Managing Ocular Surface Disease in Glaucoma Treatment: A Systematic Review
Abstract
1. Introduction


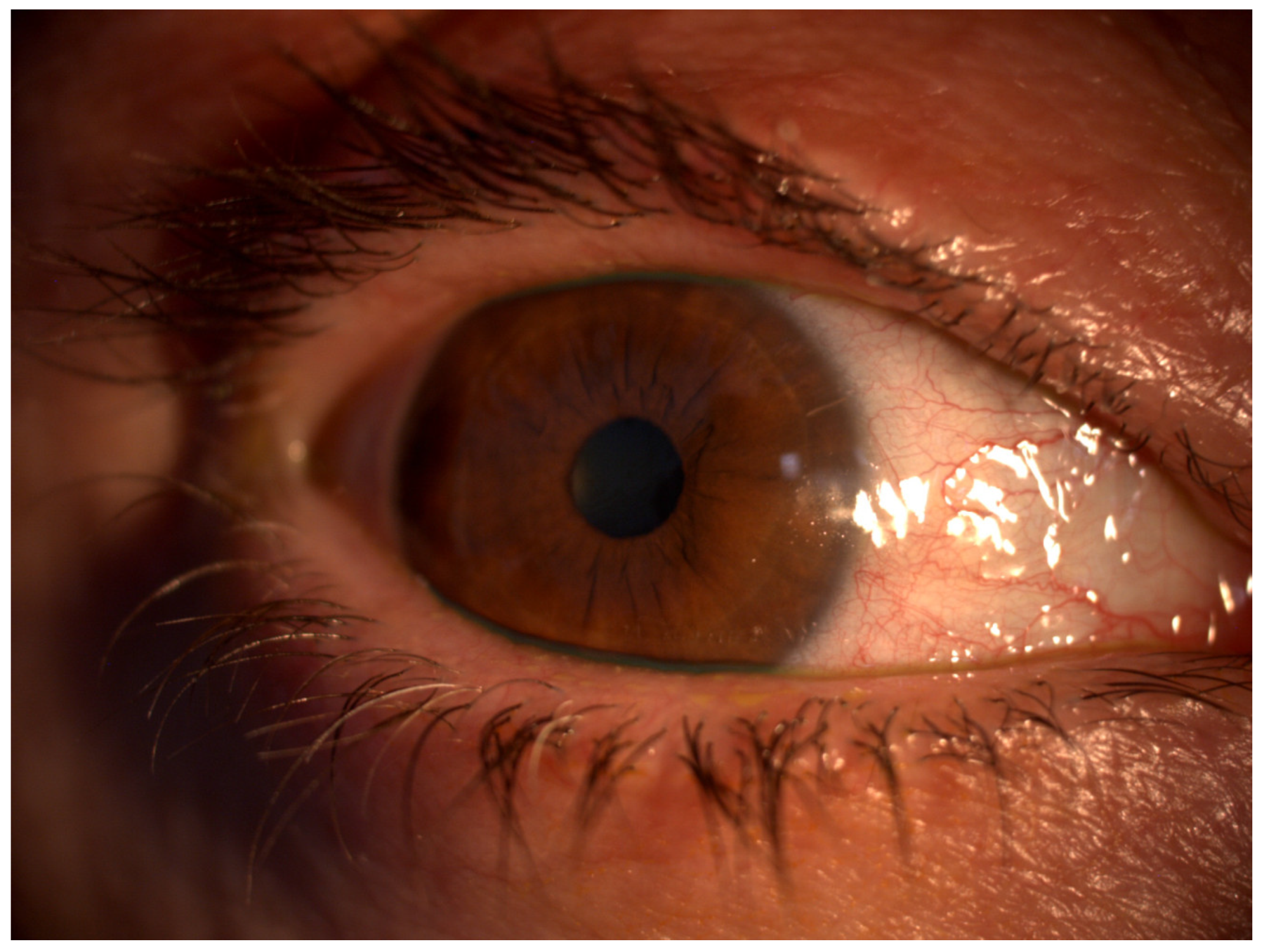
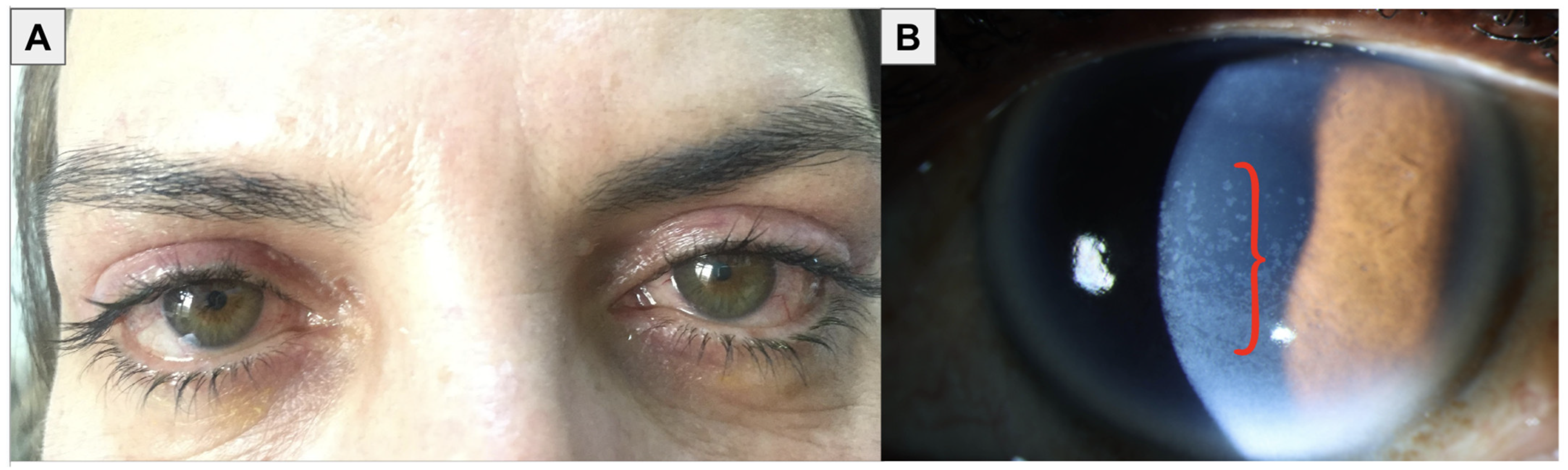
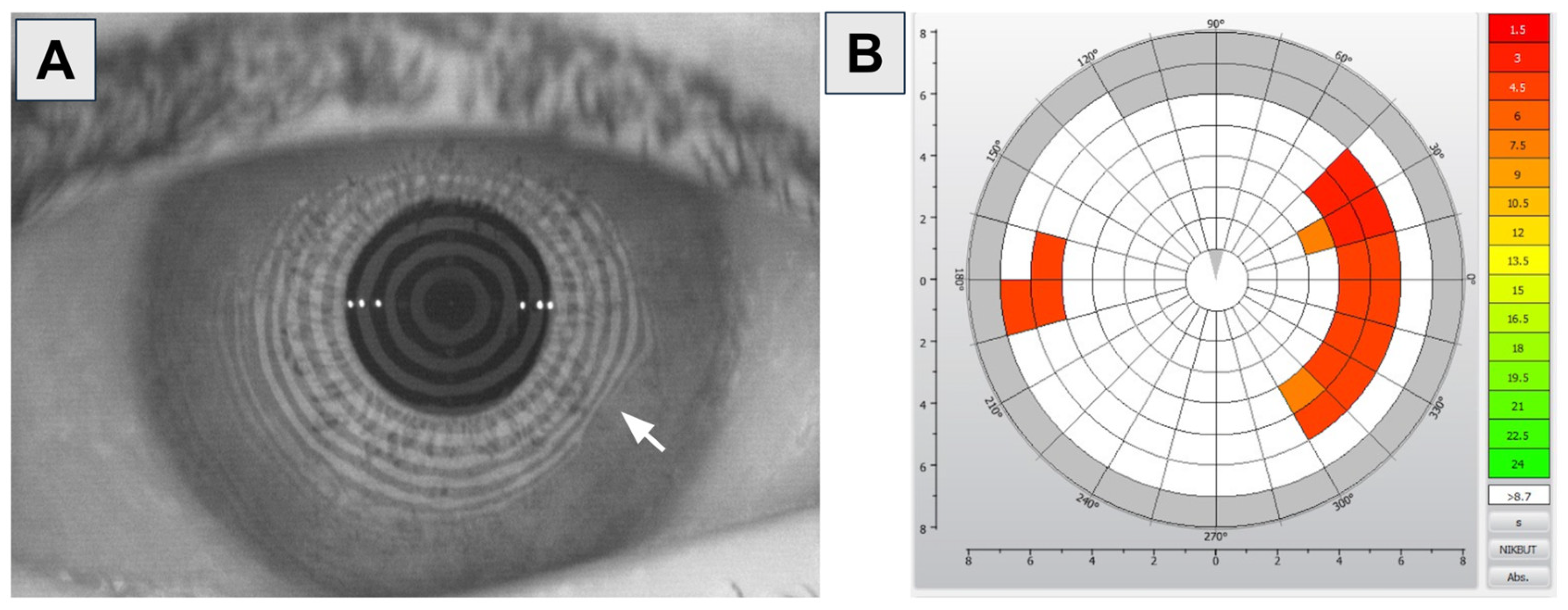
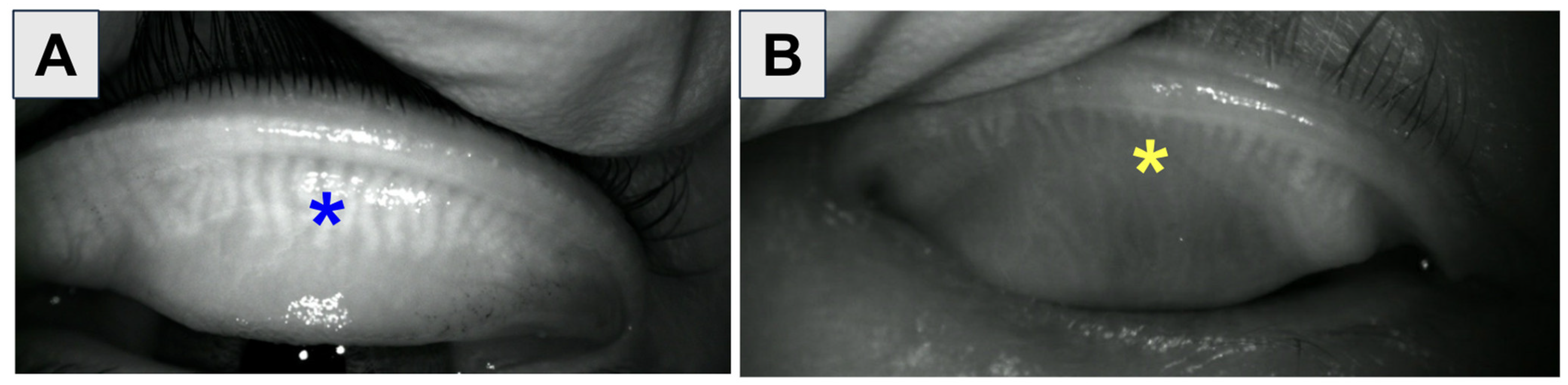
1.1. Diagnosis of OSD
1.2. Previous Research
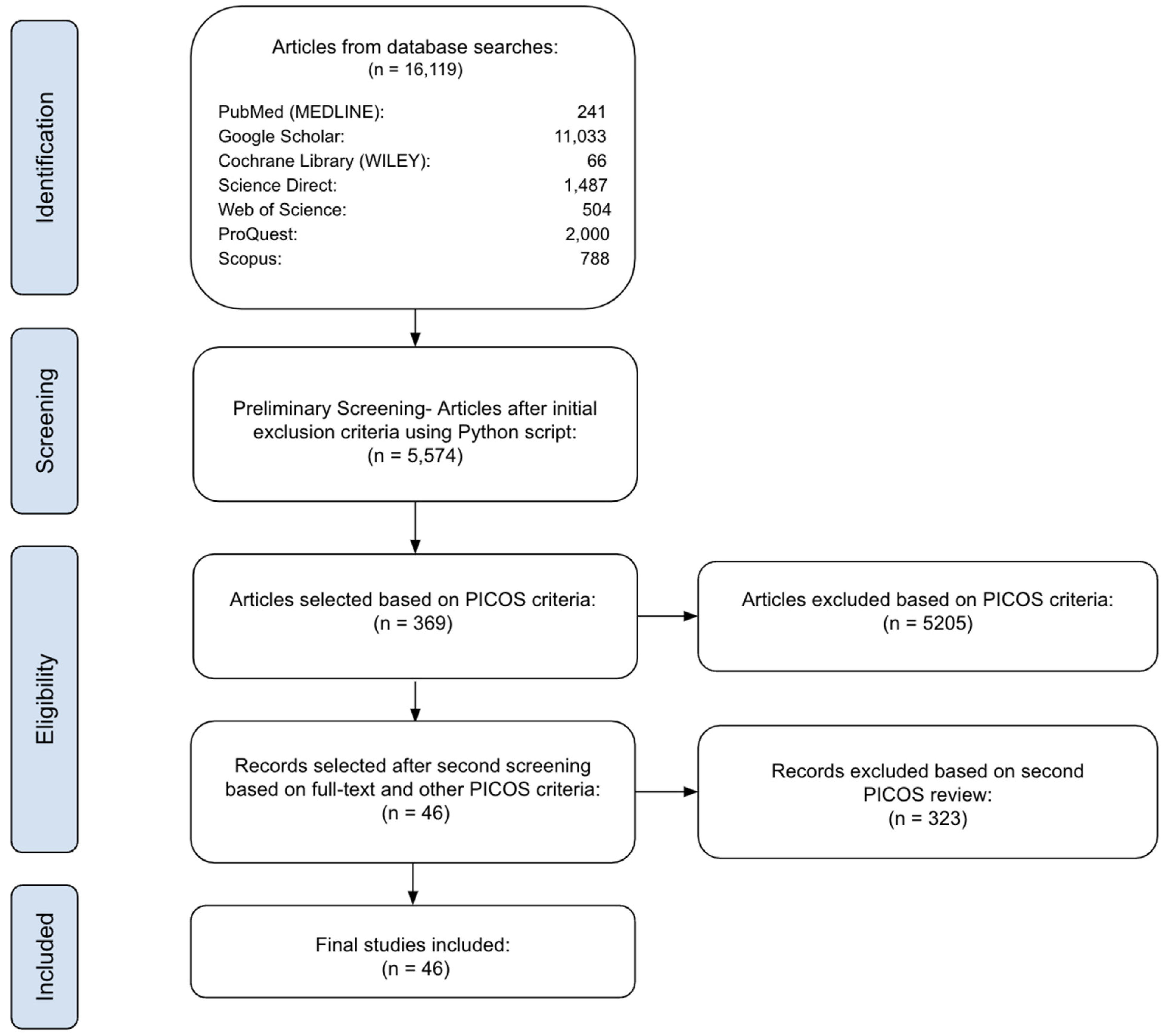
2. Materials and Methods
2.1. Initial Search
2.2. Preliminary Screening
2.3. Eligibility Assessment
3. Results
3.1. Active Ingredients
3.1.1. Beta-Adrenergic Blockers
3.1.2. Prostaglandin Analogs
3.1.3. Alpha-Adrenergic Agonists
3.1.4. Carbonic Anhydrase Inhibitors
3.1.5. Cholinergic Agonists
3.1.6. Latanoprostene Bunod
3.1.7. Netarsudil
3.2. Preservatives
3.2.1. Detergents
3.2.2. Oxidative Agents
3.2.3. Ionic Tamponade Agents
3.3. Penetration Enhancers
4. Discussion
4.1. Management of OSD Caused by Glaucoma Medications
4.1.1. Step 1: Modify Glaucoma Therapy
4.1.2. Step 2: Ocular Surface Lubrication, Anti-Inflammatory Treatment, and Other Supplemental Therapies
Anti-Inflammatory Treatment (Cyclosporine A and Topical Steroids)
Omega-3 Fatty Acid Supplementation
Vitamin A Eye Gel
Autologous Serum Eye Drops
Cryopreserved Amniotic Membranes
4.1.3. Step 3: Surgical Treatment
4.2. Future Directions in the Management of Ocular Surface Diseases
4.2.1. Sustained-Release Drug Delivery Systems
Extraocular Drug Delivery Platforms
