Enhancing Bone Formation Through bFGF-Loaded Mesenchymal Stromal Cell Spheroids During Fracture Healing in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
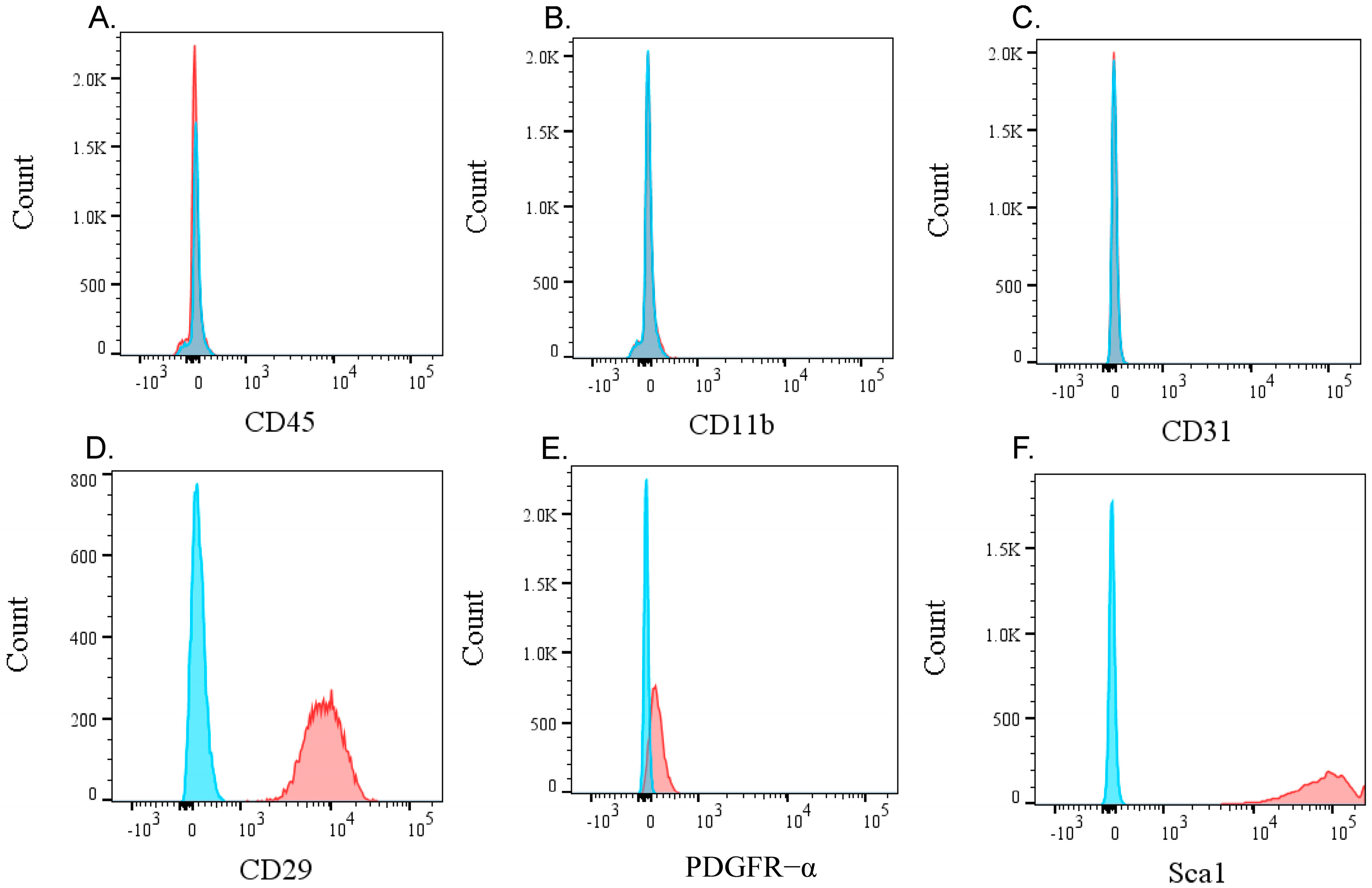
2.2. Flow Cytometric Analysis
2.3. qPCR Analysis
2.4. Binding Assay
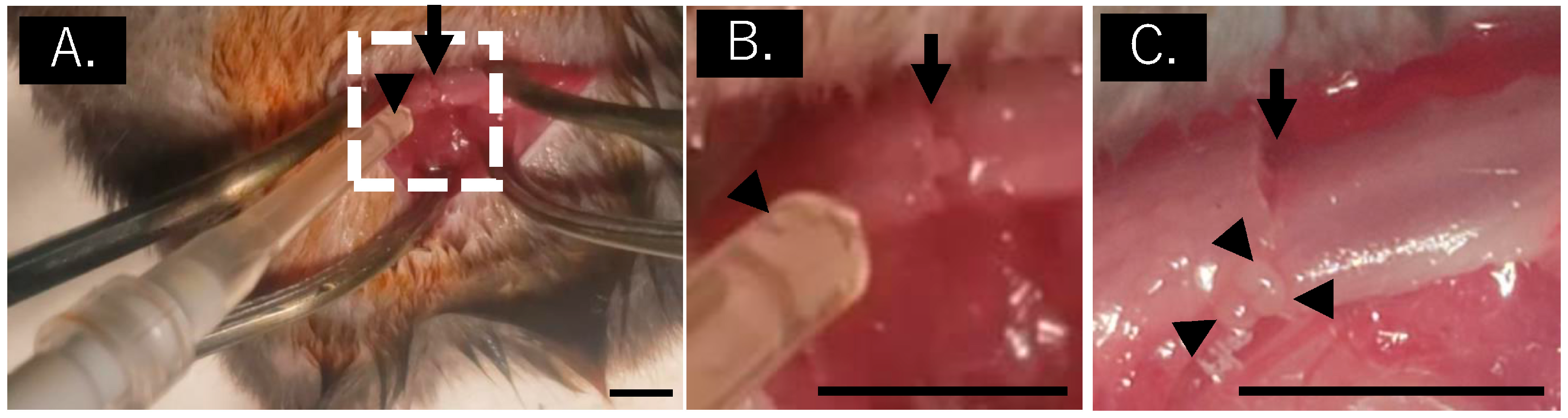
2.5. Mouse Fracture Model
2.6. Determination of New Bone Volume and Bone Mineral Content
2.7. Statistical Analysis
3. Results
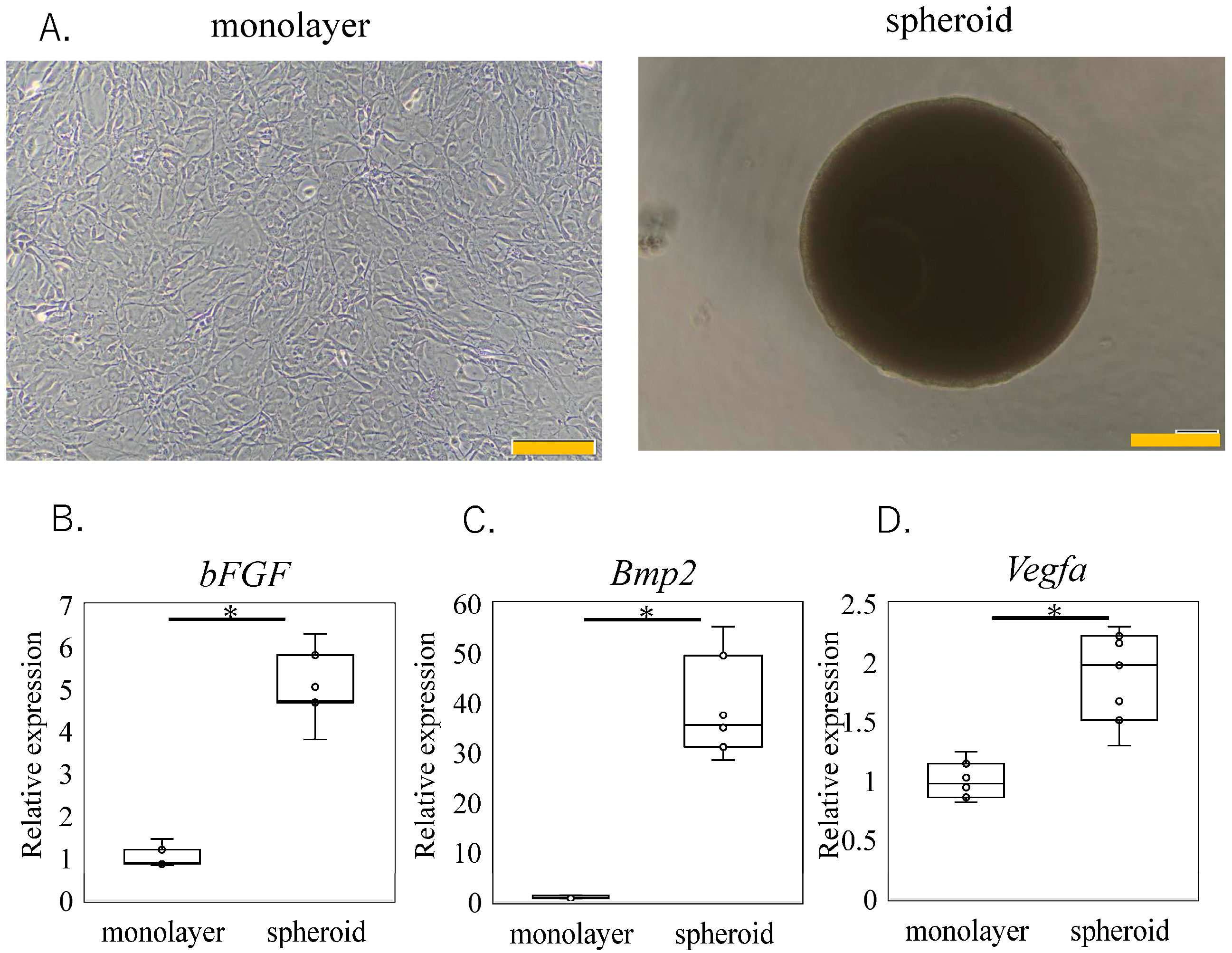
3.1. Effect of Spheroid Size on Trophic Factor Expression
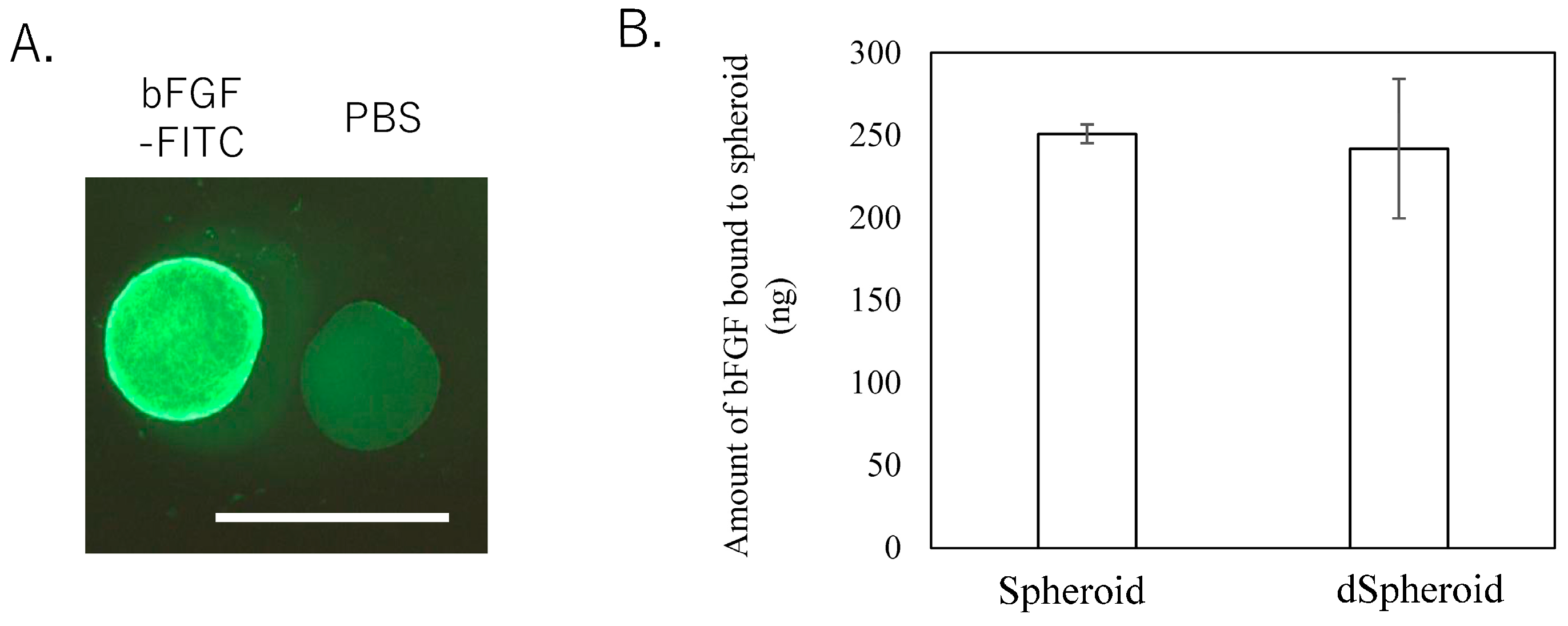
3.2. bFGF Binding Assay
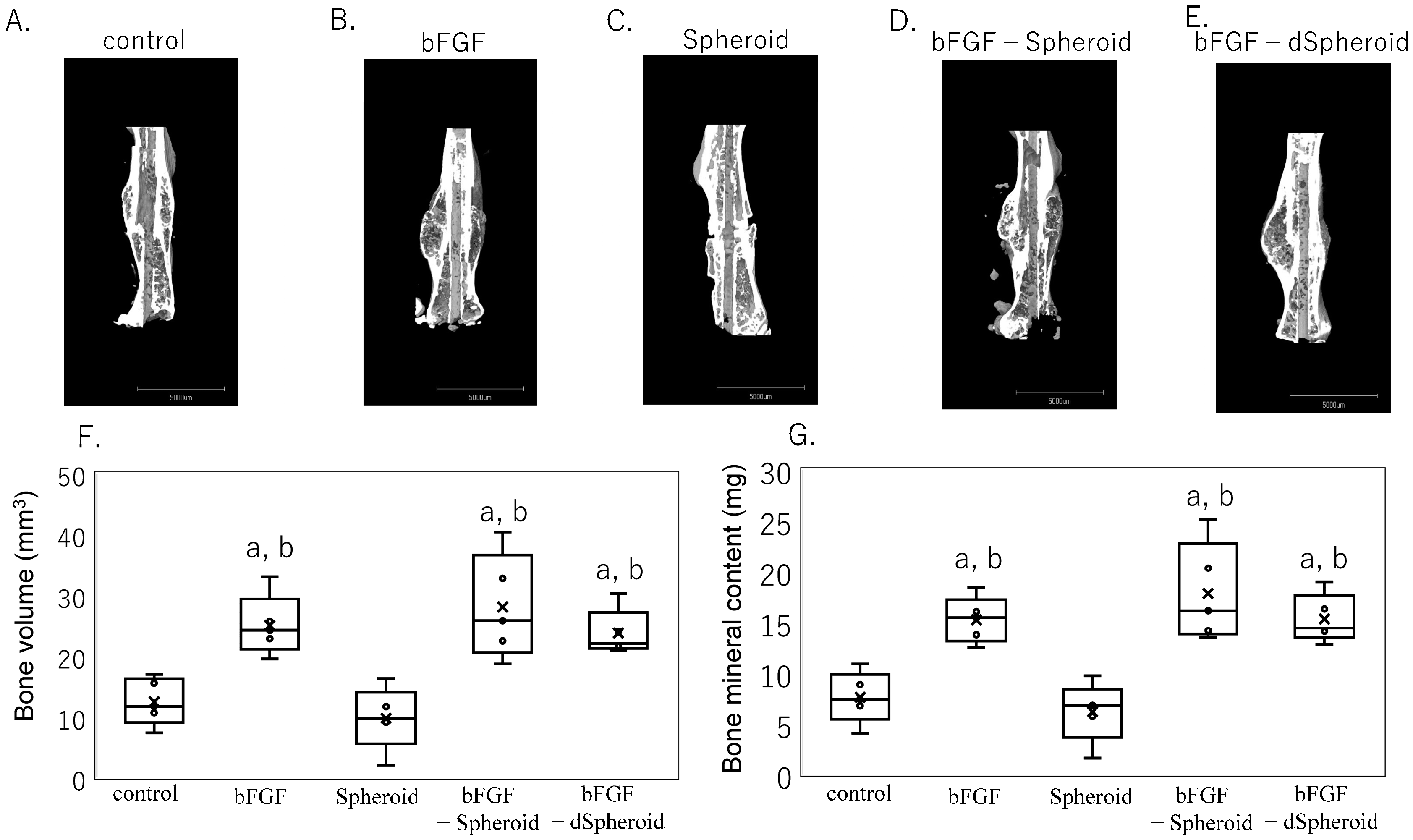
3.3. Effect of High-Dose bFGF-Loaded Spheroids on Bone Volume and Bone Mineral Content After Fracture
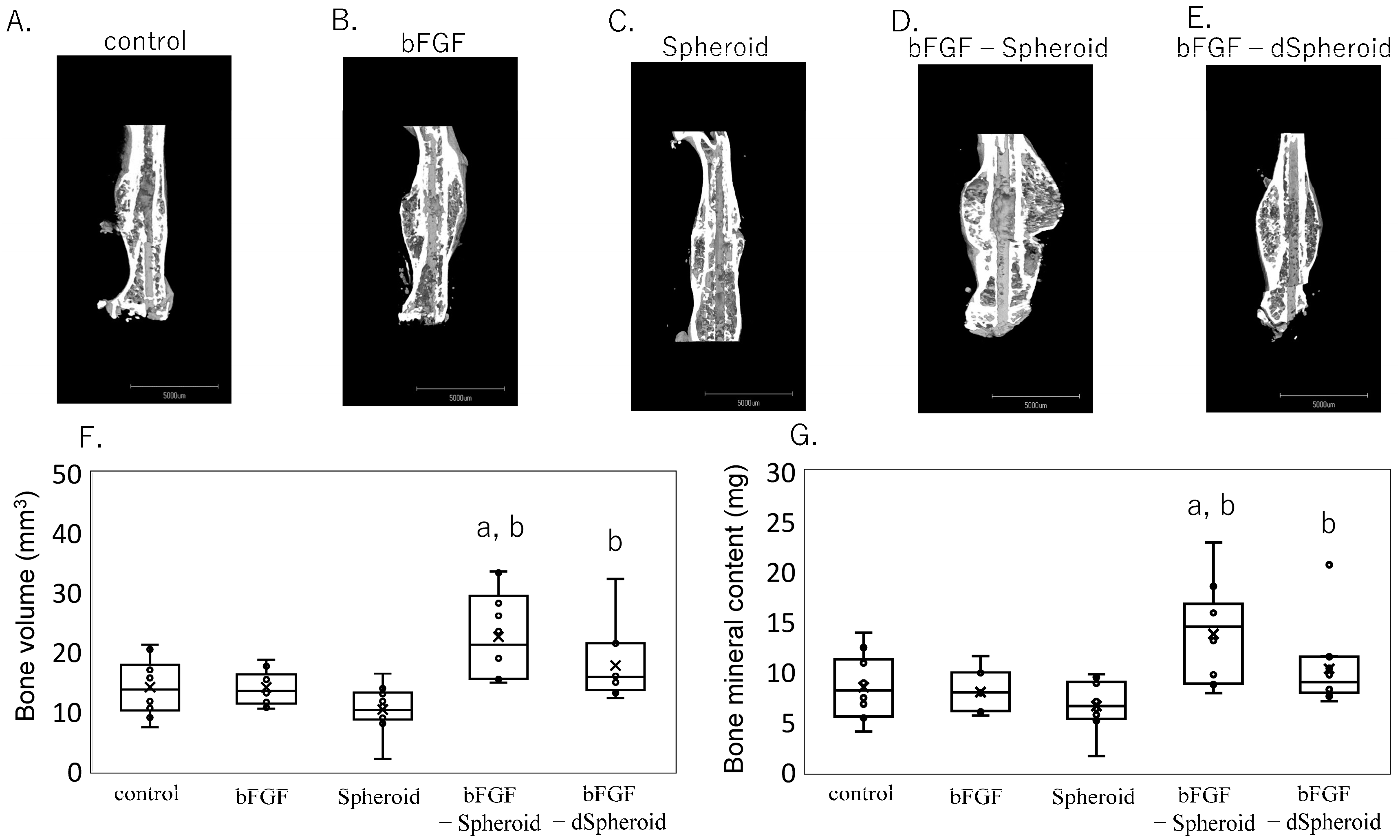
3.4. Effect of Low-Dose bFGF-Loaded Spheroids on Bone Volume and Bone Mineral Content After Fracture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhidu, S.; Ying, T.; Rui, J.; Chao, Z. Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: Challenges and opportunities. Stem Cell Res. Ther. 2024, 15, 266. [Google Scholar] [CrossRef] [PubMed]
- Kupcova Skalnikova, H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013, 95, 2196–2211. [Google Scholar] [CrossRef] [PubMed]
- Mijiritsky, E.; Ferroni, L.; Gardin, C.; Bressan, E.; Zanette, G.; Piattelli, A.; Zavan, B. Porcine Bone Scaffolds Adsorb Growth Factors Secreted by MSCs and Improve Bone Tissue Repair. Materials 2017, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, A.; Mineo, A.; Shoji, S.; Inoue, G.; Saito, W.; Sekiguchi, H.; Takaso, M.; Uchida, K. Effect