Characterization of MSC Growth, Differentiation, and EV Production in CNF Hydrogels Under Static and Dynamic Cultures in Hypoxic and Normoxic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.1.1. adMSCs
2.1.2. PBMCs
2.2. Preparation of to-CNFs
2.3. Cell Encapsulation in to-CNF Hydrogels Spheres for In Vitro Cultivation
2.4. to-CNF Hydrogel Sphere Cultivation in Bioreactors
2.5. Live/Dead Cell Visualization
2.6. Cell Metabolic Activity Assessment
2.7. DNA Quantification
2.8. Isolation of Extracellular Vesicles
2.9. Protein Concentration Assay
2.10. Nanoparticle Tracking Analysis
2.11. PBMC Co-Culture with adMSCs or adMSC-EVs
2.12. Flow Cytometry Analysis
2.13. Extracellular Vesicle Surface Marker Analysis
2.14. Adipogenic Differentiation
2.15. Intracellular Lipid Droplet Staining
2.16. Mechanical Characterization of to-CNF Hydrogels
2.17. Statistical Analysis
3. Results
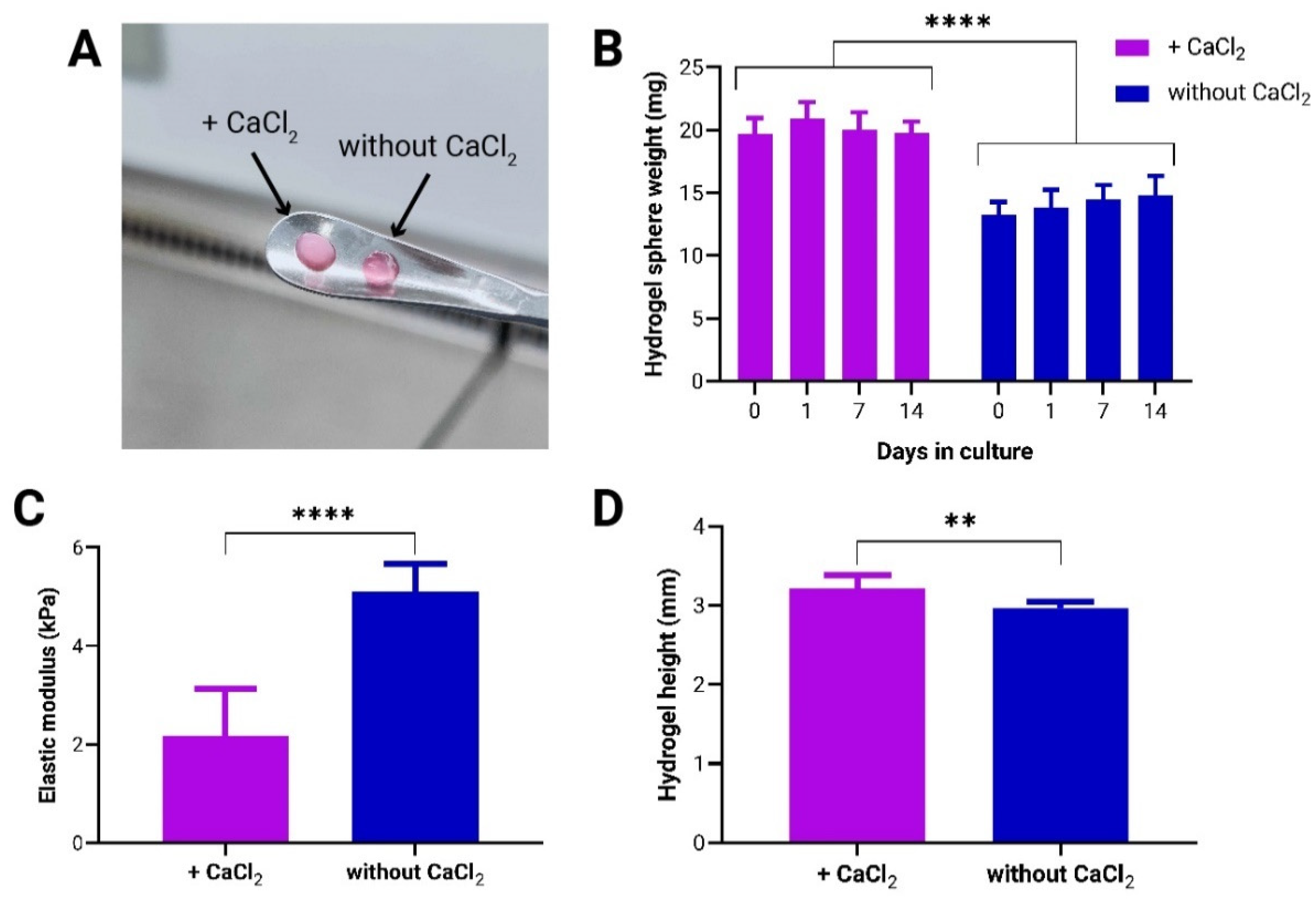
3.1. Effect of CaCl2-Induced Crosslinking on Mechanical Properties of to-CNF Hydrogel Spheres and Growth Dynamics of Encapsulated adMSCs
3.2. Dynamic Cultivation Enhances adMSC Proliferation and Protein Secretion in to-CNF Hydrogel Spheres
3.3. Hypoxia Enhances adMSC Proliferation and Protein Secretion in to-CNF Hydrogel Spheres
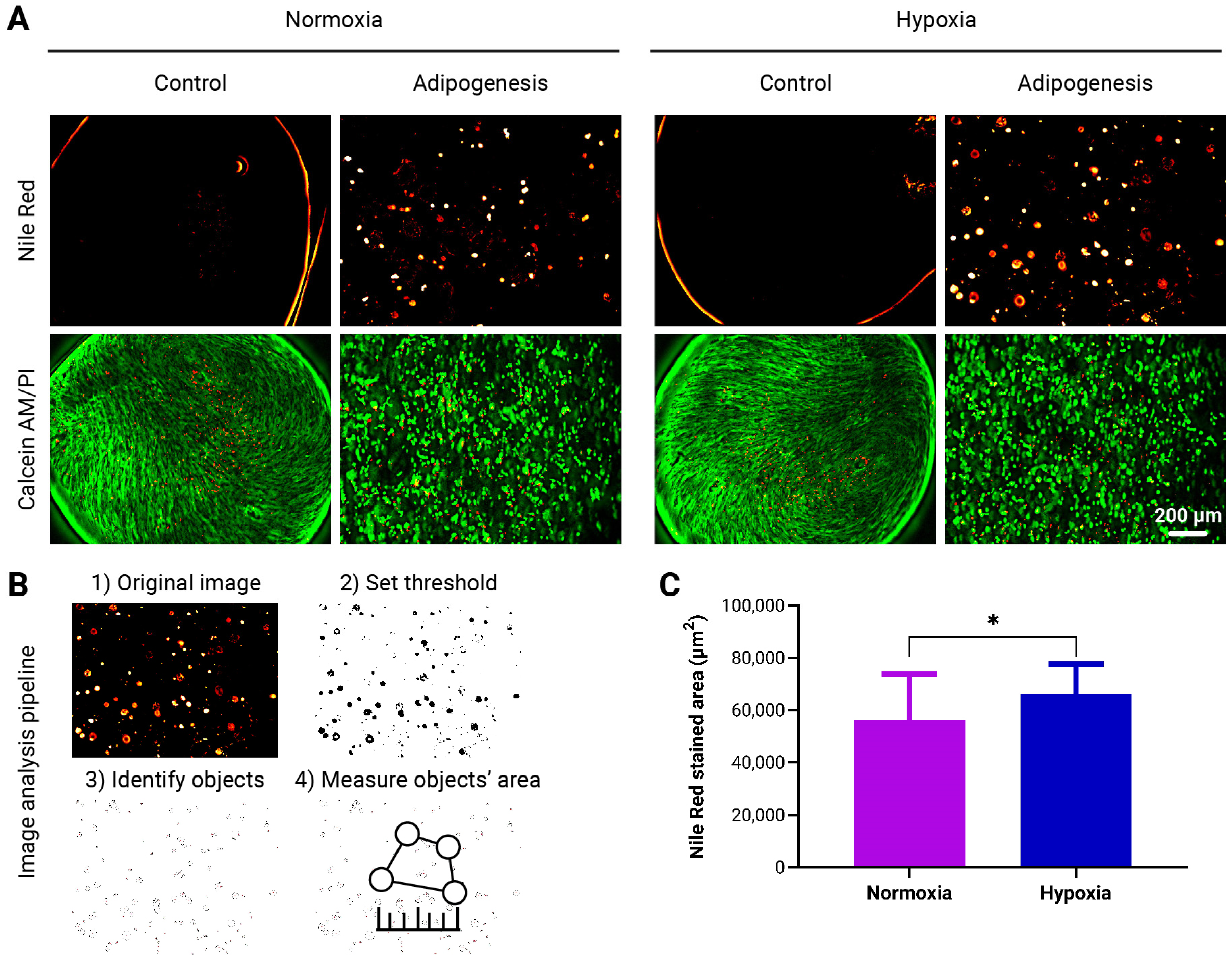
3.4. Enhanced Adipogenic Differentiation of adMSCs in to-CNF Hydrogel Spheres Under Hypoxic Conditions
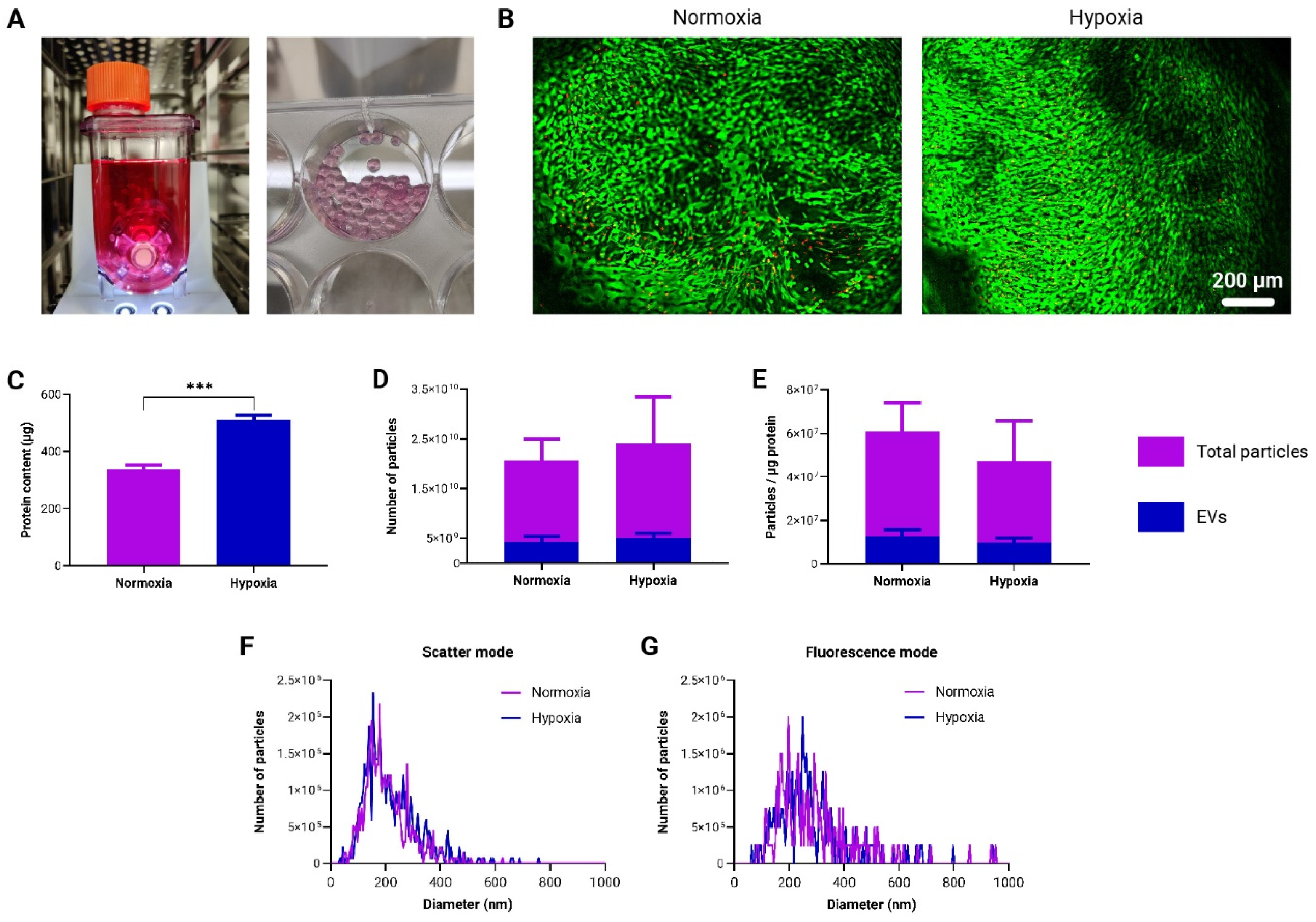
3.5. Generation and Characterization of Immunomodulatory EVs by to-CNF Hydrogel Sphere-Encapsulated adMSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Song, S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch. Pharm. Res. 2012, 35, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Khayambashi, P.; Iyer, J.; Pillai, S.; Upadhyay, A.; Zhang, Y.; Tran, S.D. Hydrogel Encapsulation of Mesenchymal Stem Cells and Their Derived Exosomes for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 684. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Yu, W.; Li, S.; Guan, X.; Zhang, N.; Xie, X.; Zhang, K.; Bai, Y. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel-assisted 3D culture system for bone regeneration. Biomater. Adv. 2022, 133, 112646. [Google Scholar] [CrossRef]