Intraocular Drug Delivery Systems
4.2.2. Innovative Technological Devices
Intense Pulsed Light Therapy
Thermal Pulsation Devices
Photobiomodulation
4.2.3. Other Emerging Therapies
Nanoparticles
Gene Therapy
Stem Cell Applications
Umbilical Cord Blood Serum Eye Drops
Acupuncture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Pooja Prajwal, M.R.; Gopalakrishna, H.N.; Kateel, R. An exploratory study on the drug utilization pattern in glaucoma patients at a tertiary care hospital. J. App. Pharm. Sci. 2013, 3, 151–155. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Efron, N.; Hirayama, M.; Horwath-Winter, J.; Kim, T.; Mehta, J.S.; Messmer, E.M.; Pepose, J.S.; et al. TFOS DEWS II iatrogenic report. Ocul. Surf. 2017, 15, 511–538. [Google Scholar] [CrossRef]
- Ruiz-Lozano, R.E.; Azar, N.S.; Mousa, H.M.; Quiroga-Garza, M.E.; Komai, S.; Wheelock-Gutierrez, L.; Cartes, C.; Perez, V.L. Ocular surface disease: A known yet overlooked side effect of topical glaucoma therapy. Front. Toxicol. 2023, 5, 1067942. [Google Scholar] [CrossRef]
- Baudouin, C.; Liang, H.; Hamard, P.; Riancho, L.; Creuzot-Garcher, C.; Warnet, J.M.; Brignole-Baudouin, F. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology 2008, 115, 109–115. [Google Scholar] [CrossRef]
