The Buccal Fat Pad: A Unique Human Anatomical Structure and Rich and Easily Accessible Source of Mesenchymal Stem Cells for Tissue Repair
Abstract
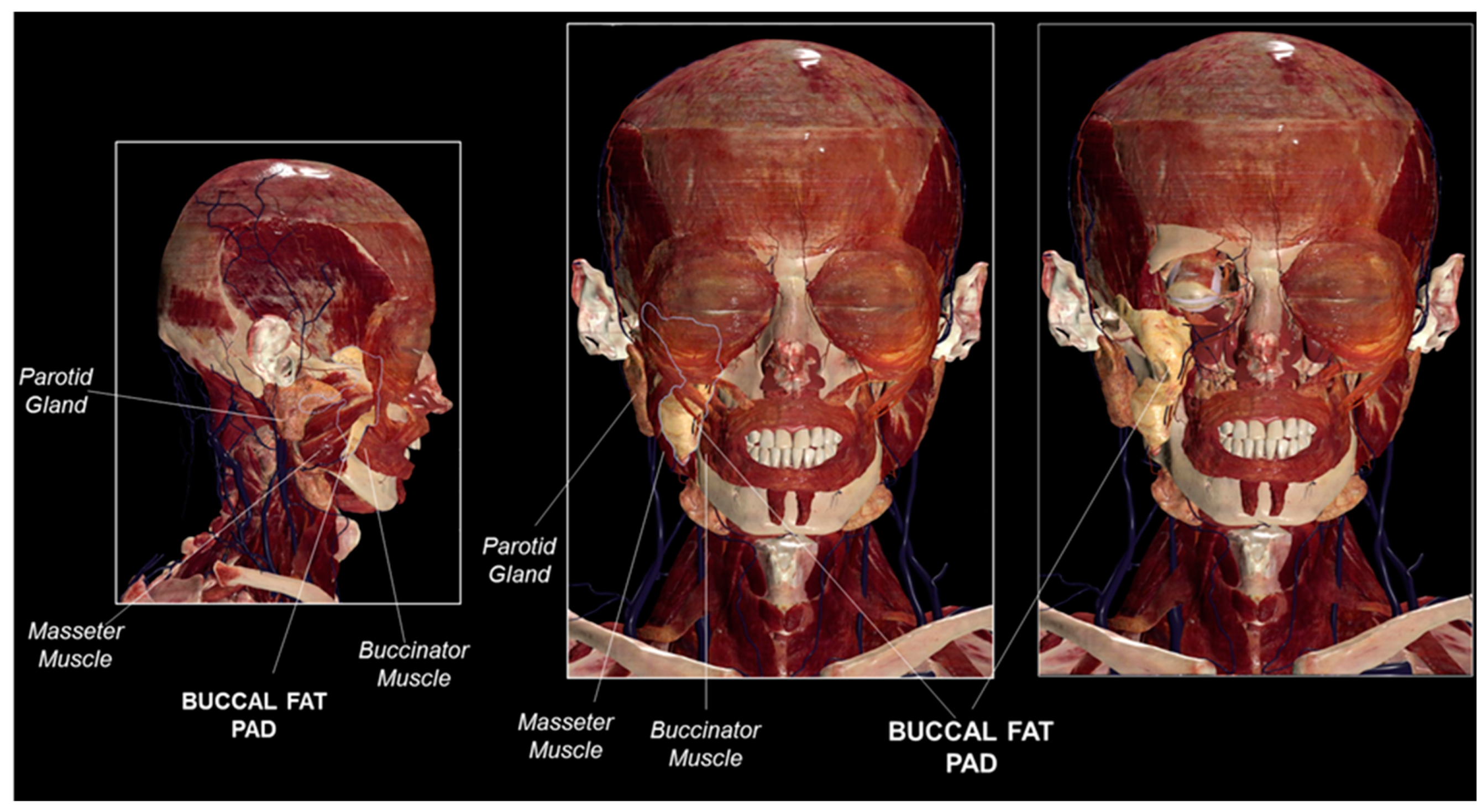
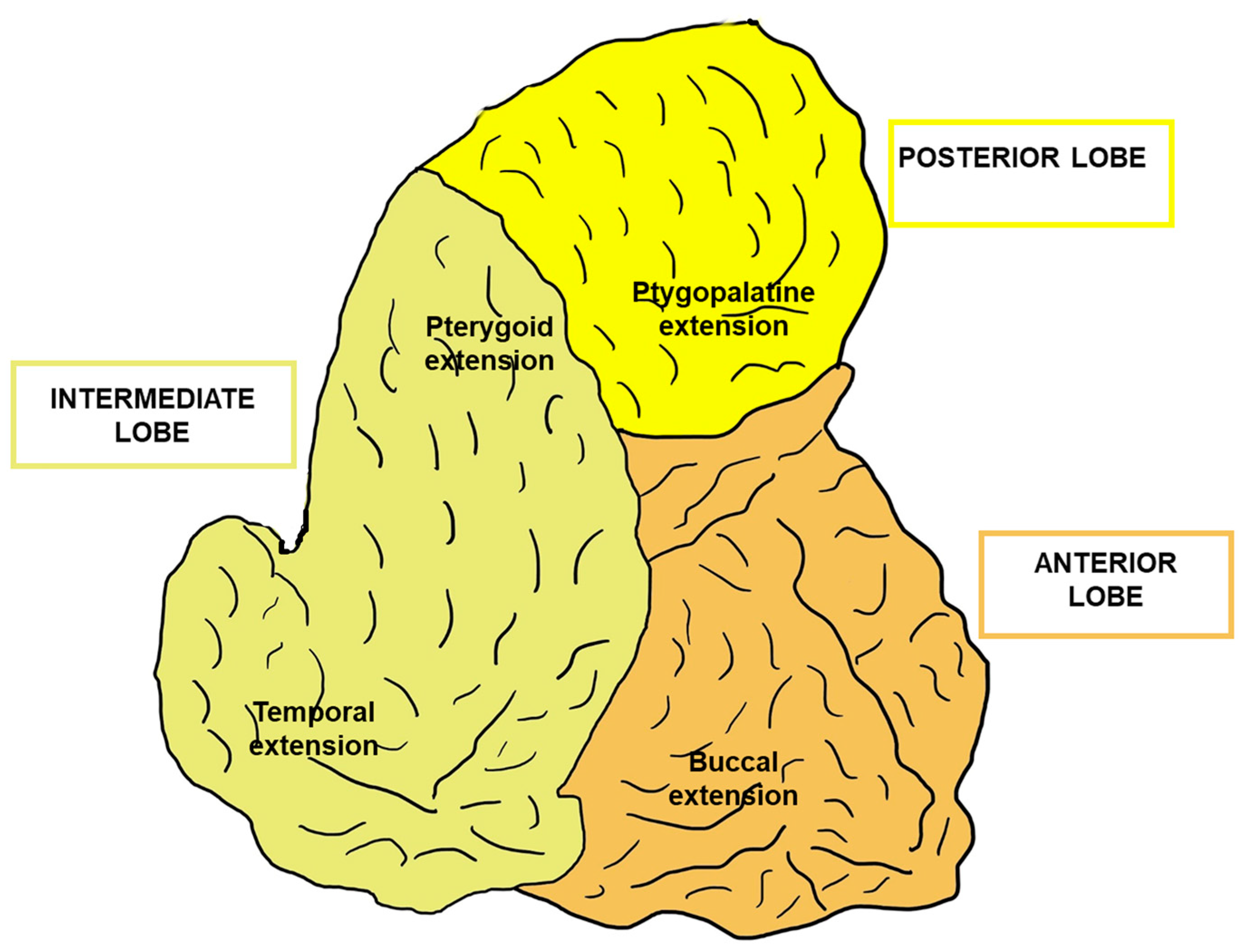
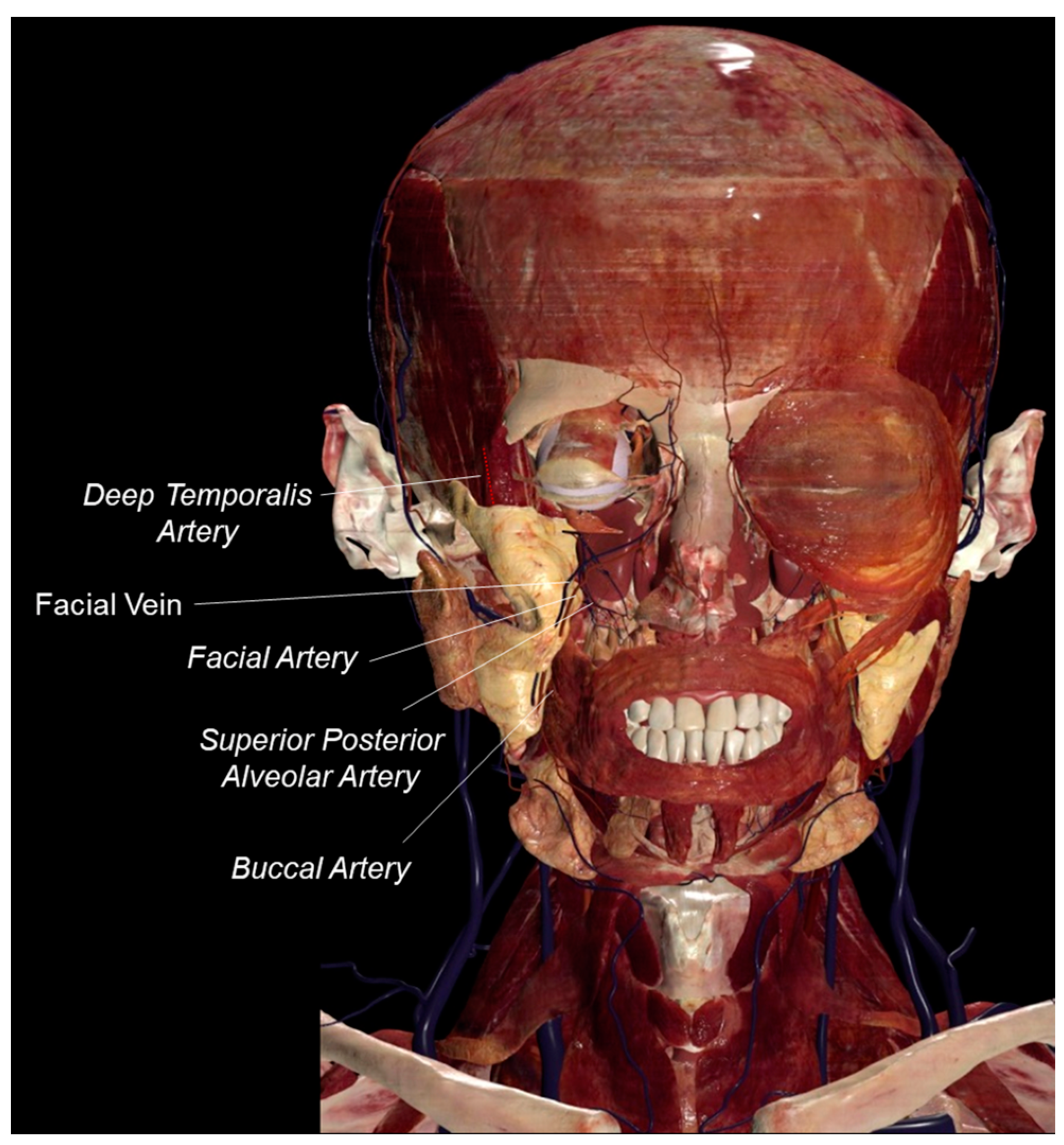
1. Introduction
2. The Buccal Fat Pad as a Stem Cell Source
3. Relevance of the Buccal Fat Pad in Surgery
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marzano, U.G. Lorenz Heister’s “molar gland”. Plast. Reconstr. Surg. 2005, 115, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.F.; de Souza, H.C.; Martins, M.S.; Oliveira, M.N.; Benfatti, C.A.; de Souza Magini, R. Versatility and importance of bichat’s fat pad in dentistry: Case reports of its use in occlusal trauma. J. Contemp. Dent. Pract. 2018, 19, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Bichat, X. Anatomie Générale, Applique’e la Physiologie et Àla Médecine; Brosson: Paris, France, 1801. [Google Scholar]
- Chouikh, F.; Dierks, E.J. The buccal fat pad flap. Oral Maxillofac. Surg. Clin. N. Am. 2021, 33, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Egyedi, P. Utilization of the buccal fat pad for closure of oro-antral and/or oro-nasal communications. J. Oral Maxillofac. Surg. 1977, 5, 241–2444. [Google Scholar] [CrossRef] [PubMed]
- Tideman, H.; Bosanquet, A.; Scott, J. Use of the buccal fat pad as a pedicled graft. J. Oral Maxillofac. Surg. 1986, 44, 435–440. [Google Scholar] [CrossRef]
- Tostevin, P.M.; Ellis, H. The buccal pad of fat: A review. Clin Anat. 1995, 8, 403–406. [Google Scholar] [CrossRef]
- Vieira, G.M.; Jorge, F.D.; Franco, E.J.; Dias, L.D.C.; Guimarães, M.D.C.M.; Oliveira, L.A. Lesions of the parotid gland and buccal artery after buccal fat pad reduction. J. Craniofacial Surg. 2019, 30, 790–792. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, W.; Kim, S.G. The use of the buccal fat pad flap for oral reconstruction. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 5. [Google Scholar] [CrossRef]
- O’Brien, J.X.; Ashton, M.W.; Rozen, W.M.; Ross, R.; Mendelson, B.C. New perspectives on the surgical anatomy and nomenclature of the temporal region: Literature review and dissection study. Plast. Reconstr. Surg. 2013, 131, 510–522. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Stuzin, J.M.; Savetsky, I.L.; Avashia, Y.J.; Agrawal, N.A.; Prada, M. the role of the buccal fat pad in facial aesthetic surgery. Plast. Reconstr. Surg. 2021, 148, 334–338. [Google Scholar] [CrossRef]
- Lin, M.J.; Hazan, E.; John, A.M.; Dubin, D.P.; Younessi, S.; Khorasani, H. Buccal Fat Pad Reduction with Intraoperative Fat Transfer to the Temple. Cutis 2022, 109, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Yan, Y.P.; Qi, K.M.; Wang, J.Q.; Liu, Z.F. Anatomical structure of the buccal fat pad and its clinical adaptations. Plast. Reconstr. Surg. 2002, 109, 2509–2518, discussion 2519–2520. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.; Serra, M. Buccal fat pad reduction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yousuf, S.; Tubbs, R.S.; Wartmann, C.T.; Kapos, T.; Cohen-Gadol, A.A.; Loukas, M. A review of the gross anatomy, functions, pathology, and clinical uses of the buccal fat pad. Surg. Radiol. Anat. 2010, 32, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, K.F.; de Lima Sousa, M.G.; Dos Santos Passos, A.; Farias, R.J.; Guerra, J.M.; Costa, F.W.G.; Sousa, F.B.; Silva, P.G.B.; Cetira Filho, E.L. The impact of partially removing the Bichat fat pad in the linear facial measurements, satisfaction with facial aesthetics and quality of life: A single-arm CONSORT-guided clinical trial. Clin. Oral Investig. 2023, 27, 249–262. [Google Scholar] [CrossRef]
