Recombinant Plasminogen Activator of the Sandworm (Perinereis aibuhitensis) Expression in Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.1.1. Reagents
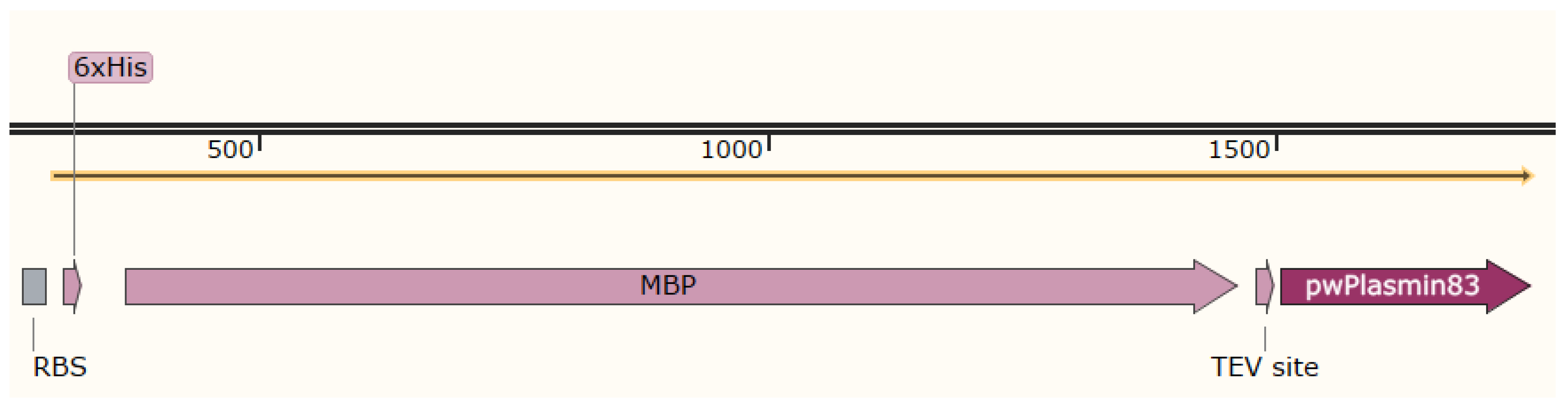
2.1.2. Plasmid
2.2. Plasmid Transformation and Protein Expression
2.2.1. Plasmid Transformation
2.2.2. Validation of Shaking-Flask-Induced Expression
2.2.3. Inclusion Body Collection and Washing
2.2.4. Inclusion Body Denaturation and Renaturation
2.3. Ni Column Gravity Column Purification
2.4. Measurement of Enzyme Activity
2.4.1. Urokinase Standard Curve
2.4.2. Fibrinolytic Enzyme Activity Test
2.4.3. Plasminogen Activator Enzyme Activity Test
2.4.4. TEVase Digestion
2.4.5. Enzyme Marker Readings
2.5. Detection of Protein Amount by SDS-PAGE and Grayscale Scanning
2.5.1. Loading and Electrophoresis
2.5.2. Dyeing and Decolorization
2.5.3. Imaging and Analysis
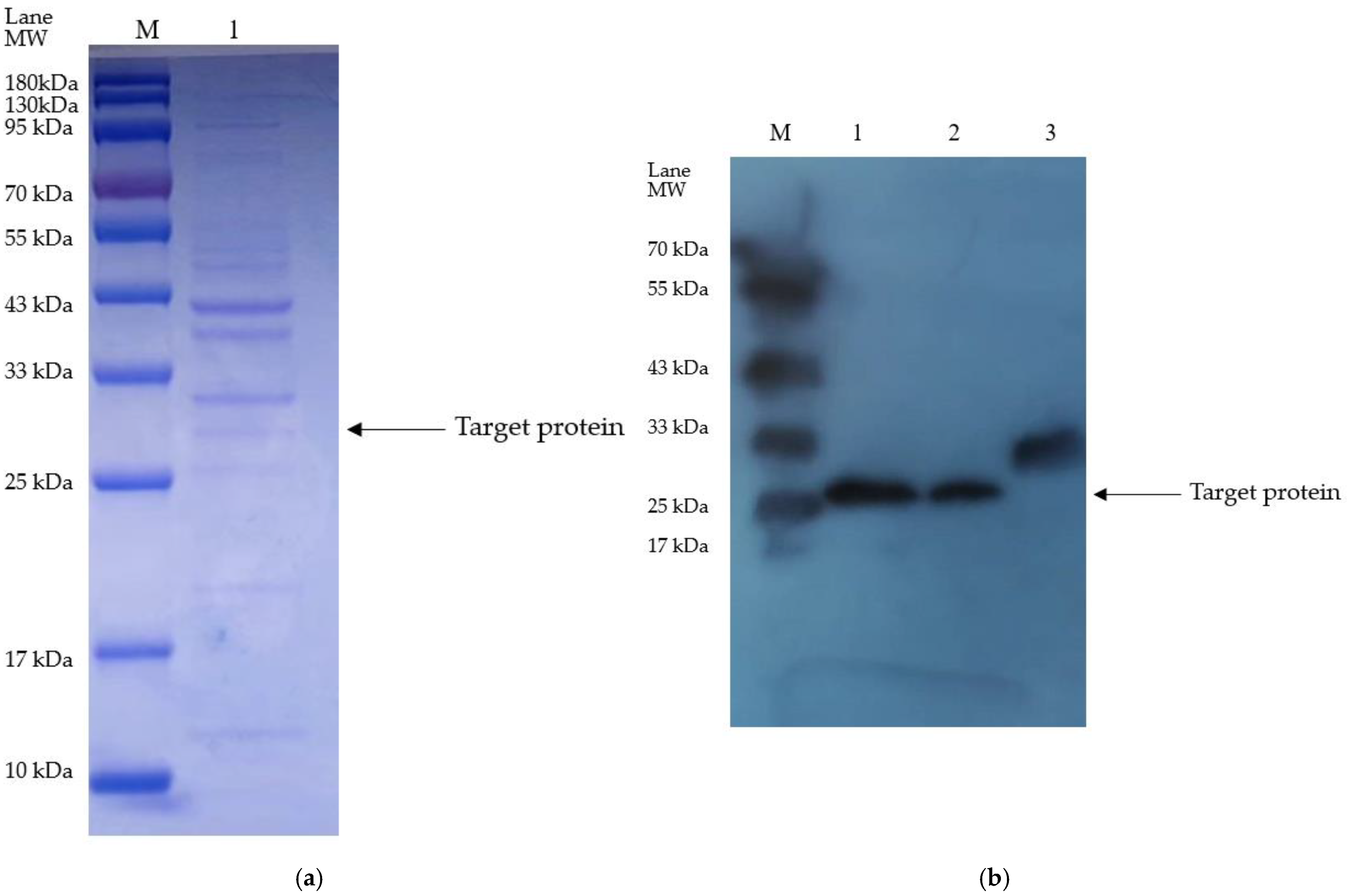
2.6. Western Blotting
3. Results
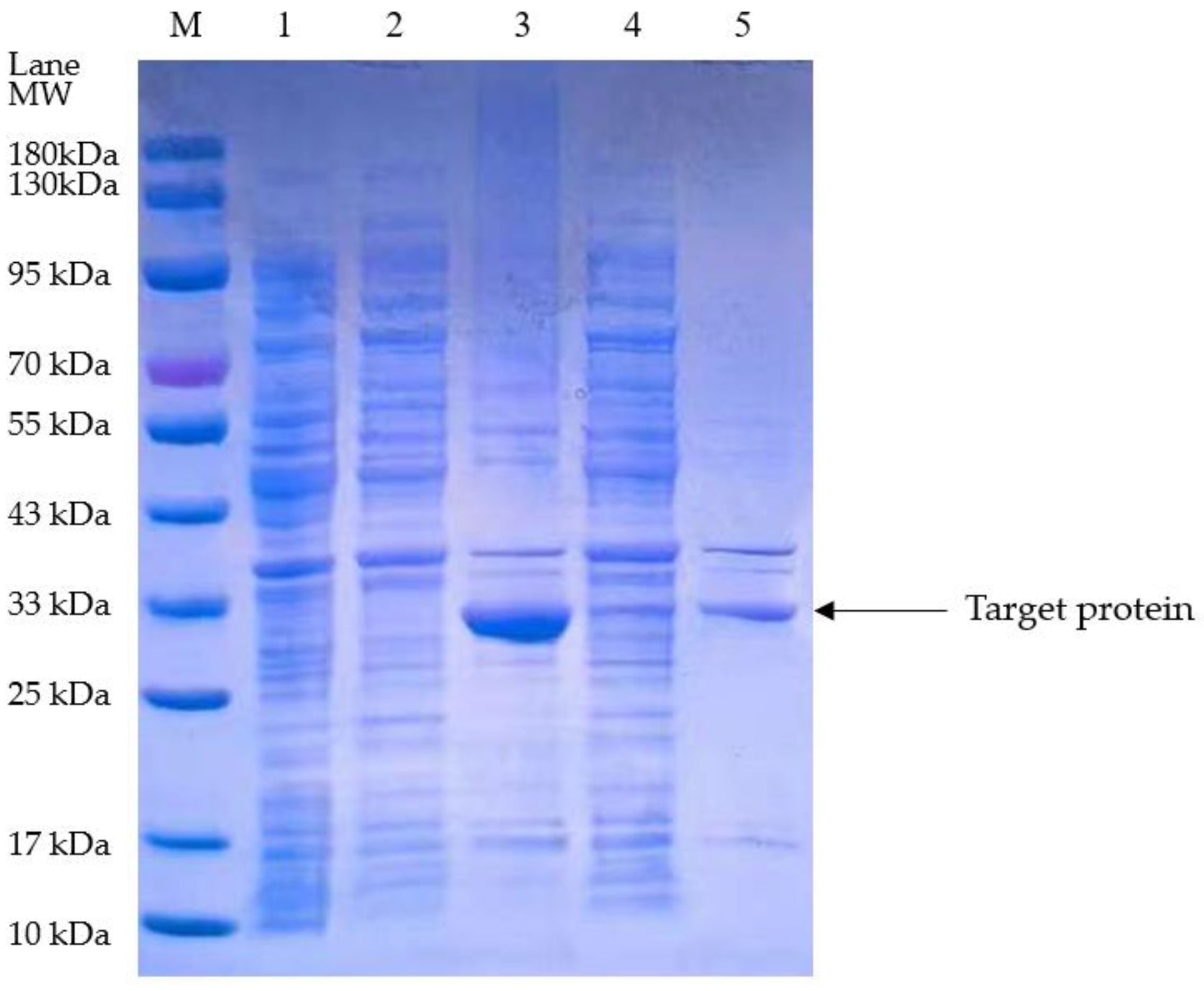
3.1. Isolation and Purification of the Plasminogen Activator from E. coli
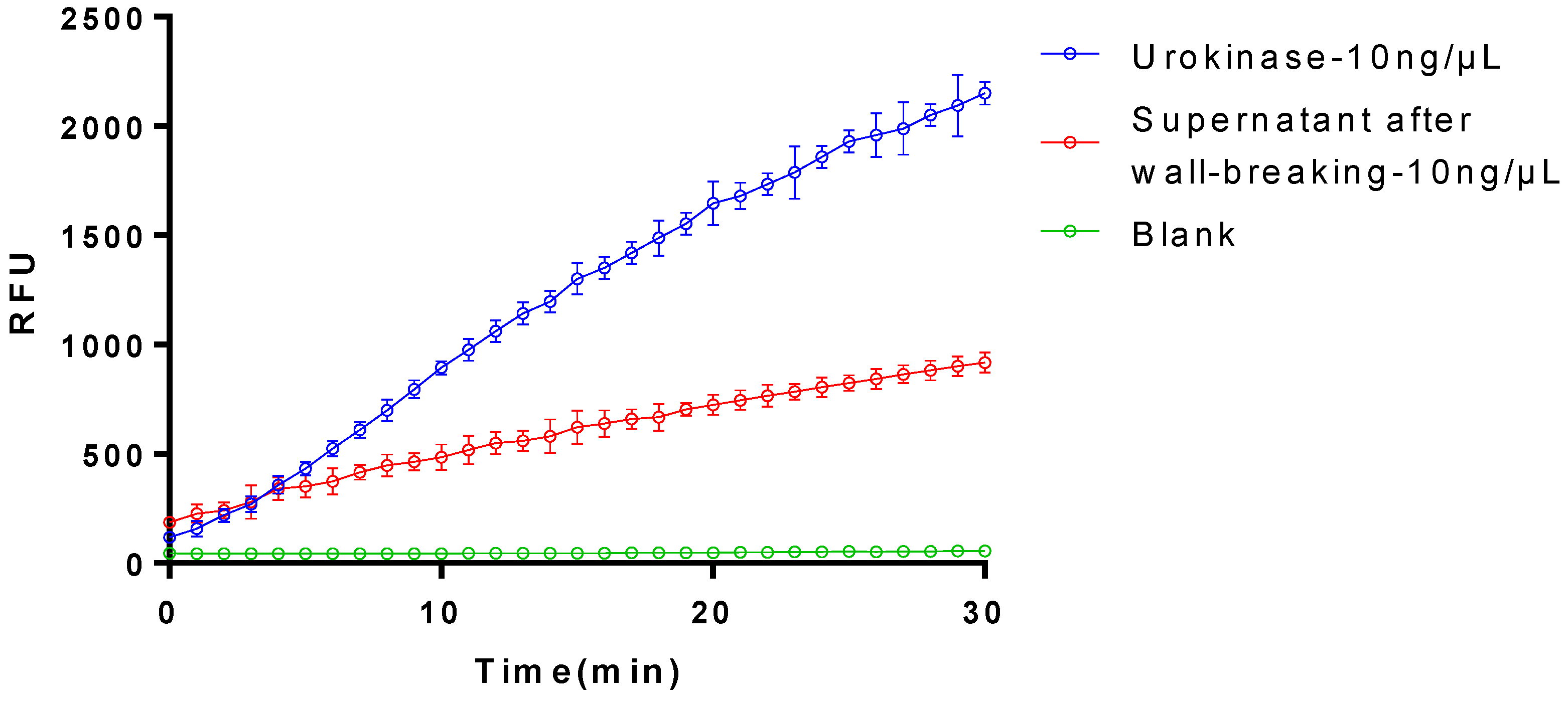
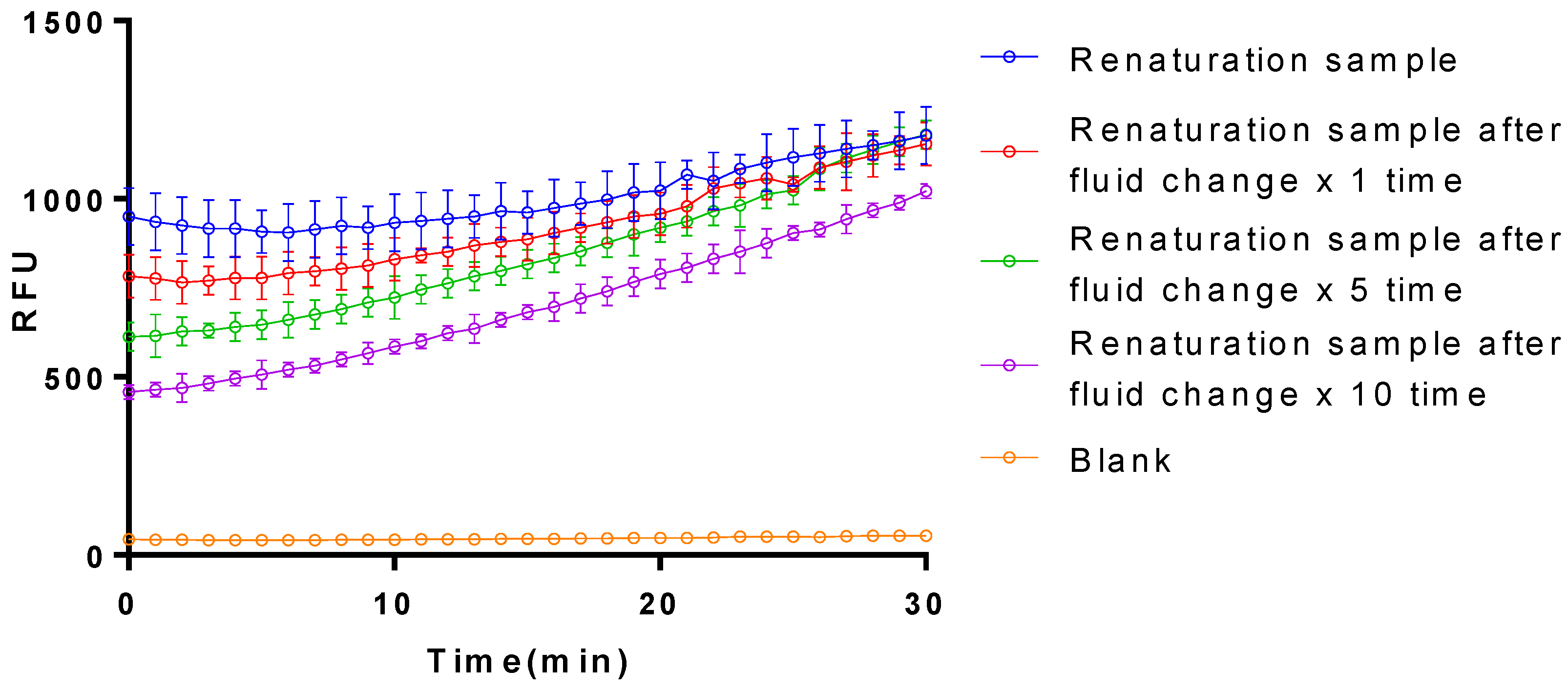
3.2. The Activation on Plasminogen of the Purified Plasminogen Activator
3.3. Isolation and Purification of the Optimized Plasminogen Activator from E. coli
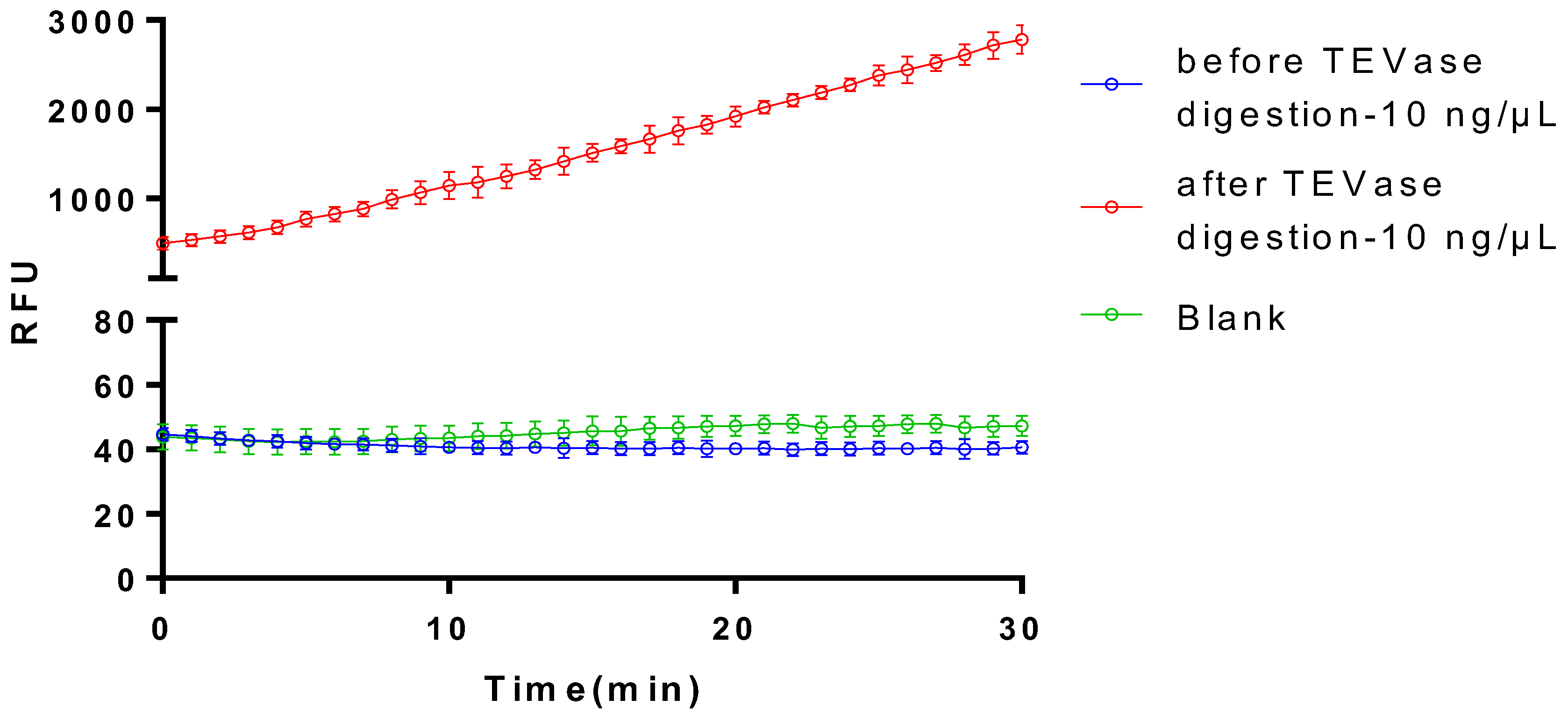