- Roda, M.; Corazza, I.; Bacchi Reggiani, M.L.; Pellegrini, M.; Taroni, L.; Giannaccare, G.; Versura, P. Dry eye disease and tear cytokine levels-a meta-analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.S.; Wei, Y.; Ying, G.S.; Maguire, M.G.; Asbell, P.A. Association of tear cytokine concentrations with symptoms and signs of dry eye disease: Baseline data from the Dry Eye Assessment and Management (DREAM) study. Curr. Eye Res. 2023, 48, 339–347. [Google Scholar] [CrossRef]
- Scarpellini, C.; Ramos Llorca, A.; Lanthier, C.; Klejborowska, G.; Augustyns, K. The potential role of regulated cell death in dry eye diseases and ocular surface dysfunction. Int. J. Mol. Sci. 2023, 24, 731. [Google Scholar] [CrossRef]
- Fineide, F.; Magnø, M.; Dahlø, K.; Kolko, M.; Heegaard, S.; Vehof, J.; Utheim, T.P. Topical glaucoma medications-possible implications on the meibomian glands. Acta Ophthalmol. 2024, 102, 1–14. [Google Scholar] [CrossRef]
- Kolko, M.; Gazzard, G.; Baudouin, C.; Beier, S.; Brignole-Baudouin, F.; Cvenkel, B.; Fineide, F.; Hedengran, A.; Hommer, A.; Jespersen, E.; et al. Impact of glaucoma medications on the ocular surface and how ocular surface disease can influence glaucoma treatment. Ocul. Surf. 2023, 29, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Akpek, E.K.; Ahmad, S. Glaucoma and ocular surface disease: More than meets the eye. Clin. Ophthalmol. 2022, 16, 3641–3649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Vadoothker, S.; Munir, W.M.; Saeedi, O. Ocular surface disease and glaucoma medications: A clinical approach. Eye Contact Lens 2019, 45, 11–18. [Google Scholar] [CrossRef]