- Moura, L.B.; Spin, J.R.; Spin-Neto, R.; Pereira-Filho, V.A. Buccal fat pad removal to improve facial aesthetics: An established technique? Med. Oral Patol. Oral Cir. Bucal 2018, 23, e478–e484. [Google Scholar] [CrossRef]
- Durbeej, B.; Eriksson, L.A. On the formation of cyclobutane pyrimidine dimers in UV-irradiated DNA: Why are thymines more reactive? Photochem. Photobiol. 2003, 78, 159–167. [Google Scholar] [CrossRef]
- Loukas, M.; Kapos, T.; Louis, R. G; Jr; Wartman, C.; Jones, A.; Hallner, B. Gross anatomical, CT and MRI analyses of the buccal fat pad with special emphasis on volumetric variations. Surg. Radiol. Anat. 2006, 28, 254–260. [Google Scholar] [CrossRef]
- Jackson, I.T. Buccal fat pad removal. Aesthetic Surg. J. 2003, 23, 484–485. [Google Scholar] [CrossRef]
- Sagayaraj, A.; Jyothi, N.D.; Mohiyuddin, S.M.A.; Deo, R.P.; Padiyar, B.V. Role of buccal pad of fat in reconstruction of the buccal mucosa defects. Indian J. Otolaryngol. Head Neck Surg. 2017, 69, 20–23. [Google Scholar] [CrossRef]
- Conti, G.; Bertossi, D.; Dai Prè, E.; Cavallini, C.; Scupoli, M.T.; Ricciardi, G.; Parnigotto, P.; Saban, Y.; Sbarbati, A.; Nocini, P. Regenerative potential of the Bichat fat pad determined by the quantification of multilineage differentiating stress enduring cells. Eur. J. Histochem. 2018, 62, 2900. [Google Scholar] [CrossRef]
- Tzikas, T.L. Lipografting: Autologous fat grafting for total facial rejuvenation. Facial Plast. Surg. 2004, 20, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Takeshita, Y.; Kugimoto, T.; Harada, H.; Park, J.S.; Tubbs, R.S.; Iwanaga, J. Anatomy of the buccal space: Surgical and radiological perspectives. J. Craniofac. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Santiago, B.M.; Damasceno, L.M.; Primo, L.G. Bilateral protrusion of the buccal fat pad into the mouth of an infant: Report of a case. J. Clin. Pediatr. Dent. 2005, 29, 181–184. [Google Scholar] [CrossRef]
- Gierloff, M.; Stöhring, C.; Buder, T.; Gassling, V.; Açil, Y.; Wiltfang, J. Aging changes of the midfacial fat compartments: A computed tomographic study. Plast. Reconstr. Surg. 2012, 129, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Ugradar, S.; Kim, J.S.; Massry, G. A Review of Midface Aging. Ophthalmic Plast. Reconstr. Surg. 2023, 39, 123–131. [Google Scholar] [CrossRef]
- van Noorden, C.J.F.; Breznik, B.; Novak, M.; van Dijck, A.J.; Tanan, S.; Vittori, M.; Bogataj, U.; Bakker, N.; Khoury, J.D.; Molenaar, R.J.; et al. Cell biology meets cell metabolism: Energy production is similar in stem cells and in cancer stem cells in brain and bone marrow. J. Histochem. Cytochem. 2022, 70, 29–51. [Google Scholar] [CrossRef]
- Farré-Guasch, E.; Martí-Pagè, C.; Hernádez-Alfaro, F.; Klein-Nulend, J.; Casals, N. Buccal fat pad, an oral access source of human adipose stem cells with potential for osteochondral tissue engineering: An in vitro study. Tissue Eng. Part C Methods 2010, 16, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.; Alamillos, F.; García-López, A.; Sánchez, J.; Peñalba, M. The buccal fat pad flap in oral reconstruction. Head Neck 2001, 23, 383–388. [Google Scholar] [CrossRef]
- Baldin, A.V.; Telich Tarriba, J.E.; Velázquez Zabaleta, E.; Apellaniz Campo, A.; Martinez Wagner, R.; Cardenas-Mejía, A. Surgical resection of vascular lesions involving the buccal fat pad. J. Craniofac. Surg. 2018, 29, e459–e461. [Google Scholar] [CrossRef]