of spheroid size on gene expression profiles of a mouse mesenchymal stem cell line in spheroid culture. Biomed. Mater. Eng. 2023, 34, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Han, Y.S.; Lee, S.H. Long-Duration Three-Dimensional Spheroid Culture Promotes Angiogenic Activities of Adipose-Derived Mesenchymal Stem Cells. Biomol. Ther. 2016, 24, 260–267. [Google Scholar] [CrossRef]
- Ho, S.S.; Hung, B.P.; Heyrani, N.; Lee, M.A.; Leach, J.K. Hypoxic Preconditioning of Mesenchymal Stem Cells with Subsequent Spheroid Formation Accelerates Repair of Segmental Bone Defects. Stem Cells 2018, 36, 1393–1403. [Google Scholar] [CrossRef]
- Inglis, S.; Kanczler, J.M.; Oreffo, R.O.C. 3D human bone marrow stromal and endothelial cell spheres promote bone healing in an osteogenic niche. FASEB J. 2019, 33, 3279–3290. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Ohno, J.; Sato, A.; Kido, H.; Fukushima, T. Mesenchymal stem cell spheroids exhibit enhanced in-vitro and in-vivo osteoregenerative potential. BMC Biotechnol. 2014, 14, 105. [Google Scholar] [CrossRef]
- Findeisen, L.; Bolte, J.; Vater, C.; Petzold, C.; Quade, M.; Muller, L.; Goodman, S.B.; Zwingenberger, S. Cell spheroids are as effective as single cells suspensions in the treatment of critical-sized bone defects. BMC Musculoskelet. Disord. 2021, 22, 401. [Google Scholar] [CrossRef]
- Wagener, N.; Lehmann, W.; Boker, K.O.; Rohner, E.; Di Fazio, P. Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants. J. Clin. Med. 2022, 11, 6218. [Google Scholar] [CrossRef]