- Garcia-Terraza, A.L.; Jimenez-Collado, D.; Sanchez-Sanoja, F.; Arteaga-Rivera, J.Y.; Morales Flores, N.; Pérez-Solórzano, S.; Garfias, Y.; Graue-Hernández, E.O.; Navas, A. Reliability, repeatability, and accordance between three different corneal diagnostic imaging devices for evaluating the ocular surface. Front. Med. 2022, 9, 893688. [Google Scholar] [CrossRef]
- Schmidl, D.; Schlatter, A.; Chua, J.; Tan, B.; Garhöfer, G.; Schmetterer, L. Novel approaches for imaging-based diagnosis of ocular surface disease. Diagnostics 2020, 10, 589. [Google Scholar] [CrossRef] [PubMed]
- Andole, S.; Senthil, S. Ocular surface disease and anti-glaucoma medications: Various features, diagnosis, and management guidelines. Semin. Ophthalmol. 2023, 38, 158–166. [Google Scholar] [CrossRef]
- Scelfo, C.; ElSheikh, R.H.; Shamim, M.M.; Abbasian, J.; Ghaffarieh, A.; Elhusseiny, A.M. Ocular surface disease in glaucoma patients. Curr. Eye Res. 2023, 48, 219–230. [Google Scholar] [CrossRef]
- Cochrane Library. What Is PICO? Available online: https://www.cochranelibrary.com/about-pico (accessed on 1 October 2024).
- Harzing, A.W. Publish or Perish. Available online: https://harzing.com/resources/publish-or-perish (accessed on 22 August 2024).