- Alarcón-Apablaza, J.; Prieto, R.; Rojas, M.; Fuentes, R. Potential of oral cavity stem cells for bone regeneration: A scoping review. Cells 2023, 12, 1392. [Google Scholar] [CrossRef]
- Surovtseva, M.A.; Kim, I.I.; Bondarenko, N.A.; Ostapets, S.V.; Drovosekov, M.N.; Kosareva, O.S.; Poveshchenko, O.V. Buccal mesenchymal stromal cells as a source of osseointegration of titanium implants. Bull. Exp. Biol. Med. 2024, 176, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Ariano, A.; Posa, F.; Storlino, G.; Mori, G. Molecules inducing dental stem cells differentiation and bone regeneration: State of the art. Int. J. Mol. Sci. 2023, 24, 9897. [Google Scholar] [CrossRef] [PubMed]
- Dehghani Nazhvani, F.; Mohammadi Amirabad, L.; Azari, A.; Namazi, H.; Hosseinzadeh, S.; Samanipour, R.; Khojasteh, A.; Golchin, A.; Hashemi, S. Effects of in vitro low oxygen tension preconditioning of buccal fat pad stem cells on in vivo articular cartilage tissue repair. Life Sci. 2021, 280, 119728. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Cavagnetto, D.; Tasinato, F.; Petrillo, S.; Ruffinatti, F.A.; Mela, L.; Carossa, M.; Munaron, L.; Roato, I.; Mussano, F. Isolation and characterization of buccal fat pad and dental pulp MSCs from the same donor. Biomedicines 2021, 9, 265. [Google Scholar] [CrossRef]
- Hashemi, S.; Mohammadi Amirabad, L.; Farzad-Mohajeri, S.; Rezai Rad, M.; Fahimipour, F.; Ardeshirylajimi, A.; Dashtimoghadam, E.; Salehi, M.; Soleimani, M.; Dehghan, M.M.; et al. Comparison of osteogenic differentiation potential of induced pluripotent stem cells and buccal fat pad stem cells on 3D-printed HA/β-TCP collagen-coated scaffolds. Cell Tissue Res. 2021, 384, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Martinez, L.; Yemisci, M.; Dalkara, T. Pericyte morphology and function. Histol. Histopathol. 2021, 36, 633–643. [Google Scholar] [CrossRef]
- Watson, D.C.; Bayik, D.; Storevik, S.; Moreino, S.S.; Sprowls, S.A.; Han, J.; Augustsson, M.T.; Lauko, A.; Sravya, P.; Røsland, G.V.; et al. GAP43-dependent mitochondria transfer from astrocytes enhances glioblastoma tumorigenicity. Nat. Cancer 2023, 4, 648–664. [Google Scholar] [CrossRef]
- van Noorden, C.J.F.; Yetkin-Arik, B.; Serrano Martinez, P.; Bakker, N.; van Breest Smallenburg, M.E.; Schlingemann, R.O.; Klaassen, I.; Majc, B.; Habic, A.; Bogataj, U.; et al. New insights in ATP synthesis as therapeutic target in cancer and angiogenic ocular diseases. J. Histochem. Cytochem. 2024, 72, 329–352. [Google Scholar] [CrossRef]
- Conti, G.; Zingaretti, N.; Amuso, D.; Dai Prè, E.; Brandi, J.; Cecconi, D.; Manfredi, M.; Marengo, E.; Boschi, F.; Riccio, M.; et al. Proteomic and ultrastructural analysis of cellulite-new findings on an old topic. Int J. Mol Sci. 2020, 21, 2077. [Google Scholar] [CrossRef]
- Quintero Sierra, L.A.; Biswas, R.; Conti, A.; Busato, A.; Ossanna, R.; Zingaretti, N.; Parodi, P.C.; Conti, G.; Riccio, M.; Sbarbati, A.; et al. Highly pluripotent adipose-derived stem cell-enriched nanofat: A novel translational system in stem cell therapy. Cell Transplant. 2023, 32, 9636897231175968. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Nik, N.; Rezai Rad, M.; Kheiri, L.; Nazeman, P.; Nadjmi, N.; Khojasteh, A. Buccal fat pad as a potential source of stem cells for bone regeneration: A literature review. Stem. Cells Int. 2017, 2017, 8354640. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Johnson, N.; Abdal-Hay, A.; Moran, C.S.; Salomon, C.; Ivanovski, S. Effects of periodontal cells-derived extracellular vesicles on mesenchymal stromal cell function. J. Periodontal Res. 2023, 58, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Honda, Y.; Momota, Y.; Tran, S.D. Dedifferentiated fat (DFAT) cells: A cell source for oral and maxillofacial tissue engineering. Oral Dis. 2018, 24, 1161–1167. [Google Scholar] [CrossRef]