- Born, L.J.; McLoughlin, S.T.; Dutta, D.; Mahadik, B.; Jia, X.; Fisher, J.P.; Jay, S.M. Sustained released of bioactive mesenchymal stromal cell-derived extracellular vesicles from 3D-printed gelatin methacrylate hydrogels. J. Biomed. Mater. Res. A 2022, 110, 1190–1198. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Troy, E.; Tilbury, M.A.; Power, A.M.; Wall, J.G. Nature-Based Biomaterials and Their Application in Biomedicine. Polymers 2021, 13, 3321. [Google Scholar] [CrossRef]
- Duarte, A.C.; Costa, E.C.; Filipe, H.A.L.; Saraiva, S.M.; Jacinto, T.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Animal-derived products in science and current alternatives. Biomater. Adv. 2023, 151, 213428. [Google Scholar] [CrossRef] [PubMed]
- Sanandiya, N.D.; Vasudevan, J.; Das, R.; Lim, C.T.; Fernandez, J.G. Stimuli-responsive injectable cellulose thixogel for cell encapsulation. Int. J. Biol. Macromol. 2019, 130, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Nikolits, I.; Radwan, S.; Liebner, F.; Dietrich, W.; Egger, D.; Chariyev-Prinz, F.; Kasper, C. Hydrogels from TEMPO-Oxidized Nanofibrillated Cellulose Support In Vitro Cultivation of Encapsulated Human Mesenchymal Stem Cells. ACS Appl. Bio Mater. 2023, 6, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Zander, N.E.; Dong, H.; Steele, J.; Grant, J.T. Metal Cation Cross-Linked Nanocellulose Hydrogels as Tissue Engineering Substrates. ACS Appl. Mater. Interfaces 2014, 6, 18502–18510. [Google Scholar] [CrossRef]