- Khan, S.N.; Bostrom, M.P.; Lane, J.M. Bone growth factors. Orthop. Clin. N. Am. 2000, 31, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kawaguchi, H.; Hanada, K.; Aoyama, I.; Hiyama, Y.; Nakamura, T.; Kuzutani, K.; Tamura, M.; Kurokawa, T.; Nakamura, K. Single local injection of recombinant fibroblast growth factor-2 stimulates healing of segmental bone defects in rabbits. J. Orthop. Res. 1998, 16, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Kurokawa, T.; Hanada, K.; Hiyama, Y.; Tamura, M.; Ogata, E.; Matsumoto, T. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology 1994, 135, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Nakamura, K.; Tabata, Y.; Ikada, Y.; Aoyama, I.; Anzai, J.; Nakamura, T.; Hiyama, Y.; Tamura, M. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. J. Clin. Endocrinol. Metab. 2001, 86, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kawaguchi, H.; Aoyama, I.; Hanada, K.; Hiyama, Y.; Awa, T.; Tamura, M.; Kurokawa, T. Stimulation of bone formation by intraosseous application of recombinant basic fibroblast growth factor in normal and ovariectomized rabbits. J. Orthop. Res. 1997, 15, 307–313. [Google Scholar] [CrossRef]
- Radomsky, M.L.; Aufdemorte, T.B.; Swain, L.D.; Fox, W.C.; Spiro, R.C.; Poser, J.W. Novel formulation of fibroblast growth factor-2 in a hyaluronan gel accelerates fracture healing in nonhuman primates. J. Orthop. Res. 1999, 17, 607–614. [Google Scholar] [CrossRef]