- Seider, N.; Miller, B.; Beiran, I. Topical glaucoma therapy as a risk factor for nasolacrimal duct obstruction. Am. J. Ophthalmol. 2008, 145, 120–123. [Google Scholar] [CrossRef]
- Kuppens, E.V.; de Jong, C.A.; Stolwijk, T.R.; de Keizer, R.J.; van Best, J.A. Effect of timolol with and without preservative on the basal tear turnover in glaucoma. Br. J. Ophthalmol. 1995, 79, 339–342. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, X.; Yang, L.; Zhou, Q.; Li, Y. β-blocker eye drops affect ocular surface through β2 adrenoceptor of corneal limbal stem cells. BMC Ophthalmol. 2021, 21, 419. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Agnifili, L.; Fasanella, V.; Curcio, C.; Brescia, L.; Lanzini, M.; Fresina, M.; Mastropasqua, L.; Marchini, G. Corneoscleral limbus in glaucoma patients: In vivo confocal microscopy and immunocytological study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2050–2058. [Google Scholar] [CrossRef]
- Inoue, K.; Okugawa, K.; Kato, S.; Inoue, Y.; Tomita, G.; Oshika, T.; Amano, S. Ocular factors relevant to anti-glaucomatous eyedrop-related keratoepitheliopathy. J. Glaucoma 2003, 12, 480–485. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zhou, D.; Zhao, Y.; Duan, X. A narrative review of ocular surface disease related to anti-glaucomatous medications. Ophthalmol. Ther. 2022, 11, 1681–1704. [Google Scholar] [CrossRef]
- Rolle, T.; Spinetta, R.; Nuzzi, R. Long term safety and tolerability of Tafluprost 0.0015% vs. Timolol 0.1% preservative-free in ocular hypertensive and in primary open-angle glaucoma patients: A cross sectional study. BMC Ophthalmol. 2017, 17, 136. [Google Scholar] [CrossRef]
- Russ, H.H.; Costa, V.P.; Ferreira, F.M.; Valgas, S.R.; Correa Neto, M.A.; Strobel, E.; Truppel, J.H. Conjunctival changes induced by prostaglandin analogues and timolol maleate: A histomorphometric study. Arq. Bras. Oftalmol. 2007, 70, 910–916. [Google Scholar] [CrossRef][Green Version]
- Yoshino, T.; Fukuchi, T.; Togano, T.; Seki, M.; Ikegaki, H.; Abe, H. Eyelid and eyelash changes due to prostaglandin analog therapy in unilateral treatment cases. Jpn. J. Ophthalmol. 2013, 57, 172–178. [Google Scholar] [CrossRef]
- Yamada, H.; Yoneda, M.; Gosho, M.; Kato, T.; Zako, M. Bimatoprost, latanoprost, and tafluprost induce differential expression of matrix metalloproteinases and tissue inhibitor of metalloproteinases. BMC Ophthalmol. 2016, 16, 26. [Google Scholar] [CrossRef]
- Mocan, M.C.; Uzunosmanoglu, E.; Kocabeyoglu, S.; Karakaya, J.; Irkec, M. The association of chronic topical prostaglandin analog use with meibomian gland dysfunction. J. Glaucoma 2016, 25, 770–774. [Google Scholar] [CrossRef]
- Yeh, P.H.; Cheng, Y.C.; Shie, S.S.; Lee, Y.S.; Shen, S.C.; Chen, H.S.; Wu, W.C.; Su, W.W. Brimonidine related acute follicular conjunctivitis: Onset time and clinical presentations, a long-term follow-up. Medicine 2021, 100, e26724. [Google Scholar] [CrossRef]
- Trotta, D.; Zucchelli, M.; Salladini, C.; Ballerini, P.; Rossi, C.; Aricò, M. Brimonidine eye drops within the reach of children: A possible foe. Children 2024, 11, 317. [Google Scholar] [CrossRef]
- Rohrschneider, K.; Koch, H.-R. [Effects of acetazolamide (Diamox®, Glaupax®) on tear production]. Klin. Monatsblätter für Augenheilkd. [Clin. Mon. Newslett. Ophthalmol.] 1991, 199, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Terai, N.; Müller-Holz, M.; Spoerl, E.; Pillunat, L.E. Short-term effect of topical antiglaucoma medication on tear-film stability, tear secretion, and corneal sensitivity in healthy subjects. Clin. Ophthalmol. 2011, 5, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Skaat, A.; Rosman, M.S.; Chien, J.L.; Mogil, R.S.; Ren, R.; Liebmann, J.M.; Ritch, R.; Park, S.C. Effect of pilocarpine hydrochloride on the schlemm canal in healthy eyes and eyes with open-angle glaucoma. JAMA Ophthalmol. 2016, 134, 976–981. [Google Scholar] [CrossRef]
- Zhang, Y.; Kam, W.R.; Liu, Y.; Chen, X.; Sullivan, D.A. Influence of pilocarpine and timolol on human meibomian gland epithelial cells. Cornea 2017, 36, 719–724. [Google Scholar] [CrossRef]
- Hartenbaum, D.; Maloney, S.; Vaccarelli, L.; Liss, C.; Wilson, H.; Gormley, G.J. Comparison of dorzolamide and pilocarpine as adjunctive therapy in patients with open-angle glaucoma and ocular hypertension. Clin. Ther. 1999, 21, 1533–1538. [Google Scholar] [CrossRef]
- Mehran, N.A.; Sinha, S.; Razeghinejad, R. New glaucoma medications: Latanoprostene bunod, netarsudil, and fixed combination netarsudil-latanoprost. Eye 2020, 34, 72–88. [Google Scholar] [CrossRef]
- Patton, G.N.; Lee, H.J. Chemical insights into topical agents in intraocular pressure management: From glaucoma etiopathology to therapeutic approaches. Pharmaceutics 2024, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Bhatnagar, K.R.; Shakrawal, J.; Tandon, M.; Jaisingh, K.; Pandey, L.; Roy, F. Ocular surface changes in primary open-angle glaucoma on anti-glaucoma medications versus treatment-naïve patients. Indian J. Ophthalmol. 2024, 72, 374–380. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, Y.; Yang, Y.; Fan, Y.; Liu, P.; Yu, K.; Yu, M. Wide corneal epithelial thickness mapping in eyes with topical antiglaucoma therapy using optical coherence tomography. Transl. Vis. Sci. Technol. 2022, 11, 4. [Google Scholar] [CrossRef]