- Shiraishi, T.; Sumita, Y.; Wakamastu, Y.; Nagai, K.; Asahina, I. Formation of engineered bone with adipose stromal cells from buccal fat pad. J. Dent. Res. 2012, 91, 592–597. [Google Scholar] [CrossRef]
- Broccaioli, E.; Niada, S.; Rasperini, G.; Ferreira, L.M.; Arrigoni, E.; Yenagi, V.; Brini, A.T. Mesenchymal stem cells from Bichat’s fat pad: In vitro comparison with adipose-derived stem cells from subcutaneous tissue. BioRes. Open Access 2013, 2, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.J.; Wang, D.H.; Wang, C.Y.; Poongodi, R.; Liou, N.H.; Liu, J.C.; Hsu, M.L.; Hong, P.D.; Yang, S.F.; Liu, M.L. Correction to: Osteogenic prospective of deriving human dental stem cells in collagen matrix boost. J. Mater. Sci. Mater. Med. 2018, 29, 100. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Biro, R.; Kovacova, V.; Babikova, M.; Zemanova, N.; Mondockova, V.; Omelka, R. Current knowledge of bone-derived factor osteocalcin: Its role in the management and treatment of diabetes mellitus, osteoporosis, osteopetrosis and inflammatory joint diseases. J. Mol. Med. 2024, 102, 435–452. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Zarei, M.; Abbasi, H.; Webster, T.J.; Beheshtizadeh, N. Promoting osteogenesis and bone regeneration employing icariin-loaded nanoplatforms. J. Biol. Eng. 2024, 18, 29. [Google Scholar] [CrossRef]
- Meshram, M.; Anchlia, S.; Shah, H.; Vyas, S.; Dhuvad, J.; Sagarka, L. Buccal fat pad-derived stem cells for repair of maxillofacial bony defects. J. Maxillofac. Oral Surg. 2019, 18, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.O.; Moon, S.H.; Jeong, H.C.; Yi, J.Y.; Lee, T.H.; Shim, S.H.; Rhee, Y.H.; Lee, S.H.; Oh, S.J.; Lee, M.Y.; et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, E3281–E3290. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Boonyuen, S.; Apinyauppatham, K.; Pripatnanont, P. Effects of oral cavity stem cell sources and serum-free cell culture on hydrogel encapsulation of mesenchymal stem cells for bone regeneration: An in vitro investigation. Bioengineering 2024, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Karimi, M.A.; Karimi, M.; Hodjat, M.; Pour, M.S.; Karimi, A.; Chiniforush, N. A comparative evaluation of the effects of 635 nm laser on cell proliferation and osteogenic differentiation of buccal fat pad mesenchymal stem cells. Photochem. Photobiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Rezaie Rad, M.; Barikani, H.; Chiniforush, N.; Akbari, S. Effect of 980 nm photobiomodulation delivered by a handpiece with Gaussian vs. Flat-Top profiles on proliferation and differentiation of buccal fat pad stem cells. Photochem. Photobiol. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mei, Z.; Li, J.; Gimble, J.M. Isolation of human adipose-derived stromal/stem cells from lipoaspirates. Methods Mol. Biol. 2024, 2783, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Zhidkov, R.; Panin, A.; Drobyshev, A.; Demura, T.; Avraamova, S.; Aleksandrov, P.; Kolesnikova, A.; Darawsheh, H.; Turkina, A.; Redko, N.; et al. Morphological evaluation and immunohistochemical analysis of the reparative potential of the buccal fat pad. Medicina 2024, 60, 567. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Tudela-Mulero, M.R.; Navarro, J.A.; Buendía, A.J.; Mercado-Díaz, A.M. Use of buccal fat pad-derived stem cells cultured on bioceramics for repair of critical-sized mandibular defects in healthy and osteoporotic rats. Clin. Oral Investig. 2022, 26, 5389–5408. [Google Scholar] [CrossRef]