3.4. The Activiation of Plasminogen of the Recombinant Plasminogen Activator after the TEVase Digestion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, R.; Li, Q.; Liu, J.; Jiang, X.; Hong, M. Effects of sand worm protease on platelet aggregation and blood rheology. Chin. J. N. Drugs 2009, 18, 930–933. [Google Scholar] [CrossRef]
- Yao, Z.; Kim, J.A.; Kim, J.H. Gene Cloning, Expression, and Properties of a Fibrinolytic Enzyme Secreted by Bacillus pumilus BS15 Isolated from Gul (Oyster) Jeotgal. Biotechnol. Bioprocess Eng. 2018, 23, 293–301. [Google Scholar] [CrossRef]
- Cao, Q. Analysis of Nutrient Composition of Cultured Double-Toothed Periphyton Sandworms and Its Isolation and Purification of Fibrinolytic Enzymes. Master’s Thesis, Qingdao University of Science and Technology, Qingdao, China, 2016. [Google Scholar]
- Castellino, F.J. A unique enzyme—Protein substrate modifier reaction: Plasmin/streptokinase interaction. Trends Biochem. Sci. 1979, 4, 1–5. [Google Scholar] [CrossRef]
- Chen, S.; Ding, L. Progress and prospects of research on the bidentate periphyton sandworm. Aquaculture 2019, 40, 44–47. [Google Scholar] [CrossRef]
- Fu, Q. Study on the Ex Vivo and In Vivo Thrombolytic Effects of a Novel Synthetic Leech Peptide Hirulogs. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2017. [Google Scholar]
- Ge, C.; Chai, Y.; Wang, H.; Kan, M.; Sun, X.; Yang, L. Metabolic response of the bidentate periphyton sandworm, Bombyx mori, to complex pollution: Monitoring species/restoration species discrimination. Chin. Agron. Bull. 2016, 32, 74–77. [Google Scholar]