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, N. On the use of ion-crosslinked nanocellulose hydrogels for wound healing solutions: Physicochemical properties and application-oriented biocompatibility studies. Carbohydr. Polym. 2017, 174, 299–308. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, D.X.; Choy, S.; Nguyen, H.-L.; Cha, H.J.; Hwang, D.S. 3D cellulose nanofiber scaffold with homogeneous cell population and long-term proliferation. Cellulose 2018, 25, 7299–7314. [Google Scholar] [CrossRef]
- Egger, D.; Tripisciano, C.; Weber, V.; Dominici, M.; Kasper, C. Dynamic Cultivation of Mesenchymal Stem Cell Aggregates. Bioengineering 2018, 5, 48. [Google Scholar] [CrossRef]
- Clementi, A.; Egger, D.; Charwat, V.; Kasper, C. Cell Culture Conditions: Cultivation of Stem Cells Under Dynamic Conditions. In Cell Engineering and Regeneration; Gimble, J.M., Marolt, D., Oreffo, R., Redl, H., Wolbank, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–33. [Google Scholar] [CrossRef]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef]
- Ivanovic, Z. Hypoxia or in situ normoxia: The stem cell paradigm. J. Cell. Physiol. 2009, 219, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Ejtehadifar, M.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Dehdilani, N.; Abbasi, P.; Molaeipour, Z.; Saleh, M. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv. Pharm. Bull. 2015, 5, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Escribano, V.; Torrecillas-Baena, B.; Camacho-Cardenosa, M.; Dorado, G.; Gálvez-Moreno, M.; Casado-Díaz, A. Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles. World J. Stem Cells 2022, 14, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef]
- Zavala, G.; Ramos, M.-P.; Figueroa-Valdés, A.I.; Cisternas, P.; Wyneken, U.; Hernández, M.; Toa, P.; Salmons, B.; Dangerfield, J.; Gunzburg, W.H.; et al. Semipermeable Cellulose Beads Allow Selective and Continuous Release of Small Extracellular Vesicles (sEV) from Encapsulated Cells. Front. Pharmacol. 2020, 11, 679. [Google Scholar] [CrossRef]
- Colao, I.L.; Corteling, R.; Bracewell, D.; Wall, I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol. Med. 2018, 24, 242–256. [Google Scholar] [CrossRef]