- Gholami, L.; Afshar, S.; Arkian, A.; Saeidijam, M.; Hendi, S.S.; Mahmoudi, R.; Khorsandi, K.; Hashemzehi, H.; Fekrazad, R. NIR irradiation of human buccal fat pad adipose stem cells and its effect on TRP ion channels. Lasers Med. Sci. 2022, 37, 3681–3692. [Google Scholar] [CrossRef]
- Khazaei, S.; Khademi, A.; Torabinejad, M.; Nasr Esfahani, M.H.; Khazaei, M.; Razavi, S.M. Improving pulp revascularization outcomes with buccal fat autotransplantation. J. Tissue Eng. Regen. Med. 2020, 14, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, V.; Lecce, M.; Marenzi, G.; Cabaro, S.; Ambrosio, M.R.; Sammartino, G.; Misso, S.; Migliaccio, T.; Liguoro, P.; Oriente, F.; et al. Platelet-rich plasma counteracts detrimental effect of high-glucose concentrations on mesenchymal stem cells from Bichat fat pad. J. Tissue Eng. Regen. Med. 2020, 14, 701–713. [Google Scholar] [CrossRef]
- Nokhbatolfoghahaei, H.; Bohlouli, M.; Paknejad, Z.; Rad, M.R.; Amirabad, L.M.; Salehi-Nik, N.; Khani, M.M.; Shahriari, S.; Nadjmi, N.; Ebrahimpour, A.; et al. Bioreactor cultivation condition for engineered bone tissue: Effect of various bioreactor designs on extra cellular matrix synthesis. J. Biomed. Mater. Res. A 2020, 108, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, F.; Hesami, N.; Rad, M.R.; Nazeman, P.; Fahimipour, F.; Khojasteh, A. Improved bone regeneration through amniotic membrane loaded with buccal fat pad-derived MSCs as an adjuvant in maxillomandibular reconstruction. J. Craniomaxillofac. Surg. 2019, 47, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.S.; Soleimanifar, F.; Ardeshirylajimi, A.; Vakilian, S.; Mossahebi-Mohammadi, M.; Enderami, S.E.; Khojasteh, A.; Zare Karizi, S. In vitro osteogenic differentiation of stem cells with different sources on composite scaffold containing natural bioceramic and polycaprolactone. Artif. Cells Nanomed. Biotechnol. 2019, 47, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Hosseinpour, S.; Rezai Rad, M.; Alikhasi, M.; Zadeh, H.H. Buccal fat pad-derived stem cells with anorganic bovine bone mineral scaffold for augmentation of atrophic posterior mandible: An exploratory prospective clinical study. Clin. Implant. Dent. Relat. Res. 2019, 21, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, H.; Razmkhah, M.; Kiany, F.; Chenari, N.; Haghshenas, M.R.; Ghaderi, A. Comparison of osteogenic and chondrogenic differentiation ability of buccal fat pad derived mesenchymal stem cells and gingival derived cells. J. Dent. (Shiraz.) 2018, 19, 124–131. [Google Scholar] [PubMed]
- Khojasteh, A.; Kheiri, L.; Behnia, H.; Tehranchi, A.; Nazeman, P.; Nadjmi, N.; Soleimani, M. Lateral ramus cortical bone plate in alveolar cleft osteoplasty with concomitant use of buccal fat pad derived cells and autogenous bone: Phase I clinical trial. BioMed. Res. Int. 2017, 2017, 6560234. [Google Scholar] [CrossRef]
- Rezai Rad, M.; Bohloli, M.; Akhavan Rahnama, M.; Anbarlou, A.; Nazeman, P.; Khojasteh, A. Impact of tissue harvesting sites on the cellular behaviors of adipose-derived stem cells: Implication for bone tissue engineering. Stem Cells Int. 2017, 2017, 2156478. [Google Scholar] [CrossRef]
