Effects of Genipin Crosslinking of Porcine Perilimbal Sclera on Mechanical Properties and Intraocular Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Preparation
2.2. Mechanical Stress–Strain and Relaxation Tests
2.3. Whole Globe Perfusions
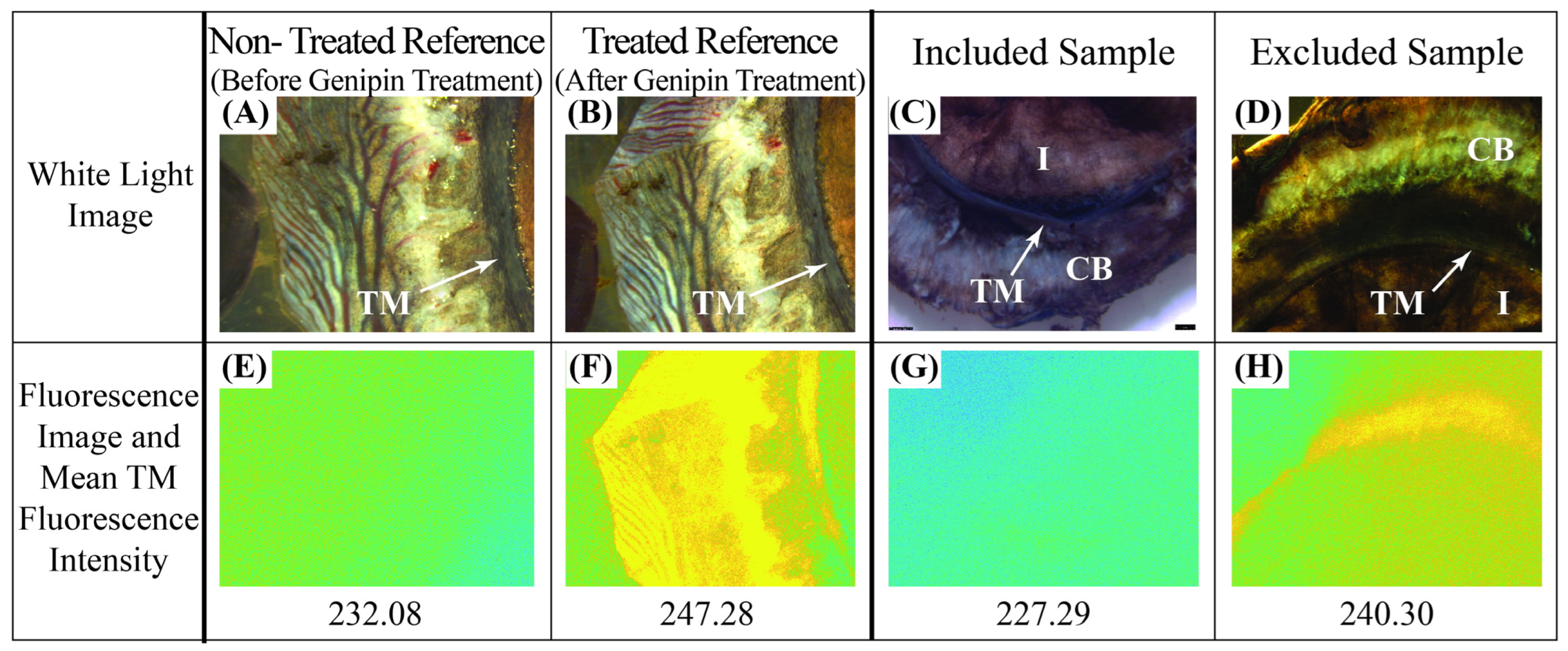
2.4. Fluorescence Assay
2.5. Statistical Methods
3. Results
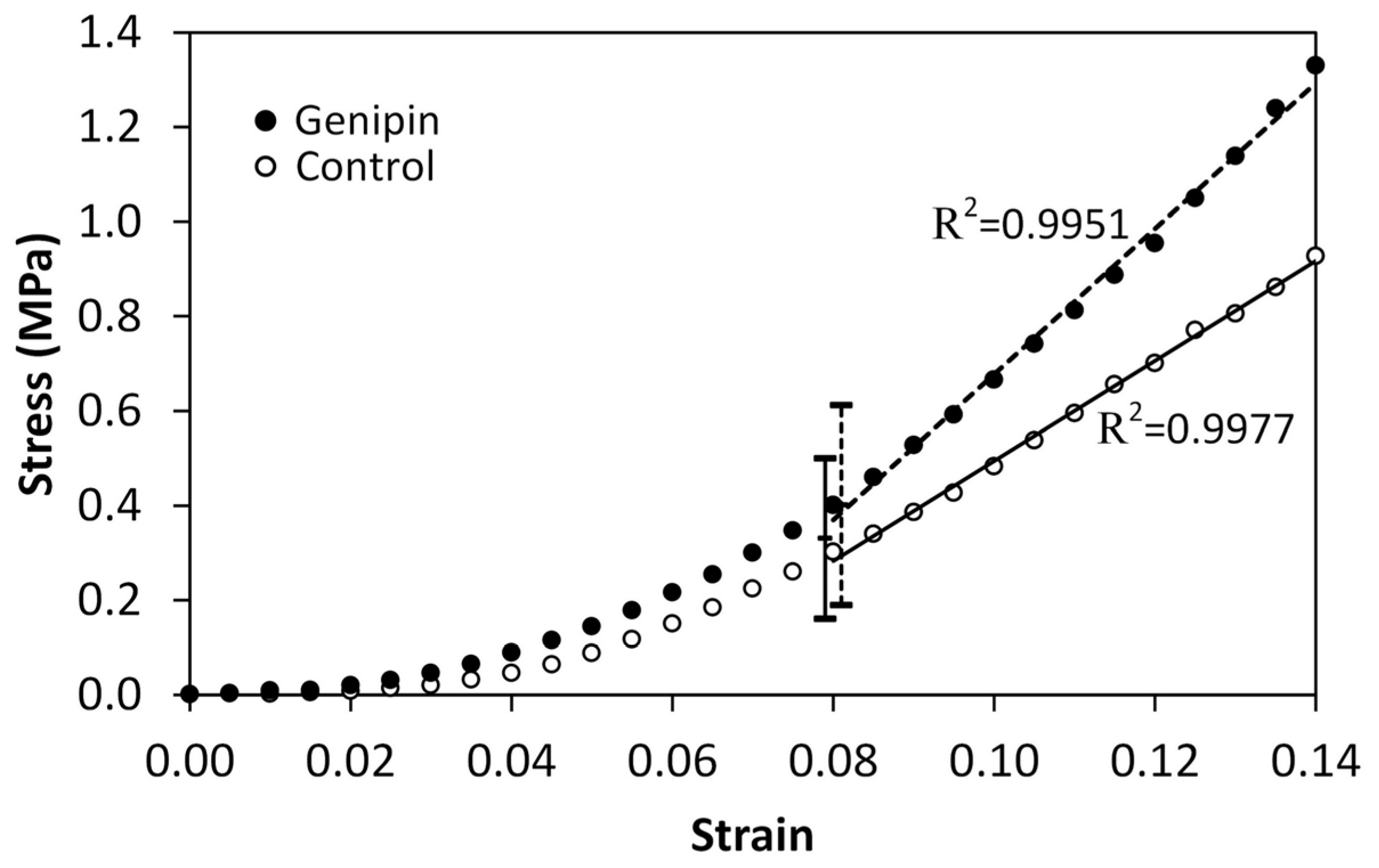
3.1. Mechanical Tests
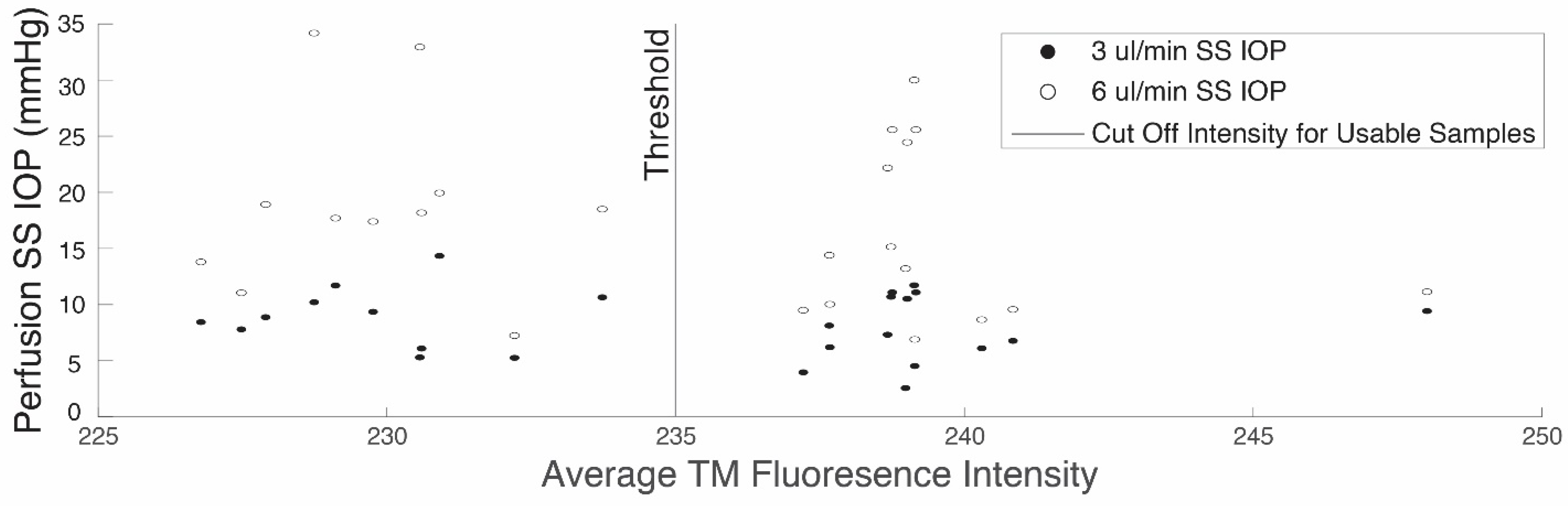
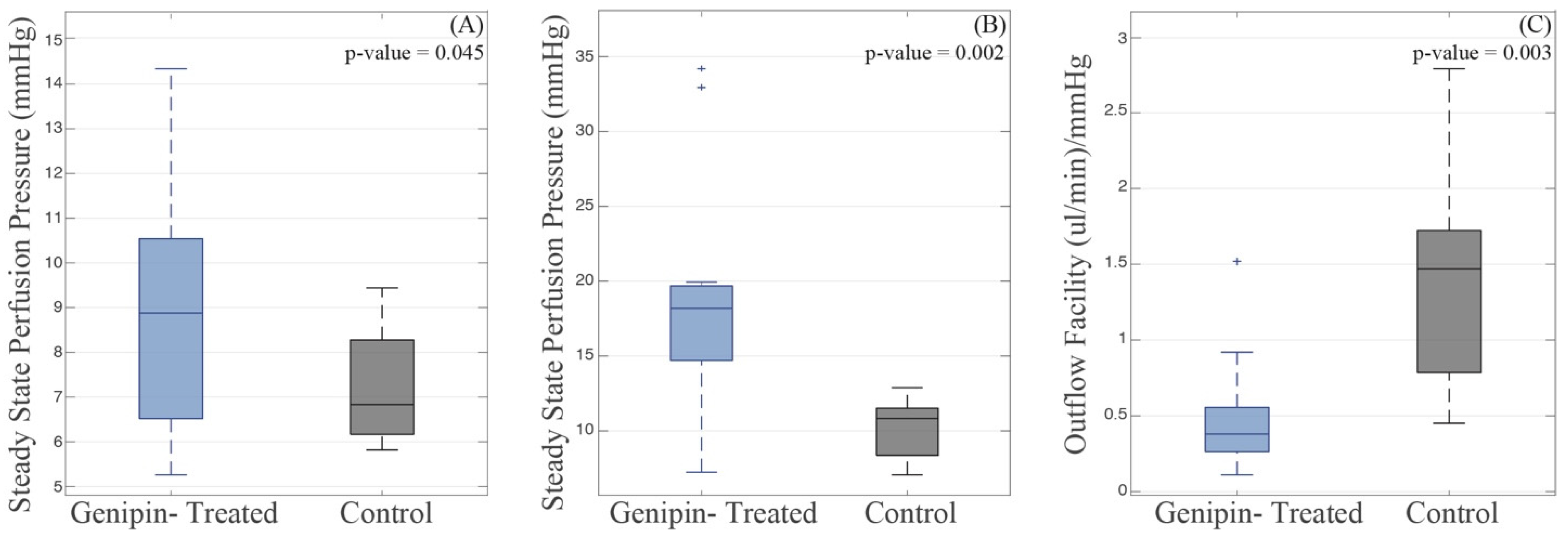
3.2. Whole Globe Perfusions and Fluorescence Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watson, P.G.; Young, R.D. Scleral structure, organisation and disease. A review. Exp. Eye Res. 2004, 78, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Boote, C.; Sigal, I.A.; Grytz, R.; Hua, Y.; Nguyen, T.D.; Girard, M.J.A. Scleral structure and biomechanics. Prog. Retin. Eye Res. 2020, 74, 100773. [Google Scholar] [CrossRef] [PubMed]
- Man, X.; Arroyo, E.; Dunbar, M.; Reed, D.M.; Shah, N.; Kagemann, L.; Kim, W.; Moroi, S.E.; Argento, A. Perilimbal sclera mechanical properties: Impact on intraocular pressure in porcine eyes. PLoS ONE 2018, 13, e0195882. [Google Scholar] [CrossRef] [PubMed]
- Bisplinghoff, J.A.; McNally, C.; Manoogian, S.J.; Duma, S.M. Dynamic material properties of the human sclera. J. Biomech. 2009, 42, 1493–1497. [Google Scholar] [CrossRef]
- Campbell, I.C.; Lovald, S.; Garcia, M.; Coudrillier, B. Biomechanical Properties of the Sclera. In Ocular Rigidity, Biomechanics and Hydrodynamics of the Eye; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 77–105. [Google Scholar] [CrossRef]
- Wilson, A.; Jones, J.; Tyrer, J.R.; Marshall, J. An interferometric ex vivo study of corneal biomechanics under physiologically representative loading, highlighting the role of the limbus in pressure compensation. Eye Vis. 2020, 7, 43. [Google Scholar] [CrossRef]
- Zvietcovich, F.; Nair, A.; Singh, M.; Aglyamov, S.R.; Twa, M.D.; Larin, K.V. Dynamic Optical Coherence Elastography of the Anterior Eye: Understanding the Biomechanics of the Limbus. Invest. Ophthalmol. Vis. Sci. 2020, 61, 7. [Google Scholar] [CrossRef]
- Gutsmann, T.; Fantner, G.E.; Kindt, J.H.; Venturoni, M.; Danielsen, S.; Hansma, P.K. Force Spectroscopy of Collagen Fibers to Investigate Their Mechanical Properties and Structural Organization. Biophys. J. 2004, 86, 3186–3193. [Google Scholar] [CrossRef]
- Svensson, R.B.; Mulder, H.; Kovanen, V.; Magnusson, S.P. Fracture mechanics of collagen fibrils: Influence of natural cross-links. Biophys. J. 2013, 104, 2476–2484. [Google Scholar] [CrossRef]
- Christiansen, D.L.; Huang, E.K.; Silver, F.H. Assembly of type I collagen: Fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol. 2000, 19, 409–420. [Google Scholar] [CrossRef]