- Egger, D.; Spitz, S.; Fischer, M.; Handschuh, S.; Glösmann, M.; Friemert, B.; Egerbacher, M.; Kasper, C. Application of a Parallelizable Perfusion Bioreactor for Physiologic 3D Cell Culture. Cells Tissues Organs 2017, 203, 316–326. [Google Scholar] [CrossRef]
- Dong, H.; Snyder, J.F.; Williams, K.S.; Andzelm, J.W. Cation-Induced Hydrogels of Cellulose Nanofibrils with Tunable Moduli. Biomacromolecules 2013, 14, 3338–3345. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.; Tae, G. Mesenchymal stem cell-encapsulated cellulose nanofiber microbeads and enhanced biological activities by hyaluronic acid incorporation. Carbohydr. Polym. 2022, 280, 119026. [Google Scholar] [CrossRef] [PubMed]
- Llobet, L.; Montoya, J.; López-Gallardo, E.; Ruiz-Pesini, E. Side Effects of Culture Media Antibiotics on Cell Differentiation. Tissue Eng. Part C Methods 2015, 21, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef]
- Pachler, K.; Ketterl, N.; Desgeorges, A.; Dunai, Z.A.; Laner-Plamberger, S.; Streif, D.; Strunk, D.; Rohde, E.; Gimona, M. An In Vitro Potency Assay for Monitoring the Immunomodulatory Potential of Stromal Cell-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2017, 18, 1413. [Google Scholar] [CrossRef]
- Adlerz, K.; Trempel, M.; Rowley, J.A.; Ahsan, T. Increasing yield of msc-evs in scalable xeno-free manufacturing. Cytotherapy 2019, 21, S58. [Google Scholar] [CrossRef]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef]
- Fitzka, M.; Rennhofer, H.; Catoor, D.; Reiterer, M.; Lichtenegger, H.; Checchia, S.; di Michiel, M.; Irrasch, D.; Gruenewald, T.A.; Mayer, H. High Speed In Situ Synchrotron Observation of Cyclic Deformation and Phase Transformation of Superelastic Nitinol at Ultrasonic Frequency. Exp. Mech. 2020, 60, 317–328. [Google Scholar] [CrossRef]
- Webber, J.; Clayton, A. How pure are your vesicles? J. Extracell. Vesicles 2013, 2, 19861. [Google Scholar] [CrossRef]