- Gou, Y.; Zhou, J. Progress of glucokinase research. Adv. Cardiovasc. Dis. 2014, 35, 695–699. [Google Scholar] [CrossRef]
- Hu, S.; Gao, R.; Liu, L.; Zhu, M.; Wang, W.; Wang, Y.; Wu, Z.; Li, H.; Gu, D.; Yang, Y.; et al. China Cardiovascular Disease Report; Encyclopedia of China Press: Beijing, China, 2018. [Google Scholar]
- Huang, L.; Duan, L.; Li, R.; Wang, B. Antioxidant activity of extracts from the sand worm, Bombyx mori. Chin. Mar. Drugs 2007, 2, 19–22. [Google Scholar] [CrossRef]
- Zhao, C.; Ju, J. Cloning, Expression and Activity Analysis of a Novel Fibrinolytic Serine Protease from Arenicola cristata. J. Ocean. Univ. China 2015, 14, 533–540. [Google Scholar] [CrossRef]
- Li, A.; Zhang, C.; Wang, J.; Wang, J.; Jiang, H.; Li, J.; Ma, X.; Zhang, W.; Lu, Y. Cloning, expression, purification and bioactivity evaluation of a thrombin-like enzyme from Deinagkistrodon acutus venom gland library. Biotechnol. Lett. 2018, 40, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S. The 2017 ESC STEMI guidelines. Eur. Heart J. 2018, 39, 79–82. [Google Scholar] [CrossRef]