- Roeder, B.A.; Kokini, K.; Sturgis, J.E.; Robinson, J.P.; Voytik-Harbin, S.L. Tensile Mechanical Properties of Three-Dimensional Type I Collagen Extracellular Matrices with Varied Microstructure. J. Biomech. Eng. 2002, 124, 214–222. [Google Scholar] [CrossRef]
- McBrien, N.A.; Jobling, A.I.; Gentle, A. Biomechanics of the sclera in myopia: Extracellular and cellular factors. Optom. Vis. Sci. 2009, 86, E23–E30. [Google Scholar] [CrossRef] [PubMed]
- Papi, M.; Paoletti, P.; Geraghty, B.; Akhtar, R. Nanoscale characterization of the biomechanical properties of collagen fibrils in the sclera. Appl. Phys. Lett. 2014, 104, 103703. [Google Scholar] [CrossRef]
- Hammond, M.A.; Gallant, M.A.; Burr, D.B.; Wallace, J.M. Nanoscale changes in collagen are reflected in physical and mechanical properties of bone at the microscale in diabetic rats. Bone 2014, 60, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Mao, W.; Wordinger, R.J.; Clark, A.F. Transforming Growth Factor–β Induces Extracellular Matrix Protein Cross-Linking Lysyl Oxidase (LOX) Genes in Human Trabecular Meshwork Cells. Investig. Opthalmol. Vis. Sci. 2011, 52, 5240. [Google Scholar] [CrossRef]
- Ellingson, A.; Pancheri, N.; Schiele, N. Regulators of collagen crosslinking in developing and adult tendons. Eur. Cells Mater. 2022, 43, 130–152. [Google Scholar] [CrossRef]
- Bailey, A.J.; Paul, R.G.; Knott, L. Mechanisms of maturation and ageing of collagen. Mech. Ageing Dev. 1998, 106, 1–56. [Google Scholar] [CrossRef]
- Liu, B.; McNally, S.; Kilpatrick, J.I.; Jarvis, S.P.; O’Brien, C.J. Aging and ocular tissue stiffness in glaucoma. Surv. Ophthalmol. 2018, 63, 56–74. [Google Scholar] [CrossRef]
- Kandarakis, S.A.; Piperi, C.; Topouzis, F.; Papavassiliou, A.G. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Prog. Retin. Eye Res. 2014, 42, 85–102. [Google Scholar] [CrossRef]
- Schultz, D.S.; Lotz, J.C.; Lee, S.M.; Trinidad, M.L.; Stewart, J.M. Structural Factors That Mediate Scleral Stiffness. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4232–4236. [Google Scholar] [CrossRef]
- Blackburn, B.J.; Rollins, A.M.; Dupps, W.J. Biomechanics of Ophthalmic Crosslinking. Transl. Vis. Sci. Technol. 2021, 10, 8. [Google Scholar] [CrossRef]
- Campbell, I.C.; Hannon, B.G.; Read, A.T.; Sherwood, J.M.; Schwaner, S.A.; Ethier, C.R. Quantification of the efficacy of collagen cross-linking agents to induce stiffening of rat sclera. J. R. Soc. Interface 2017, 14, 20170014. [Google Scholar] [CrossRef]