- Khojasteh, A.; Sadeghi, N. Application of buccal fat pad-derived stem cells in combination with autogenous iliac bone graft in the treatment of maxillomandibular atrophy: A preliminary human study. Int. J. Oral Maxillofac. Surg. 2016, 45, 864–871. [Google Scholar] [CrossRef]
- Tsurumachi, N.; Akita, D.; Kano, K.; Matsumoto, T.; Toriumi, T.; Kazama, T.; Oki, Y.; Tamura, Y.; Tonogi, M.; Isokawa, K.; et al. Small buccal fat pad cells have high osteogenic differentiation potential. Tissue Eng. Part C Methods 2016, 22, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Ardeshirylajimi, A.; Mossahebi-Mohammadi, M.; Vakilian, S.; Langroudi, L.; Seyedjafari, E.; Atashi, A.; Soleimani, M. Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic-coated electrospun poly (L-lactide) nanofibres. Cell Prolif. 2015, 48, 47–58. [Google Scholar] [CrossRef]
- Sezgin, B.; Tatar, S.; Boge, M.; Ozmen, S.; Yavuzer, R. The excision of the buccal fat pad for cheek refinement: Volumetric considerations. Aesthet. Surg. J. 2019, 39, 585–592. [Google Scholar] [CrossRef]
- Grillo, R.; de la Puente Dongo, J.L.; de Moura Moreira, L.; Dos Santos Queiroz, A.G.; Teixeira, R.G. Effectiveness of bandage in the incidence of major complications on bichectomy: Literature review and case series of 643 bichectomies. Oral Maxillofac. Surg. 2022, 26, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Turhal, G.; Topal, P.Ö.A.; Tanrıverdi, O.H.; Göde, S. Orbital abscess a rare complication after bichectomy surgery. Eur. Arch. Otorhinolaryngol. 2023, 280, 3479–3483. [Google Scholar] [CrossRef] [PubMed]
- Weissler, J.M.; Mohamed, O.; Gryskiewicz, J.M.; Chopra, K. An algorithmic approach to managing parotid duct injury following buccal fat pad removal. Aesthet. Surg. J. Open Forum 2022, 4, ojac032. [Google Scholar] [CrossRef] [PubMed]
- Bereczki-Temistocle, D.L.; Gurzu, S.; Ioan, I.; Vlad, G.M.; Beresescu, G.; Ormenisan, A. Successful closure of an oro-antral communication, after three previously failed attempts, using Bichat fat pad flap: A case report. World J. Plast. Surg. 2023, 12, 90–94. [Google Scholar] [CrossRef]
- Bradley, P. Buccal pad of fat and its applications in oral and maxillofacial surgery: A review of published literature (February) 2004 to (July) 2009. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 146. [Google Scholar] [CrossRef]
- Sharma, S.; Mehra, H.; Gupta, H.; Agarwal, R.; Gangwar, A.; Kumar, A. Comparison of the efficacy of amniotic membrane versus Buccal fat pad in treatment of oral submucous fibrosis. J. Maxillofac. Oral Surg. 2023, 22, 525–532. [Google Scholar] [CrossRef]
- Nelke, K.; Morawska, A.; Błaszczyk, B.; Janeczek, M.; Pasicka, E.; Łukaszewski, M.; Żak, K.; Dobrzyński, M. Anatomical and surgical implications of the usage of Bichat fat pad in oroantral communication, maxillary, palatal, and related surgeries-narrative review. J. Clin. Med. 2023, 12, 4909. [Google Scholar] [CrossRef]
- Rapidis, A.D.; Alexandridis, C.A.; Eleftheriadis, E.; Angelopoulos, A.P. The use of the buccal fat pad for reconstruction of oral defects: Review of the literature and report of 15 cases. J. Oral Maxillofac. Surg. 2000, 58, 158–163. [Google Scholar] [CrossRef]
- Mannelli, G.; Arcuri, F.; Comini, L.V.; Valente, D.; Spinelli, G. Buccal fat pad: Report of 24 Cases and literature review of 1,635 cases of oral defect reconstruction. ORL J. Otorhinolaryngol. Relat. Spec. 2019, 81, 24–35. [Google Scholar] [CrossRef]