- Ihara, M.; Matsuura, N.; Yamashita, A. High-resolution Native-PAGE for membrane proteins capable of fluorescence detection and hydrodynamic state evaluation. Anal. Biochem. 2011, 412, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Matsuura, Y.; Sumi, H.; Hori, K.; Miyazawa, K. A fibrinolytic enzyme from the green alga Codium latum activates plasminogen. Fisheries Science 2002, 68, 455–457. [Google Scholar] [CrossRef][Green Version]
- Krishnamurthy, A.; Belur, P.D. A novel fibrinolytic serine metalloprotease from the marine Serratia marcescens subs askesis: Purification and characterization. Int. J. Biol. Macromol. 2018, 112, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Stump, D.C.; Thienpont, M.; Collen, D. Urokinase-related proteins in human urine. Isolation and characterization of single-chain urokinase (pro-urokinase) and urokinase-inhibitor complex. J. Biol. Chem. 1986, 261, 1267–1273. [Google Scholar] [CrossRef]
- Draxler, D.F.; Sashindranath, M.; Medcalf, R.L. Plasmin: A Modulator of Immune Function. Semin Thromb Hemost. 2017, 43, 143–153. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Loy, J.A.; Tang, J.; Zhang, X.C. Crystal Structure of the Catalytic Domain of Human Plasmin Complexed with Streptokinase. Science 1998, 281, 1662–1665. [Google Scholar] [CrossRef]
- Bokarewa, M.; Tarkowski, A. Human α-defensins neutralize fibrinolytic activity exerted by staphyloma kinase. Thromb. Hemost. 2004, 91, 991–999. [Google Scholar]
- Feuerstein, G.Z.; Nichols, A.J.; Valocik, R.E.; Gagnon, R. Cardio protection and Thrombolysis by Antitemplate in Anesthetized Dogs. J. Cardiovasc. Pharmacol. 1995, 25, 625–633. [Google Scholar] [CrossRef]