- Kimball, E.C.; Nguyen, C.; Steinhart, M.R.; Nguyen, T.D.; Pease, M.E.; Oglesby, E.N.; Oveson, B.C.; Quigley, H.A. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp. Eye Res. 2014, 128, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Vellara, H.R.; Patel, D.V. Biomechanical properties of the keratoconic cornea: A review. Clin. Exp. Optom. 2015, 98, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Saw, S.-M.; Matsumura, S.; Hoang, Q.V. Prevention and management of myopia and myopic pathology. Invest. Ophthalmol. Vis. Sci. 2019, 60, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Corpuz, C.C.C. Effects of scleral cross-linking using genipin on the process of form-deprivation myopia in the guinea pig: A randomized controlled experimental study. BMC Ophthalmol. 2015, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Yoo, J.S.; Kim, Y.J.; Kim, S.H.; Choi, S.H. Study on genipin: A new alternative natural crosslinking agent for fixing heterograft tissue. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 197. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, K.; Lin, W.; Li, D. Ring-opening polymerization of genipin and its long-range crosslinking effect on collagen hydrogel. J. Biomed. Mater. Res. Part. A 2013, 101A, 385–393. [Google Scholar] [CrossRef]
- Avila, M.Y.; Navia, J.L. Effect of genipin collagen crosslinking on porcine corneas. J. Cataract. Refract. Surg. 2010, 36, 659–664. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Paul, G.R.; Attenburrow, G. Cross-linking of extruded collagen fibers-A biomimetic three-dimensional scaffold for tissue engineering applications. J. Biomed. Mater. Res. Part. A 2009, 89A, 895–908. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Larsen, J.; Krasieva, T.B.; Lyubovitsky, J.G. Effect of genipin crosslinking on the optical spectral properties and structures of collagen hydrogels. ACS Appl. Mater. Interfaces 2011, 3, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Alekya, B.; Rao, S.; Pandya, H.J. Engineering approaches for characterizing soft tissue mechanical properties: A review. Clin. Biomech. 2019, 69, 127–140. [Google Scholar] [CrossRef]
- Liu, T.X.; Wang, Z. Collagen crosslinking of porcine sclera using genipin. Acta Ophthalmol. 2013, 91, e253–e257. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-W.; Chang, Y.; Chiu, C.-T.; Chen, C.-N.; Liang, H.-C. Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J. Biomed. Mater. Res. 1999, 47, 116–126. [Google Scholar] [CrossRef]
- Sung, H.-W.; Chang, Y.; Chiu, C.-T.; Chen, C.-N.; Liang, H.-C. Mechanical properties of a porcine aortic valve fixed with a naturally occurring crosslinking agent. Biomaterials 1999, 20, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-X.; Luo, X.; Gu, Y.-W.; Yang, B.; Wang, Z. Correlation of discoloration and biomechanical properties in porcine sclera induced by genipin. Int. J. Ophthalmol. 2014, 7, 621. [Google Scholar]