- Azoidis, I.; Metcalfe, J.; Reynolds, J.; Keeton, S.; Hakki, S.S.; Sheard, J.; Widera, D. Three-dimensional cell culture of human mesenchymal stem cells in nanofibrillar cellulose hydrogels. MRS Commun. 2017, 7, 458–465. [Google Scholar] [CrossRef]
- Sheard, J.J.; Bicer, M.; Meng, Y.; Frigo, A.; Aguilar, R.M.; Vallance, T.M.; Iandolo, D.; Widera, D. Optically Transparent Anionic Nanofibrillar Cellulose Is Cytocompatible with Human Adipose Tissue-Derived Stem Cells and Allows Simple Imaging in 3D. Stem Cells Int. 2019, 2019, 3106929. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Jo, M.K.; Cho, Y.S.; Yang, S.H. Diffusion-Controlled Crystallization of Calcium Phosphate in a Hydrogel toward a Homogeneous Octacalcium Phosphate/Agarose Composite. ACS Omega 2022, 7, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lei, L.; Song, Q.; Li, X. Calcium ion cross-linking alginate/dexamethasone sodium phosphate hybrid hydrogel for extended drug release. Colloids Surf. B Biointerfaces 2019, 175, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Witt, F.; Duda, G.N.; Bergmann, C.; Petersen, A. Cyclic mechanical loading enables solute transport and oxygen supply in bone healing: An in vitro investigation. Tissue Eng. Part A 2014, 20, 486–493. [Google Scholar] [CrossRef]
- Shou, Y.; Teo, X.Y.; Wu, K.Z.; Bai, B.; Kumar, A.R.K.; Low, J.; Le, Z.; Tay, A. Dynamic Stimulations with Bioengineered Extracellular Matrix-Mimicking Hydrogels for Mechano Cell Reprogramming and Therapy. Adv. Sci. 2023, 10, 2300670. [Google Scholar] [CrossRef]
- Sinha, R.; Verdonschot, N.; Koopman, B.; Rouwkema, J. Tuning Cell and Tissue Development by Combining Multiple Mechanical Signals. Tissue Eng. Part B Rev. 2017, 23, 494–504. [Google Scholar] [CrossRef]