- Liang, J.F.; Li, Y.T.; Yang, V.C. The Potential Mechanism for the Effect of Heparin on Tissue plasminogen Activator–Mediated plasminogen Activation. Thromb. Res. 2004, 97, 349–358. [Google Scholar] [CrossRef]
- He, F.; Chao, J.; Yang, D.; Zhang, X.; Yang, C.; Xu, Z.; Tian, J.; Tian, Y. Optimization of fermentation conditions for production of neutral metalloprotease by Bacillus subtilis SCK6 and its application in goatskin-dehairing. Prep. Biochem. Biotechnol. 2022, 52, 789–799. [Google Scholar] [CrossRef]
- Mulder, M.; Kohnert, U.; Fische, S.; van Hinsbergh, V.W.M.; Verheijen, J.H. The interaction of recombinant tissue type plasminogen activator and recombinant plasminogen activator (r-PA/BM 06.022) with human endothelial cells. Blood Coagul. Fibrinolysis 1997, 8, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Modi, N.B.; Eppler, S.; Breed, J.; Cannon, C.P.; Braunwald, E.; Love, T.W. Pharmacokinetics of a slower clearing tissue plasminogen activator variant, TNK-tPA, in patients with acute myocardial infarction. Thromb. Haemostasias 1998, 79, 134–139. [Google Scholar] [CrossRef]
- Wang, T. The Study about Fibrinolytic Active Protease Optimal Purification Process and Enzymatic Properties Exploration of Clamworm (Perinereis aibuhitensis Grub). Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2020. [Google Scholar]