- Levy, A.M.; Fazio, M.A.; Grytz, R. Experimental myopia increases and scleral crosslinking using genipin inhibits cyclic softening in the tree shrew sclera. Ophthalmic Physiol. Opt. 2018, 38, 246–256. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J. Ultrasonic Measurement of Scleral Cross-Sectional Strains During Elevations of Intraocular Pressure: Method Validation and Initial Results in Posterior Porcine Sclera. J. Biomech. Eng. 2012, 134, 091007. [Google Scholar] [CrossRef]
- Fazio, M.A.; Grytz, R.; Bruno, L.; Girard, M.J.A.; Gardiner, S.; Girkin, C.A.; Downs, J.C. Regional Variations in Mechanical Strain in the Posterior Human Sclera. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5326. [Google Scholar] [CrossRef]
- Ni, L.; Riesterer, J.; Wang, H.; Berry, L.; Blackburn, K.; Chuang, J.; Kim, W.; Xu, G.; Moroi, S.E.; Argento, A. Method for the biomechanical analysis of aqueous veins and perilimbal sclera by three-dimensional photoacoustic imaging and strain field calculation. Sci. Rep. 2021, 11, 22108. [Google Scholar] [CrossRef]
- Lari, D.R.; Schultz, D.S.; Wang, A.S.; Lee, O.-T.; Stewart, J.M. Scleral mechanics: Comparing whole globe inflation and uniaxial testing. Exp. Eye Res. 2012, 94, 128. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Waxman, S.; Wang, C.; Atta, S.; Loewen, R.; Loewen, N.A. Dose-dependent effects of netarsudil, a Rho-kinase inhibitor, on the distal outflow tract. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.; Wang, C.; Dang, Y.; Hong, Y.; Esfandiari, H.; Shah, P.; Lathrop, K.L.; Loewen, R.T.; Loewen, N.A. Structure–Function Changes of the Porcine Distal Outflow Tract in Response to Nitric Oxide. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4886. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shi, H.-M.; Cong, L.; Lu, Z.-Z.; Ye, W.; Zhang, Y.-Y. Outflow facility efficacy of five drugs in enucleated porcine eyes by a method of constant-pressure perfusion. Int. J. Clin. Exp. Med. 2015, 8, 7184. [Google Scholar]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. Part. A 2008, 87A, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Almog, J.; Cohen, Y.; Azoury, M.; Hahn, T.-R. Genipin—A novel fingerprint reagent with colorimetric and fluorogenic activity. J. Forensic Sci. 2004, 49, JFS2003321. [Google Scholar] [CrossRef]
- Macaya, D.; Ng, K.K.; Spector, M. Injectable Collagen-Genipin Gel for the Treatment of Spinal Cord Injury: In Vitro Studies. Adv. Funct. Mater. 2011, 21, 4788–4797. [Google Scholar] [CrossRef]
- Matcham, S.; Novakovic, K. Fluorescence Imaging in Genipin Crosslinked Chitosan–Poly(vinyl pyrrolidone) Hydrogels. Polymers 2016, 8, 385. [Google Scholar] [CrossRef]
- Hannon, B.G.; Schwaner, S.A.; Boazak, E.M.; Gerberich, B.G.; Winger, E.J.; Prausnitz, M.R.; Ethier, C.R. Sustained scleral stiffening in rats after a single genipin treatment. J. R. Soc. Interface 2019, 16, 20190427. [Google Scholar] [CrossRef]