- Huang, L.; Li, R.; Liu, W.; Dai, J.; Du, Z.; Wang, X.; Ma, J.; Zhao, J. Dynamic culture of a thermosensitive collagen hydrogel as an extracellular matrix improves the construction of tissue-engineered peripheral nerve. Neural Regen. Res. 2014, 9, 1371–1378. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.-Y.; Zhu, H.-J.; Lai, J.-W.; Sun, S.-S.; Lin, Y.; Li, X.-L.; Guo, Z.-B.; Lv, Z.; Meng, H.; et al. Mechano-biomimetic hydrogel 3D cell cultivation as a strategy to improve mammalian cell protein expression. Mater. Today Bio 2023, 21, 100732. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Majore, I.; Kasper, C.; Hass, R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun. Signal. 2010, 8, 18. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Chun, S.Y.; Ha, Y.S.; Kim, D.H.; Kim, J.; Song, P.H.; Kim, H.T.; Yoo, E.S.; Kim, B.S.; Kwon, T.G. Hypoxia Enhances Cell Properties of Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2017, 14, 595–604. [Google Scholar] [CrossRef]
- Fábián, Z. The Effects of Hypoxia on the Immune-Modulatory Properties of Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2019, 2019, 2509606. [Google Scholar] [CrossRef] [PubMed]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Li, L.; Wu, J.; Huang, T.; Zhang, Y.; Cao, J.; Ma, T.; Chen, J.; Zhang, C.; Zhang, X.; et al. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater. Sci. 2022, 10, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Rodriguez, L.A.; Walker, K.P.; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Parfejevs, V.; Sagini, K.; Buss, A.; Sobolevska, K.; Llorente, A.; Riekstina, U.; Abols, A. Adult Stem Cell-Derived Extracellular Vesicles in Cancer Treatment: Opportunities and Challenges. Cells 2020, 9, 1171. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Franklin, J.L.; Coffey, R.J. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat. Protocols 2023, 18, 1462–1487. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Conlon, M.M.; Rider, M.A.; Brownstein, N.C.; Meckes Jr, D.G. Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. J. Extracell. Vesicles 2016, 5, 31295. [Google Scholar] [CrossRef]