- Pai, V.; Reddy, L.S.H. Prevalence of ocular surface disease in patients with glaucoma on topical medications. Asian J. Ophthalmol. 2018, 16, 101–109. [Google Scholar] [CrossRef]
- Pérez-Bartolomé, F.; Martínez-de-la-Casa, J.M.; Arriola-Villalobos, P.; Fernández-Pérez, C.; Polo, V.; García-Feijoó, J. Ocular surface disease in patients under topical treatment for glaucoma. Eur. J. Ophthalmol. 2017, 27, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Cvenkel, B.; Štunf, Š.; Srebotnik Kirbiš, I.; Strojan Fležar, M. Symptoms and signs of ocular surface disease related to topical medication in patients with glaucoma. Clin. Ophthalmol. 2015, 9, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Saade, C.E.; Lari, H.B.; Berezina, T.L.; Fechtner, R.D.; Khouri, A.S. Topical glaucoma therapy and ocular surface disease: A prospective, controlled cohort study. Can. J. Ophthalmol. 2015, 50, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Van Went, C.; Alalwani, H.; Brasnu, E.; Pham, J.; Hamard, P.; Baudouin, C.; Labbé, A. [Corneal sensitivity in patients treated medically for glaucoma or ocular hypertension]. J. Fr. Ophtalmol. 2011, 34, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, R.D.; Godfrey, D.G.; Budenz, D.; Stewart, J.A.; Stewart, W.C.; Jasek, M.C. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea 2010, 29, 618–621. [Google Scholar] [CrossRef]
- Jaenen, N.; Baudouin, C.; Pouliquen, P.; Manni, G.; Figueiredo, A.; Zeyen, T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur. J. Ophthalmol. 2007, 17, 341–349. [Google Scholar] [CrossRef]
- Pisella, P.J.; Pouliquen, P.; Baudouin, C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br. J. Ophthalmol. 2002, 86, 418–423. [Google Scholar] [CrossRef]
- Petounis, A.D.; Akritopoulos, P. Influence of topical and systemic beta-blockers on tear production. Int. Ophthalmol. 1989, 13, 75–80. [Google Scholar] [CrossRef]
- Kaur, I.P.; Lal, S.; Rana, C.; Kakkar, S.; Singh, H. Ocular preservatives: Associated risks and newer options. Cutan. Ocul. Toxicol. 2009, 28, 93–103. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Silva, F.Q.; Blender, N.; Tran, T.; Vantipalli, S. Ocular benzalkonium chloride exposure: Problems and solutions. Eye 2022, 36, 361–368. [Google Scholar] [CrossRef]
- Debbasch, C.; Brignole, F.; Pisella, P.J.; Warnet, J.M.; Rat, P.; Baudouin, C. Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 642–652. [Google Scholar]
- Zhang, R.; Park, M.; Richardson, A.; Tedla, N.; Pandzic, E.; de Paiva, C.S.; Watson, S.; Wakefield, D.; Di Girolamo, N. Dose-dependent benzalkonium chloride toxicity imparts ocular surface epithelial changes with features of dry eye disease. Ocul. Surf. 2020, 18, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.; Supramaniam, G.; Samsudin, A.; Juana, A.; Zahari, M.; Choo, M.M. Ocular surface disease in glaucoma: Effect of polypharmacy and preservatives. Optom. Vis. Sci. 2015, 92, e222–e226. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.B.; Grierson, I.; Millar, L.; Hitchings, R.A. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology 1989, 96, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, T.; Ichhpujani, P.; Vohra, S. Ocular surface disease with BAK preserved travoprost and polyquaternium 1(Polyquad) preserved travoprost. Rom. J. Ophthalmol. 2019, 63, 249–256. [Google Scholar] [CrossRef]
- Ammar, D.A.; Kahook, M.Y. Effects of benzalkonium chloride- or polyquad-preserved fixed combination glaucoma medications on human trabecular meshwork cells. Mol. Vis. 2011, 17, 1806–1813. [Google Scholar]
- Katz, L.J. Twelve-month evaluation of brimonidine-purite versus brimonidine in patients with glaucoma or ocular hypertension. J. Glaucoma 2002, 11, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G., Jr.; Fain, J.M.; Lovelace, C.; Gelotte, K.M. Effectiveness of ophthalmic solution preservatives: A comparison of latanoprost with 0.02% benzalkonium chloride and travoprost with the sofZia preservative system. BMC Ophthalmol. 2011, 11, 8. [Google Scholar] [CrossRef]
- Kanamoto, T.; Kiuchi, Y.; Tanito, M.; Mizoue, S.; Naito, T.; Teranishi, S.; Hirooka, K.; Rimayanti, U. Comparison of the toxicity profile of benzalkonium chloride-preserved tafluprost and SofZia-preserved travoprost applied to the ocular surface. J. Ocul. Pharmacol. Ther. 2015, 31, 156–164. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef]
- Uusitalo, H.; Chen, E.; Pfeiffer, N.; Brignole-Baudouin, F.; Kaarniranta, K.; Leino, M.; Puska, P.; Palmgren, E.; Hamacher, T.; Hofmann, G.; et al. Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol. 2010, 88, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Iester, M.; Telani, S.; Frezzotti, P.; Motolese, I.; Figus, M.; Fogagnolo, P.; Perdicchi, A. Ocular surface changes in glaucomatous patients treated with and without preservatives beta-blockers. J. Ocul. Pharmacol. Ther. 2014, 30, 476–481. [Google Scholar] [CrossRef]