- Tondelli, T.; Götschi, T.; Camenzind, R.S.; Snedeker, J.G. Assessing the effects of intratendinous genipin injections: Mechanical augmentation and spatial distribution in an ex vivo degenerative tendon model. PLoS ONE 2020, 15, e0231619. [Google Scholar] [CrossRef]
- Brazuna, R.; Alonso, R.S.; Salomão, M.Q.; Fernandes, B.F.; Ambrósio, R. Ocular Biomechanics and Glaucoma. Vision 2023, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.C.; Suh, J.K.F.; Thomas, K.A.; Bellezza, A.J.; Burgoyne, C.F.; Hart, R.T. Viscoelastic Characterization of Peripapillary Sclera: Material Properties by Quadrant in Rabbit and Monkey Eyes. J. Biomech. Eng. 2003, 125, 124–131. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E. Collagen crosslinking of human and porcine sclera. J. Cataract. Refract. Surg. 2004, 30, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.; Kassem, W.; Jones, S.W. Strain-rate sensitivity of porcine and ovine corneas. Acta Bioeng. Biomech. 2011, 13, 25–36. [Google Scholar] [PubMed]
- Xu, B.; Li, H.; Zhang, Y. Understanding the viscoelastic behavior of collagen matrices through relaxation time distribution spectrum. Biomatter 2013, 3, e24651. [Google Scholar] [CrossRef]
- Sit, A.J.; McLaren, J.W. Measurement of episcleral venous pressure. Exp. Eye Res. 2011, 93, 291–298. [Google Scholar] [CrossRef]
- Grant, W. Experimental aqueous perfusion in enucleated human eyes. Arch. Ophthalmol. 1963, 69, 783–801. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Bao, F.; Lopes, B.T.; Wang, L.; Eliasy, A.; Abass, A.; Elsheikh, A. Review of ex-vivo characterisation of corneal biomechanics. Med. Nov. Technol. Devices 2021, 11, 100074. [Google Scholar] [CrossRef]
- Stamper, R.L.; Lieberman, M.F.; Drake, M.V.; Becker, B. Becker-Shaffer’s Diagnosis and Therapy of the Glaucomas; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Camras, L.J.; Stamer, W.D.; Epstein, D.; Gonzalez, P.; Yuan, F. Differential effects of trabecular meshwork stiffness on outflow facility in normal human and porcine eyes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5242. [Google Scholar] [CrossRef]
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic Modulus Determination of Normal and Glaucomatous Human Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2147–2152. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Sun, Y.Y.; Acott, T.S.; Keller, K.E. Effects of induction and inhibition of matrix cross-linking on remodeling of the aqueous outflow resistance by ocular trabecular meshwork cells. Sci. Rep. 2016, 6, 30505. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, V.K.; Benoit, J.; Kasetti, R.; Zode, G.; Salemi, M.; Phinney, B.S.; Keller, K.E.; Staverosky, J.A.; Murphy, C.J.; Acott, T.; et al. Glaucomatous cell derived matrices differentially modulate non-glaucomatous trabecular meshwork cellular behavior. Acta Biomater. 