- Yiwei, A.; Chenxu, G.; Marta, G.-C.; Laura, S.S.B.; Andras, S.; Oluwapelumi, S.; Shankar, R.; Junhao, X.; Shang Jui, T.; Yi, D.; et al. Syntenin and CD63 Promote Exosome Biogenesis from the Plasma Membrane by Blocking Cargo Endocytosis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Fanaei, M.; Monk, P.N.; Partridge, L.J. The role of tetraspanins in fusion. Biochem. Soc. Trans. 2011, 39, 524–528. [Google Scholar] [CrossRef]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J.; Sullivan, M.L.G.; Stolz, D.B.; Papworth, G.D.; Zahorchak, A.F.; Logar, A.J.; Wang, Z.; Watkins, S.C.; et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004, 104, 3257–3266. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolits, I.; Chariyev-Prinz, F.; Egger, D.; Liebner, F.; Mytzka, N.; Kasper, C. Characterization of MSC Growth, Differentiation, and EV Production in CNF Hydrogels Under Static and Dynamic Cultures in Hypoxic and Normoxic Conditions. Bioengineering 2024, 11, 1050. https://doi.org/10.3390/bioengineering11101050
Nikolits I, Chariyev-Prinz F, Egger D, Liebner F, Mytzka N, Kasper C. Characterization of MSC Growth, Differentiation, and EV Production in CNF Hydrogels Under Static and Dynamic Cultures in Hypoxic and Normoxic Conditions. Bioengineering. 2024; 11(10):1050. https://doi.org/10.3390/bioengineering11101050
Chicago/Turabian StyleNikolits, Ilias, Farhad Chariyev-Prinz, Dominik Egger, Falk Liebner, Nicolas Mytzka, and Cornelia Kasper. 2024. "Characterization of MSC Growth, Differentiation, and EV Production in CNF Hydrogels Under Static and Dynamic Cultures in Hypoxic and Normoxic Conditions" Bioengineering 11, no. 10: 1050. https://doi.org/10.3390/bioengineering11101050
APA StyleNikolits, I., Chariyev-Prinz, F., Egger, D., Liebner, F., Mytzka, N., & Kasper, C. (2024). Characterization of MSC Growth, Differentiation, and EV Production in CNF Hydrogels Under Static and Dynamic Cultures in Hypoxic and Normoxic Conditions. Bioengineering, 11(10), 1050. https://doi.org/10.3390/bioengineering11101050