- Jandroković, S.; Vidas Pauk, S.; Lešin Gaćina, D.; Skegro, I.; Tomić, M.; Masnec, S.; Kuzman, T.; Kalauz, M. Tolerability in glaucoma patients switched from preserved to preservative-free prostaglandin-timolol combination: A prospective real-life study. Clin. Ophthalmol. 2022, 16, 3181–3192. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef]
- Kim, J.G.; An, J.H.; Cho, S.Y.; Lee, C.E.; Shim, K.Y.; Jun, J.H. Efficacy of topical 0.05% cyclosporine A for ocular surface disease related to topical anti-glaucoma medications. J. Ocul. Pharmacol. Ther. 2023, 39, 389–397. [Google Scholar] [CrossRef]
- Pleyer, U.; Ursell, P.G.; Rama, P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: Are they all the same? Ophthalmol. Ther. 2013, 2, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Bernabei, F.; Roda, M.; Taroni, L.; Versura, P.; Campos, E.C. Efficacy of omega-3 fatty acid supplementation for treatment of dry eye disease: A meta-analysis of randomized clinical trials. Cornea 2019, 38, 565–573. [Google Scholar] [CrossRef]
- Cui, X.; Xiang, J.; Zhu, W.; Wei, A.; Le, Q.; Xu, J.; Zhou, X. Vitamin A palmitate and carbomer gel protects the conjunctiva of patients with long-term prostaglandin analogs application. J. Glaucoma 2016, 25, 487–492. [Google Scholar] [CrossRef]
- Vazirani, J.; Sridhar, U.; Gokhale, N.; Doddigarla, V.R.; Sharma, S.; Basu, S. Autologous serum eye drops in dry eye disease: Preferred practice pattern guidelines. Indian J. Ophthalmol. 2023, 71, 1357–1363. [Google Scholar] [CrossRef]
- McDonald, M.; Janik, S.B.; Bowden, F.W.; Chokshi, A.; Singer, M.A.; Tighe, S.; Mead, O.G.; Nanda, S.; Qazi, M.A.; Dierker, D.; et al. Association of treatment duration and clinical outcomes in dry eye treatment with sutureless cryopreserved amniotic membrane. Clin. Ophthalmol. 2023, 17, 2697–2703. [Google Scholar] [CrossRef]
- Conlon, R.; Saheb, H.; Ahmed, I.I.K. Glaucoma treatment trends: A review. Can. J. Ophthalmol. 2017, 52, 114–124. [Google Scholar] [CrossRef]
- Al-Qaysi, Z.K.; Beadham, I.G.; Schwikkard, S.L.; Bear, J.C.; Al-Kinani, A.A.; Alany, R.G. Sustained release ocular drug delivery systems for glaucoma therapy. Expert Opin. Drug Deliv. 2023, 20, 905–919. [Google Scholar] [CrossRef]
- M Grover, L.; Moakes, R.; Rauz, S. Innovations in fluid-gel eye drops for treating disease of the eye: Prospects for enhancing drug retention and reducing corneal scarring. Expert Rev. Ophthalmol. 2022, 17, 175–181. [Google Scholar] [CrossRef]
- Brandt, J.D.; Sall, K.; DuBiner, H.; Benza, R.; Alster, Y.; Walker, G.; Semba, C.P. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: Results of a phase II randomized controlled study. Ophthalmology 2016, 123, 1685–1694. [Google Scholar] [CrossRef]
- Kesav, N.P.; Young, C.E.C.; Ertel, M.K.; Seibold, L.K.; Kahook, M.Y. Sustained-release drug delivery systems for the treatment of glaucoma. Int. J. Ophthalmol. 2021, 14, 148–159. [Google Scholar] [CrossRef]
- Hsu, K.H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Ross, A.E.; Tulsan, R.; Watts, A.C.; Wang, R.F.; Zurakowski, D.; Serle, J.B.; Kohane, D.S. Latanoprost-eluting contact lenses in glaucomatous monkeys. Ophthalmology 2016, 123, 2085–2092. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, A.S.; Parvathy, J. Extended wear therapeutic contact lens fabricated from timolol imprinted carboxymethyl chitosan-g-hydroxy ethyl methacrylate-g-poly acrylamide as a onetime medication for glaucoma. Eur. J. Pharm. Biopharm. 2016, 109, 61–71. [Google Scholar] [CrossRef]
- Clinical Trials. Safety and Intraocular Lowering Effect of Delivery of Travoprost Evolute® in Subjects with Elevated Intraocular Pressure. Available online: https://clinicaltrials.gov/study/NCT04962009?term=EVOLUTE&rank=2 (accessed on 1 September 2024).
- Perera, S.A.; Ting, D.S.; Nongpiur, M.E.; Chew, P.T.; Aquino, M.C.; Sng, C.C.; Ho, S.W.; Aung, T. Feasibility study of sustained-release travoprost punctum plug for intraocular pressure reduction in an Asian population. Clin. Ophthalmol. 2016, 10, 757–764. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Bacharach, J.; Brubaker, J.W.; Medeiros, F.A.; Bejanian, M.; Bernstein, P.; Robinson, M.R. Bimatoprost implant biodegradation in the Phase 3, randomized, 20-month ARTEMIS studies. J. Ocul. Pharmacol. Ther. 2023, 39, 55–62. [Google Scholar] [CrossRef]
- Clinical Trials. Safety and Efficacy of ENV515 Travoprost Extended Release (XR) in Patients with Bilateral Ocular Hypertension or Primary Open Angle Glaucoma. Available online: https://clinicaltrials.gov/study/NCT02371746?term=ENV515&rank=1 (accessed on 1 September 2024).
- Clinical Trials. Safety, and Efficacy of OTX-TIC in Participants with Open Angle Glaucoma or Ocular Hypertension. Available online: https://clinicaltrials.gov/study/NCT04360174?term=OTX-TIC&rank=1 (accessed on 1 September 2024).