2018, 71, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Zyablitskaya, M.; Takaoka, A.; Munteanu, E.L.; Nagasaki, T.; Trokel, S.L.; Paik, D.C. Evaluation of Therapeutic Tissue Crosslinking (TXL) for Myopia Using Second Harmonic Generation Signal Microscopy in Rabbit Sclera. Investig. Ophthalmol. Vis. Sci. 2017, 58, 21–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gogola, A.; Jan, N.-J.; Brazile, B.; Lam, P.; Lathrop, K.L.; Chan, K.C.; Sigal, I.A. Spatial Patterns and Age-Related Changes of the Collagen Crimp in the Human Cornea and Sclera. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2987–2998. [Google Scholar] [CrossRef]
- Wong, F.F.; Lari, D.R.; Schultz, D.S.; Stewart, J.M. Whole globe inflation testing of exogenously crosslinked sclera using genipin and methylglyoxal. Exp. Eye Res. 2012, 103, 17. [Google Scholar] [CrossRef][Green Version]
- Hannon, B.G.; Luna, C.; Feola, A.J.; Ritch, M.D.; Read, A.T.; Stinnett, S.S.; Vo, H.; Pardue, M.T.; Gonzalez, P.; Ethier, C.R. Assessment of Visual and Retinal Function Following In Vivo Genipin-Induced Scleral Crosslinking. Transl. Vis. Sci. Technol. 2020, 9, 8. [Google Scholar] [CrossRef]
- Kim, W.; Argento, A.; Rozsa, F.W.; Mallett, K. Constitutive behavior of ocular tissues over a range of strain rates. J. Biomech. Eng. 2012, 134, 061002. [Google Scholar] [CrossRef]
- Elsheikh, A.; Geraghty, B.; Alhasso, D.; Knappett, J.; Campanelli, M.; Rama, P. Regional variation in the biomechanical properties of the human sclera. Exp. Eye Res. 2010, 90, 624–633. [Google Scholar] [CrossRef]
- Fratzl, P.; Misof, K.; Zizak, I.; Rapp, G.; Amenitsch, H.; Bernstorff, S. Fibrillar Structure and Mechanical Properties of Collagen. J. Struct. Biol. 1998, 122, 119–122. [Google Scholar] [CrossRef]
- Jan, N.-J.; Lee, P.-Y.; Wallace, J.; Iasella, M.; Gogola, A.; Wang, B.; Sigal, I.A. Stretch-induced uncrimping of equatorial sclera collagen bundles. J. Biomech. Eng. 2023, 145, 054503. [Google Scholar] [CrossRef]
- Williams, C.; Liao, J.; Joyce, E.M.; Wang, B.; Leach, J.B.; Sacks, M.S.; Wong, J.Y. Altered structural and mechanical properties in decellularized rabbit carotid arteries. Acta Biomater. 2009, 5, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Buehler, M.J.; Redaelli, A. Deformation rate controls elasticity and unfolding pathway of single tropocollagen molecules. J. Mech. Behav. Biomed. Mater. 2009, 2, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Troyer, K.L.; Estep, D.J.; Puttlitz, C.M. Viscoelastic effects during loading play an integral role in soft tissue mechanics. Acta Biomater. 2012, 8, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Hatami-Marbini, H.; Rahimi, A. Collagen cross-linking treatment effects on corneal dynamic biomechanical properties. Exp. Eye Res. 2015, 135, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.C.; Suh, J.K.F.; Thomas, K.A.; Bellezza, A.J.; Hart, R.T.; Burgoyne, C.F. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 540–546. [Google Scholar] [CrossRef]