- Dolanmaz, D.; Tuz, H.; Bayraktar, S.; Metin, M.; Erdem, E.; Baykul, T. Use of pedicled buccal fat pad in the closure of oroantral communication: Analysis of 75 cases. Quintessence Int. 2004, 35, 241–246. [Google Scholar]
- Poeschl, P.W.; Baumann, A.; Russmueller, G.; Poeschl, E.; Klug, C.; Ewers, R. Closure of oroantral communications with Bichat’s buccal fat pad. J. Oral Maxillofac. Surg. 2009, 67, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Manuel, S.; Kumar, S.; Nair, P.R. The versatility in the use of buccal fat pad in the closure of oro-antral fistulas. J. Maxillofac. Oral Surg. 2015, 14, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Rahpeyma, A.; Khajehahmadi, S. Buccal fat pad graft in maxillofacial surgery. Indian J. Surg. Oncol. 2021, 12, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.; Khobaragade, B.; Ganesan, K. Use of the temporal extension of the buccal fat pad for closure of oro-antral communications. Int. J. Oral Maxillofac. Surg. 2021, 50, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.; Trujillo, O.; Kristen, Q.; Isac, K.; Zorko, J.; Fam, M.; Okonkwo, K.; Mian, A.; Thanh, H.; Koban, K.; et al. The facial adipose tissue: A revision. Facial Plast. Surg. 2016, 32, 671–682. [Google Scholar] [CrossRef]
- Kahn, J.L.; Sick, H.; Laude, M.; Koritke, J.G. La vascularisation du corps adipeux de la joue [Vascularization of the adipose body of the cheek]. Arch. Anat. Histol. Embryol. 1990, 73, 3–20. [Google Scholar]
- Singh, J.; Prasad, K.; Lalitha, R.M.; Ranganath, K. Buccal pad of fat and its applications in oral and maxillofacial surgery: A review of published literature (February) 2004 to (July) 2009. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2010, 110, 698–705. [Google Scholar] [CrossRef]
- Kumar, D.; Rattan, V.; Rai, S.; Yadav, S.; Sahu, G.R. Reconstruction of anterior maxillary defect with buccal pad fat after excision of melanotic neuroectodermal tumor of infancy. Ann. Maxillofac. Surg. 2015, 5, 234–236. [Google Scholar] [CrossRef] [PubMed]

| Reference | BFP MSC Key Findings |
|---|---|
| Arpornmaeklong et al. [53] | BFP MSCs promote bone regeneration and exhibited a higher proliferation rate than periodontal ligament stem cells |
| Etemadi et al. [54] | Osteogenic differentiation and expression of related genes are increased in BFP MSCs by the effects of a 635 nm diode laser without affecting cell proliferation |
| Homayouni et al. [55] | Osteogenic differentiation and expression of related genes are increased in BFP MSCs by the effects of 980 nm irradiation |
| Li et al. [56] | Optimized protocol of isolation of MSCs from BFP |
| Zhidkov et al. [57] | BFP MSCs are preferentially localized around capillaries and in brown fat tissue |
| Camacho-Alonso et al. [58] | BFP MSCs cultured on bioceramics improve bone regeneration in bone defects compared with bioceramics alone in healthy and osteoporotic rats |
| Gholami et al. [59] | Proliferation and osteogenic differentiation of BFP MSCs are increased by the effects of 940 nm irradiation |
| Dehghani Nazhvani et al. [35] | Hypoxia preconditioning of BFP MSCs in combination with a bilayer chitosan scaffold promote regeneration of articular cartilage defects in the absence of chondrogenic growth factors |
| Genova et al. [36] | BFP MSCs show a higher isolation rate than dental pulp MSCs and show higher expression of MSC markers and larger osteogenic differentiation capacity. |