- Tabata, Y.; Yamada, K.; Hong, L.; Miyamoto, S.; Hashimoto, N.; Ikada, Y. Skull bone regeneration in primates in response to basic fibroblast growth factor. J. Neurosurg. 1999, 91, 851–856. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Jingushi, S.; Izumi, T.; Fukunaga, M.; Matsushita, T.; Nakamura, T.; Mizuno, K.; Nakamura, T.; Nakamura, K. Local application of recombinant human fibroblast growth factor-2 on bone repair: A dose-escalation prospective trial on patients with osteotomy. J. Orthop. Res. 2007, 25, 480–487. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Oka, H.; Jingushi, S.; Izumi, T.; Fukunaga, M.; Sato, K.; Matsushita, T.; Nakamura, K.; Group, T. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. J. Bone Miner. Res. 2010, 25, 2735–2743. [Google Scholar] [CrossRef]
- Epstein, S.E.; Fuchs, S.; Zhou, Y.F.; Baffour, R.; Kornowski, R. Therapeutic interventions for enhancing collateral development by administration of growth factors: Basic principles, early results and potential hazards. Cardiovasc. Res. 2001, 49, 532–542. [Google Scholar] [CrossRef]
- Unger, E.F.; Goncalves, L.; Epstein, S.E.; Chew, E.Y.; Trapnell, C.B.; Cannon, R.O., 3rd; Quyyumi, A.A. Effects of a single intracoronary injection of basic fibroblast growth factor in stable angina pectoris. Am. J. Cardiol. 2000, 85, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, A.; Marui, A.; Yamamoto, S.; Ozeki, M.; Hirano, Y.; Yamamoto, M.; Ogawa, O.; Komeda, M.; Tabata, Y. Type I collagen can function as a reservoir of basic fibroblast growth factor. J. Control. Release 2004, 99, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, A.; Yamamoto, S.; Ozeki, M.; Noguchi, T.; Kanatani, I.; Ogawa, O.; Tabata, Y. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials 2004, 25, 4513–4520. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bai, Y.; Yin, G.; Pu, X.; Huang, Z.; Liao, X.; Chen, X.; Yao, Y. Synergistic and sequential effects of BMP-2, bFGF and VEGF on osteogenic differentiation of rat osteoblasts. J. Bone Miner. Metab. 2014, 32, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Makiguchi, T.; Yamada, M.; Yoshioka, Y.; Sugiura, H.; Koarai, A.; Chiba, S.; Fujino, N.; Tojo, Y.; Ota, C.; Kubo, H.; et al. Serum extracellular vesicular miR-21-5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 110. [Google Scholar] [CrossRef]
- Mieda, T.; Suto, N.; Iizuka, A.; Matsuura, S.; Iizuka, H.; Takagishi, K.; Nakamura, K.; Hirai, H. Mesenchymal stem cells attenuate peripheral neuronal degeneration in spinocerebellar ataxia type 1 knockin mice. J. Neurosci. Res. 2016, 94, 246–252. [Google Scholar] [CrossRef]
- Ono, M.; Ohkouchi, S.; Kanehira, M.; Tode, N.; Kobayashi, M.; Ebina, M.; Nukiwa, T.; Irokawa, T.; Ogawa, H.; Akaike, T.; et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol. Ther. 2015, 23, 549–560. [Google Scholar] [CrossRef]
- Morikawa, S.; Mabuchi, Y.; Kubota, Y.; Nagai, Y.; Niibe, K.; Hiratsu, E.; Suzuki, S.; Miyauchi-Hara, C.; Nagoshi, N.; Sunabori, T.; et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J. Exp. Med. 2009, 206, 2483–2496. [Google Scholar] [CrossRef]
- Sung, J.H.; Yang, H.M.; Park, J.B.; Choi, G.S.; Joh, J.W.; Kwon, C.H.; Chun, J.M.; Lee, S.K.; Kim, S.J. Isolation and characterization of mouse mesenchymal stem cells. Transplant. Proc. 2008, 40, 2649–2654. [Google Scholar] [CrossRef]