- Yang, L.; Van der Werf, K.; Dijkstra, P.J.; Feijen, J.; Bennink, M.L. Micromechanical analysis of native and cross-linked collagen type I fibrils supports the existence of microfibrils. J. Mech. Behav. Biomed. Mater. 2012, 6, 148–158. [Google Scholar] [CrossRef]
- Coudrillier, B.; Campbell, I.C.; Read, A.T.; Geraldes, D.M.; Vo, N.T.; Feola, A.; Mulvihill, J.; Albon, J.; Abel, R.L.; Ethier, C.R. Effects of peripapillary scleral stiffening on the deformation of the lamina cribrosa. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2666–2677. [Google Scholar] [CrossRef]
- Shaik, T.A.; Baria, E.; Wang, X.; Korinth, F.; Lagarto, J.L.; Höppener, C.; Pavone, F.S.; Deckert, V.; Popp, J.; Cicchi, R.; et al. Structural and Biochemical Changes in Pericardium upon Genipin Cross-Linking Investigated Using Nondestructive and Label-Free Imaging Techniques. Anal. Chem. 2022, 94, 1575–1584. [Google Scholar] [CrossRef]
- Lu, H.-T.; Lu, T.-W.; Chen, C.-H.; Mi, F.-L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. [Google Scholar] [CrossRef]
- Monica, M. Cell and Tissue Autofluorescence Research and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2005; Volume 11, pp. 227–256. [Google Scholar]
- Croce, A.C.; Bottiroli, G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 2461. [Google Scholar] [CrossRef]
- Lin, S.C. Vascular effects on ciliary tissue from endoscopic versus trans-scleral cyclophotocoagulation. Br. J. Ophthalmol. 2006, 90, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Qian, Q.; Wang, J.; Cheng, W.; Han, K. Target Area Extraction Algorithm for the In Vivo Fluorescence Imaging of Small Animals. ACS Omega 2020, 5, 20100–20106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Clarke, E.C.; Bilston, L.E. The effects of preconditioning strain on measured tissue properties. J. Biomech. 2009, 42, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A.; Gefen, N.; Zhu, Q.; Raghupathi, R.; Margulies, S.S. Age-Dependent Changes in Material Properties of the Brain and Braincase of the Rat. J. Neurotrauma 2003, 20, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Prevost, T.P.; Balakrishnan, A.; Suresh, S.; Socrate, S. Biomechanics of brain tissue. Acta Biomater. 2011, 7, 83–95. [Google Scholar] [CrossRef]
- Zhai, Y.; Colmenarez, J.A.; Mendoza, V.O.; Dong, P.; Nunes, K.; Suh, D.; Gu, L. Multiscale Mechanical Characterization of Cornea With AFM, SEM, and Uniaxial Tensile Test. In Proceedings of the ASME 2023 International Mechanical Engineering Congress and Exposition, New Orleans, LA, USA, 29 October–2 November 2023. [Google Scholar]
