young corneal stroma after standard corneal cross-linking treatment with different ultraviolet-A energies. Acta Biomater. 2020, 113, 438–451. [Google Scholar] [CrossRef]
- Sinha Roy, A.; Kurian, M.; Matalia, H.; Shetty, R. Air-puff associated quantification of non-linear biomechanical properties of the human cornea in vivo. J. Mech. Behav. Biomed. Mater. 2015, 48, 173–182. [Google Scholar] [CrossRef]
- Ehmke, T.; Seiler, T.G.; Fischinger, I.; Ripken, T.; Heisterkamp, A.; Frueh, B.E. Comparison of Corneal Riboflavin Gradients Using Dextran and HPMC Solutions. J. Refract. Surg. 2016, 32, 798–802. [Google Scholar] [CrossRef]
- Lynch, A.P.; Wilson, S.; Ahearne, M.J.A.O. Ultrastructural maintenance of decellularized corneas using dextran. Acta Ophthalmologica. 2015, 93, 660–668. [Google Scholar] [CrossRef]
- Chen, Y.; Jorgensen, C.S.J.C.O.R. The effects of varying Dextran solution concentration on the swelling properties of porcine cornea. Chinese ophthalmic research. 2000, 18, 109–111. [Google Scholar]
- Patel, M.R.; Desai, N.; Ambati, B.K.J.I.O.V. Effect of 10% Dextran on Posterior Membrane Dystrophic and Pseudophakic Bullous Corneas. Investig. Ophthalmol. Vis. 2005, 46, 2724. [Google Scholar]
- Hamon, L.; Daas, L.; Mäurer, S.; Weinstein, I.; Quintin, A.; Schulz, K.; Langenbucher, A.; Seitz, B. Thickness and Curvature Changes of Human Corneal Grafts in Dextran-Containing Organ Culture Medium Before Keratoplasty. Cornea 2021, 40, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, A.P.; Ivarsen, A.; Hjortdal, J. Corneal resistance to shear force after UVA-riboflavin cross-linking. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5059–5069. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.P.; Wilson, S.L.; Ahearne, M. Dextran Preserves Native Corneal Structure During Decellularization. Tissue Eng. Part C Methods 2016, 22, 561–572. [Google Scholar] [CrossRef]
- Hatami-Marbini, H.; Rahimi, A. The relation between hydration and mechanical behavior of bovine cornea in tension. J. Mech. Behav. Biomed. Mater. 2014, 36, 90–97. [Google Scholar] [CrossRef]

| 2% Dextran Group | 20% Dextran Group | CXL Group | Control Group | p | |
|---|---|---|---|---|---|
| CCT/μm | 983 ± 16 | 747 ± 21 | 877 ± 20 | 860 ± 22 | <0.001 |
| R/mm | 8.10 ± 0.87 | 8.65 ± 1.30 | 8.54 ± 1.07 | 8.16 ± 1.67 | 0.154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Hu, B.; Guo, L.; Zhang, H.; Li, L.; Jie, Y.; Tian, L. Influence of Dextran Solution and Corneal Collagen Crosslinking on Corneal Biomechanical Parameters Evaluated by Corvis ST In Vitro. Bioengineering 2024, 11, 1156. https://doi.org/10.3390/bioengineering11111156
Qin X, Hu B, Guo L, Zhang H, Li L, Jie Y, Tian L. Influence of Dextran Solution and Corneal Collagen Crosslinking on Corneal Biomechanical Parameters Evaluated by Corvis ST In Vitro. Bioengineering. 2024; 11(11):1156. https://doi.org/10.3390/bioengineering11111156
Chicago/Turabian StyleQin, Xiao, Bi Hu, Lili Guo, Haixia Zhang, Lin Li, Ying Jie, and Lei Tian. 2024. "Influence of Dextran Solution and Corneal Collagen Crosslinking on Corneal Biomechanical Parameters Evaluated by Corvis ST In Vitro" Bioengineering 11, no. 11: 1156. https://doi.org/10.3390/bioengineering11111156
APA StyleQin, X., Hu, B., Guo, L., Zhang, H., Li, L., Jie, Y., & Tian, L. (2024). Influence of Dextran Solution and Corneal Collagen Crosslinking on Corneal Biomechanical Parameters Evaluated by Corvis ST In Vitro. Bioengineering, 11(11), 1156. https://doi.org/10.3390/bioengineering11111156