- Kang, W.; Liang, Q.; Du, L.; Shang, L.; Wang, T.; Ge, S. Sequential application of bFGF and BMP-2 facilitates osteogenic differentiation of human periodontal ligament stem cells. J. Periodontal. Res. 2019, 54, 424–434. [Google Scholar] [CrossRef]
- Qi, W.; Yan, J.; Sun, H.; Wang, H. Multifunctional Nanocomposite Films for Synergistic Delivery of bFGF and BMP-2. ACS Omega 2017, 2, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Saito, W.; Uchida, K.; Ueno, M.; Matsushita, O.; Inoue, G.; Nishi, N.; Ogura, T.; Hattori, S.; Fujimaki, H.; Tanaka, K.; et al. Acceleration of bone formation during fracture healing by injectable collagen powder and human basic fibroblast growth factor containing a collagen-binding domain from Clostridium histolyticum collagenase. J. Biomed. Mater. Res. A 2014, 102, 3049–3055. [Google Scholar] [CrossRef] [PubMed]
- Al-Waeli, H.; Reboucas, A.P.; Mansour, A.; Morris, M.; Tamimi, F.; Nicolau, B. Non-steroidal anti-inflammatory drugs and bone healing in animal models-a systematic review and meta-analysis. Syst. Rev. 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Menger, M.M.; Stief, M.; Scheuer, C.; Rollmann, M.F.; Herath, S.C.; Braun, B.J.; Ehnert, S.; Nussler, A.K.; Menger, M.D.; Laschke, M.W.; et al. Diclofenac, a NSAID, delays fracture healing in aged mice. Exp. Gerontol. 2023, 178, 112201. [Google Scholar] [CrossRef]