- Yan, S.; Song, X.; Hu, X.; Yao, K.; Qu, S. A novel intraocular pressure predicting method based on hyperelastic mechanical model of cornea. J. Mech. Behav. Biomed. Mater. 2024, 153, 106475. [Google Scholar] [CrossRef]



| Test Rate (mm/min) [Strain Rate] | Treatment | n | Stress (kPa) | Tangent Modulus (MPa) | Strain Rate Dependence | |
|---|---|---|---|---|---|---|
| Stress | Tangent Modulus | |||||
| 0.5 | Control | 22 | 196.59 ± 115.47 (a) | 11.88 ± 2.84 (g) | ||
| [6.4 × 10−4/s] | Genipin | 22 | 329.06 ± 207.43 (b) | 15.62 ± 4.91 (h) | ||
| p (a,b) = 0.002 | p (g,h) = 2.9 × 10−4 | |||||
| 10 | Control | 26 | 330.95 ± 170.09 (c) | 12.50 ± 6.39 (i) | 68.3%, p (a,c) = 0.001 | 5.2%, p (g,i) > 0.05 |
| [0.0128/s] | Genipin | 26 | 401.62 ± 211.08 (d) | 17.58 ± 6.30 (j) | 22.1%, p (b,d) > 0.05 | 12.5%, p (h,j) > 0.05 |
| p (c,d) > 0.05 | p (i,j) = 6.8 × 10−5 | |||||
| 100 | Control | 21 | 484.48 ± 136.62 (e) | 17.63 ± 4.46 (k) | 146.4%, p (a,e) = 1.8 × 10−9 | 48.4%, p (g,k) = 4.4 × 10−6 |
| [0.128/s] | Genipin | 21 | 513.53 ± 254.29 (f) | 19.32 ± 5.33 (l) | 56.1%, p (b,f) = 0.006 | 23.7%, p (h,l) = 0.011 |
| p (e,f) > 0.05 | p (k,l) > 0.05 | |||||
| 0.5 mm/min Input Case (n = 13) | 100 mm/min Input Case (n = 14) | |||
|---|---|---|---|---|
| Time (s) | Control (MPa) | Genipin (MPa) | Control (MPa) | Genipin (MPa) |
| 10 | 5.01 ± 1.28 | 5.89 ± 1.46 | 3.05 ± 0.71 | 3.95 ± 0.99 |
| (p = 0.003) | (p = 0.005) | |||
| 150 | 3.61 ± 0.85 | 4.36 ± 1.03 | 1.76 ± 0.46 | 2.40 ± 0.59 |
| (p = 0.002) | (p = 0.004) | |||
| 275 | 3.25 ± 0.75 | 3.97 ± 0.91 | 1.54 ± 0.41 | 2.11 ± 0.52 |
| (p = 0.002) | (p = 0.004) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riesterer, J.; Warchock, A.; Krawczyk, E.; Ni, L.; Kim, W.; Moroi, S.E.; Xu, G.; Argento, A. Effects of Genipin Crosslinking of Porcine Perilimbal Sclera on Mechanical Properties and Intraocular Pressure. Bioengineering 2024, 11, 996. https://doi.org/10.3390/bioengineering11100996
Riesterer J, Warchock A, Krawczyk E, Ni L, Kim W, Moroi SE, Xu G, Argento A. Effects of Genipin Crosslinking of Porcine Perilimbal Sclera on Mechanical Properties and Intraocular Pressure. Bioengineering. 2024; 11(10):996. https://doi.org/10.3390/bioengineering11100996
Chicago/Turabian StyleRiesterer, John, Alexus Warchock, Erik Krawczyk, Linyu Ni, Wonsuk Kim, Sayoko E. Moroi, Guan Xu, and Alan Argento. 2024. "Effects of Genipin Crosslinking of Porcine Perilimbal Sclera on Mechanical Properties and Intraocular Pressure" Bioengineering 11, no. 10: 996. https://doi.org/10.3390/bioengineering11100996
APA StyleRiesterer, J., Warchock, A., Krawczyk, E., Ni, L., Kim, W., Moroi, S. E., Xu, G., & Argento, A. (2024). Effects of Genipin Crosslinking of Porcine Perilimbal Sclera on Mechanical Properties and Intraocular Pressure. Bioengineering, 11(10), 996. https://doi.org/10.3390/bioengineering11100996