| Khazaei e al. [60] | Large amounts of BFP MSCs are harvested and differentiate successfully into odontoblast-, osteoblast- and cementoblast-like cells. |
| D’Esposito et al. [61] | Hyperglycemia reduces BFP MSC growth and osteogenic differentiation potential, whereas platelet-rich plasma enhances their growth without impairing their osteogenic differentiation potential |
| Nokhbatolfoghahaei et al. [62] | BFP MSCs on a β-tricalcium phosphate scaffold highly express osteogenic markers |
| Akhlaghi et al. [63] | Human amniotic membranes loaded with BFP MSCs enhance bone regeneration and reduce bone resorption by creating a protective membrane |
| Hosseini et al. [64] | BFP MSCs are the best choice for bone tissue repair |
| Khojasteh et al. [65] | BFP MSCs in combination with anorganic bovine bone mineral ameliorate bone regeneration |
| Meshram et al. [51] | BFP MSCs express various adhesion molecules and are not cells of hematopoietic and angiogenic lineages. Maxillofacial bone defect repair by grafting BFP MSCs results in high bone density formation with enhanced bone trabecular formation, well-organized and well-vascularized lamellar bone with Haversian channels and osteocytes |
| Fang et al. [48] | BFP MSCs and periodontal ligament stem cells have a higher osteogenic potential than dental pulp stem cells |
| Ghaderi et al. [66] | BFP yields a greater proportion of MSCs than gingiva |
| Khojasteh et al. [67] | Phase I clinical trial shows that BFP MSCs and autogenous bone may enhance bone regeneration in alveolar cleft bone |
| Rezai Rad et al. [68] | BFP MSCs show higher proliferation rates and osteogenic capacities than adipose tissue- and bone marrow-derived stem cells. BFP MSCs express osteogenic and angiogenic markers |
| Khojasteh and Sadeghi [69] | BFP MSCs and autogenous bone increase osteogenic capacity and prevent graft resorption in patients |
| Tsurumachi et al. [70] | BFP MSCs show higher osteogenic differentiation capacity than BFP large dedifferentiated fat cells |
| Ardeshirylajimi et al. [71] | BFP MSCs show higher osteogenic differentiation capacity than adipose tissue MSCs and unrestricted somatic stem cells which is similar to that of bone marrow MSCs |
| Broccaioli et al. [47] | BFP MSCs are rather similar to subcutaneous stem cells in differentiation capacity, adherence behavior to biological and synthetic materials and osteogenic differentiation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favero, G.; van Noorden, C.J.F.; Rezzani, R. The Buccal Fat Pad: A Unique Human Anatomical Structure and Rich and Easily Accessible Source of Mesenchymal Stem Cells for Tissue Repair. Bioengineering 2024, 11, 968. https://doi.org/10.3390/bioengineering11100968
Favero G, van Noorden CJF, Rezzani R. The Buccal Fat Pad: A Unique Human Anatomical Structure and Rich and Easily Accessible Source of Mesenchymal Stem Cells for Tissue Repair. Bioengineering. 2024; 11(10):968. https://doi.org/10.3390/bioengineering11100968
Chicago/Turabian StyleFavero, Gaia, Cornelis J. F. van Noorden, and Rita Rezzani. 2024. "The Buccal Fat Pad: A Unique Human Anatomical Structure and Rich and Easily Accessible Source of Mesenchymal Stem Cells for Tissue Repair" Bioengineering 11, no. 10: 968. https://doi.org/10.3390/bioengineering11100968
APA StyleFavero, G., van Noorden, C. J. F., & Rezzani, R. (2024). The Buccal Fat Pad: A Unique Human Anatomical Structure and Rich and Easily Accessible Source of Mesenchymal Stem Cells for Tissue Repair. Bioengineering, 11(10), 968. https://doi.org/10.3390/bioengineering11100968