- Modi, A.; Raval, I.; Doshi, P.; Joshi, M.; Joshi, C.; Patel, A.K. Heterologous expression of recombinant nattokinase in Escherichia coli BL21(DE3) and media optimization for overproduction of nattokinase using RSM. Protein Expr. Purif. 2023, 203, 106198. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, R.; Zhao, B.; Song, X.; Ding, S.; Qian, D.; Wang, B. Cloning and expression of Recombinant fibrinolytic protein from Perinereis aibuhitensis Grube. Chin. J. Pharmacol. 2007, 19, 1452–1456. [Google Scholar]




| Description | Note |
|---|---|
| Diluent | 20 mM Tris-HCl, pH 7.2 |
| 1:10 (bacteria: dilution solution (w/w)) for 30 min. | |
| Homogenize | Bale breaking with high-pressure homogenizers |
| Centrifuge after breaking cell wall | 12,000 rpm, 10 min |
| Post centrifugation filtration | 0.8 µm-pore-size filter paper filtration |
| Sample adjustment | The conductance of the homogenate was adjusted to 15–17 mS/cm using 1 M NaCl |
| Reagent | Volume | ||||
|---|---|---|---|---|---|
| Assay Buffer | 48 µL | ||||
| Fibrin analogues | 2 µL | ||||
| Plasminogen | 2 µL | ||||
| Urokinase | 0.0625 U/µL | 0.125 U/µL | 0.25 U/µL | 0.5 U/µL | 1 U/µL |
| Assay Buffer | to 100 µL | ||||
| Reagent | Volume | |||
|---|---|---|---|---|
| Plasmin (10 ng/µL) | Plasminogen Activator (250 ng/μL) | Plasminogen Activator (25 ng/μL) | Plasminogen Activator (10 ng/μL) | |