- Saito, H.; Shoji, S.; Kuroda, A.; Inoue, G.; Tazawa, R.; Sekiguchi, H.; Fukushima, K.; Miyagi, M.; Takaso, M.; Uchida, K. In situ-formed hyaluronan gel/BMP-2/hydroxyapatite composite promotes bone union in refractory fracture model mice. Biomed. Mater. Eng. 2023, 34, 537–544. [Google Scholar] [CrossRef]
- Shoji, S.; Uchida, K.; Saito, W.; Sekiguchi, H.; Inoue, G.; Miyagi, M.; Kuroda, A.; Takaso, M. Acceleration of Bone Healing by In Situ-Forming Dextran-Tyramine Conjugates Containing Basic Fibroblast Growth Factor in Mice. Cureus 2020, 12, e10085. [Google Scholar] [CrossRef]
- Nakamura, S.; Ito, T.; Okamoto, K.; Mima, T.; Uchida, K.; Siddiqui, Y.D.; Ito, M.; Tai, M.; Okubo, K.; Yamashiro, K.; et al. Acceleration of bone regeneration of horizontal bone defect in rats using collagen-binding basic fibroblast growth factor combined with collagen scaffolds. J. Periodontol. 2019, 90, 1043–1052. [Google Scholar] [CrossRef]
- Yin, S.; Wu, H.; Huang, Y.; Lu, C.; Cui, J.; Li, Y.; Xue, B.; Wu, J.; Jiang, C.; Gu, X.; et al. Structurally and mechanically tuned macroporous hydrogels for scalable mesenchymal stem cell-extracellular matrix spheroid production. Proc. Natl. Acad. Sci. USA 2024, 121, e2404210121. [Google Scholar] [CrossRef]
- Wu, H.; Yin, G.; Pu, X.; Wang, J.; Liao, X.; Huang, Z. Inhibitory Effects of Combined Bone Morphogenetic Protein 2, Vascular Endothelial Growth Factor, and Basic Fibroblast Growth Factor on Osteoclast Differentiation and Activity. Tissue Eng. Part A 2021, 27, 1387–1398. [Google Scholar] [CrossRef]
| Gene | Sequence (5′-3′) | Product Size (bp) | |
|---|---|---|---|
| bFGF | F | AGAGCGACCCTCACATCAAG | 80 |
| R | ACGGTTAGCACACACTCCTT | ||
| Bmp2 | F | AAGGCACCCTTTGTATGTGG | 211 |