| Assay Buffer | 48 µL | |||
| Fibrin analogues | 2 µL | |||
| Enzyme | 25 µL | 25 µL | 25 µL | 25 µL |
| Assay Buffer | to 100 µL | |||
| Reagent | Volume | |
|---|---|---|
| Samples | Positive Reference Material | |
| Assay Buffer | 48 µL | |
| Fibrin analogues | 2 µL | |
| Plasminogen | 2 µL | 2 µL |
| Urokinase (10 ng/µL) | N/A | 25 µL |
| Plasminogen activator (10 ng/µL) | 25 µL | N/A |
| Assay Buffer | to 100 µL | |
| Individual Parts Making Up a Compound | Volumetric |
|---|---|
| TEV enzyme (10 U/µL) | 1 µL |
| sample protein | 30 µg |
| 10 × TEV Buffer | To 50 µL |
| Parameter Name | Set Value |
|---|---|
| Strip sensitivity | 0.50–5.0 (can be adjusted according to the actual situation; ensure that all visible strips are identified and only a small number of strip identifiers in the blank lane is present ≤ 5) |
| Select background mode | Peak-valley connection |
| Baseline connections | 1% (can be adjusted according to the actual situation so that the baseline is level, and the background is completely deducted) |
| Molecular weight regression model | Exponential regression |
| Quantitative approach | Lane total/relative amount (tick) |
| Results report form | The “relative quantity” and “IOD” forms are saved separately. |
| Protein amount Results | The IOD value is the gray value |
| Before Sequence Optimized | After Sequence Optimized | |
|---|---|---|
| SDS-PAGE Results | The target protein bands were not obvious | After adding DNA sequence to MBP tag: the target protein bands are clear and single |
| Protein concentration | 2 mg/mL | 2 mg/mL |
| Total protein | 330 mg | 1.2 g |
| Plasminogen activator is active | 3 U/μL | 15 U/μL |
| Yield | 10% | 85% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Diao, X.; Cheng, J.; Man, Y.; Chen, B.; Zhang, H.; Wu, W. Recombinant Plasminogen Activator of the Sandworm (Perinereis aibuhitensis) Expression in Escherichia coli. Bioengineering 2024, 11, 1030. https://doi.org/10.3390/bioengineering11101030
Song T, Diao X, Cheng J, Man Y, Chen B, Zhang H, Wu W. Recombinant Plasminogen Activator of the Sandworm (Perinereis aibuhitensis) Expression in Escherichia coli. Bioengineering. 2024; 11(10):1030. https://doi.org/10.3390/bioengineering11101030
Chicago/Turabian StyleSong, Tuo, Xiaozhen Diao, Jun Cheng, Yang Man, Boyu Chen, Haixing Zhang, and Wenhui Wu. 2024. "Recombinant Plasminogen Activator of the Sandworm (Perinereis aibuhitensis) Expression in Escherichia coli" Bioengineering 11, no. 10: 1030. https://doi.org/10.3390/bioengineering11101030
APA StyleSong, T., Diao, X., Cheng, J., Man, Y., Chen, B., Zhang, H., & Wu, W. (2024). Recombinant Plasminogen Activator of the Sandworm (Perinereis aibuhitensis) Expression in Escherichia coli. Bioengineering, 11(10), 1030. https://doi.org/10.3390/bioengineering11101030