| R | GCTAAGCTCAGTGGGGACAC | ||
| Vegfa | F | CACTGGACCCTGGCTTTACT | 79 |
| R | TCTGCTCTCCTTCTGTCGTG | ||
| Gapdh | F | AACTTTGGCATTGTGGAAGG | 223 |
| R | ACACATTGGGGGTAGGAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, K.; Saito, H.; Shoji, S.; Sekiguchi, H.; Matsumoto, M.; Ujihira, M.; Miyagi, M.; Inoue, G.; Takaso, M.; Uchida, K. Enhancing Bone Formation Through bFGF-Loaded Mesenchymal Stromal Cell Spheroids During Fracture Healing in Mice. Bioengineering 2024, 11, 1041. https://doi.org/10.3390/bioengineering11101041
Takeda K, Saito H, Shoji S, Sekiguchi H, Matsumoto M, Ujihira M, Miyagi M, Inoue G, Takaso M, Uchida K. Enhancing Bone Formation Through bFGF-Loaded Mesenchymal Stromal Cell Spheroids During Fracture Healing in Mice. Bioengineering. 2024; 11(10):1041. https://doi.org/10.3390/bioengineering11101041
Chicago/Turabian StyleTakeda, Kugo, Hiroki Saito, Shintaro Shoji, Hiroyuki Sekiguchi, Mitsuyoshi Matsumoto, Masanobu Ujihira, Masayuki Miyagi, Gen Inoue, Masashi Takaso, and Kentaro Uchida. 2024. "Enhancing Bone Formation Through bFGF-Loaded Mesenchymal Stromal Cell Spheroids During Fracture Healing in Mice" Bioengineering 11, no. 10: 1041. https://doi.org/10.3390/bioengineering11101041
APA StyleTakeda, K., Saito, H., Shoji, S., Sekiguchi, H., Matsumoto, M., Ujihira, M., Miyagi, M., Inoue, G., Takaso, M., & Uchida, K. (2024). Enhancing Bone Formation Through bFGF-Loaded Mesenchymal Stromal Cell Spheroids During Fracture Healing in Mice. Bioengineering, 11(10), 1041. https://doi.org/10.3390/bioengineering11101041