- Berdahl, J.P.; Sarkisian, S.R., Jr.; Ang, R.E.; Doan, L.V.; Kothe, A.C.; Usner, D.W.; Katz, L.J.; Navratil, T. Efficacy and safety of the travoprost intraocular implant in reducing topical iop-lowering medication burden in patients with open-angle glaucoma or ocular hypertension. Drugs 2024, 84, 83–97. [Google Scholar] [CrossRef]
- Rafiei, F.; Tabesh, H.; Farzad, F. Sustained subconjunctival drug delivery systems: Current trends and future perspectives. Int. Ophthalmol. 2020, 40, 2385–2401. [Google Scholar] [CrossRef]
- Safir, M.; Twig, G.; Mimouni, M. Dry eye disease management. BMJ 2024, 384, e077344. [Google Scholar] [CrossRef]
- Miao, S.; Yan, R.; Jia, Y.; Pan, Z. Effect of intense pulsed light therapy in dry eye disease caused by meibomian gland dysfunction: A systematic review and meta-analysis. Eye Contact Lens 2022, 48, 424–429. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, S.; Liu, X. Efficacy and safety of a vectored thermal pulsation system (Lipiflow®) in the treatment of meibomian gland dysfunction: A systematic review and meta-analysis. Graefes. Arch. Clin. Exp. Ophthalmol. 2022, 260, 25–39. [Google Scholar] [CrossRef]
- Antwi, A.; Schill, A.W.; Redfern, R.; Ritchey, E.R. Effect of low-level light therapy in individuals with dry eye disease. Ophthalmic Physiol. Opt. 2024, 1–8. [Google Scholar] [CrossRef]
- Clinical Trials. Photobiomodulation with REd vs. BluE Light (REBEL). Available online: https://clinicaltrials.gov/study/NCT06371300 (accessed on 3 September 2024).
- Ciociola, E.C.; Fernandez, E.; Kaufmann, M.; Klifto, M.R. Future directions of glaucoma treatment: Emerging gene, neuroprotection, nanomedicine, stem cell, and vascular therapies. Curr. Opin. Ophthalmol. 2024, 35, 89–96. [Google Scholar] [CrossRef]
- Occhiutto, M.L.; Maranhão, R.C.; Costa, V.P.; Konstas, A.G. Nanotechnology for medical and surgical glaucoma therapy-a review. Adv. Ther. 2020, 37, 155–199. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Patil, R.J.; Desai, A.R.; Shukla, M.R.; Vaidya, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Shah, D.O. Effect of gold nanoparticles on timolol uptake and its release kinetics from contact lenses: In vitro and in vivo evaluation. Acta Biomater. 2019, 86, 350–362. [Google Scholar] [CrossRef]
- Chern, K.J.; Nettesheim, E.R.; Reid, C.A.; Li, N.W.; Marcoe, G.J.; Lipinski, D.M. Prostaglandin-based rAAV-mediated glaucoma gene therapy in Brown Norway rats. Commun. Biol. 2022, 5, 1169. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, E.; Honda, S.; Kitamura, Y.; Namekata, K.; Kimura, A.; Guo, X.; Azuchi, Y.; Harada, C.; Murakami, A.; Matsuda, A.; et al. Vision protection and robust axon regeneration in glaucoma models by membrane-associated Trk receptors. Mol. Ther. 2023, 31, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Roubeix, C.; Godefroy, D.; Mias, C.; Sapienza, A.; Riancho, L.; Degardin, J.; Fradot, V.; Ivkovic, I.; Picaud, S.; Sennlaub, F.; et al. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res. Ther. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Vrathasha, V.; Nikonov, S.; Bell, B.A.; He, J.; Bungatavula, Y.; Uyhazi, K.E.; Murthy Chavali, V.R. Transplanted human induced pluripotent stem cells- derived retinal ganglion cells embed within mouse retinas and are electrophysiologically functional. iScience 2022, 25, 105308. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Hayashi, R.; Kudo, Y.; Li, X.; Yamaguchi, K.; Shibata, S.; Okubo, T.; Ishii, T.; Honma, Y.; Nishida, K. Ocular instillation of conditioned medium from mesenchymal stem cells is effective for dry eye syndrome by improving corneal barrier function. Sci. Rep. 2023, 13, 13100. [Google Scholar] [CrossRef]
- Wong, J.; Govindasamy, G.; Prasath, A.; Hwang, W.; Ho, A.; Yeo, S.; Tong, L. Allogeneic umbilical cord plasma eyedrops for the treatment of recalcitrant dry eye disease patients. J. Clin. Med. 2023, 12, 6750. [Google Scholar] [CrossRef]
- Prinz, J.; Maffulli, N.; Fuest, M.; Walter, P.; Hildebrand, F.; Migliorini, F. Acupuncture for the management of dry eye disease. Front. Med. 2022, 16, 975–983. [Google Scholar] [CrossRef]

| Parameter | Description |
|---|---|
| Population | Patients with glaucoma regardless of study location |
| Intervention | Focusing on patients using anti-glaucoma eye drops with or without preservatives |
| Comparison | Patients using topical eye drops with or without preservatives |
| Outcomes | OSDI, Schirmer’s test, corneal and conjunctival staining (fluorescein, lissamine green), conjunctival hyperemia, meibography, TMH, TBUT, NITBUT |
| Study Design | Cohort, cross-sectional, case-control, randomized or nonrandomized controlled (or uncontrolled) trials, or reviews |
| Medications | Mechanism of Action | Dosing & Concentrations | OSD or Other Complications | IOP Reduction |
|---|---|---|---|---|
| Beta-adrenergic blockers (timolol, levobunolol, betaxolol, metipranolol) [5,20,21,22,23,24,25,26,27] | Decrease aqueous humor (AH) production via blockade of beta-adrenergic receptors on the ciliary epithelium | Once or twice daily; 0.25–0.5% | Conjunctival goblet cell loss, MGD, SPK, and pseudo-pemphigoid cicatrizing conjunctivitis | ~20–30% |
| Prostaglandin analogues (latanoprost, bimatoprost, travoprost, tafluprost) [5,28,29,30] | Increase uveoscleral outflow by remodeling the ECM and regulating matrix metalloproteinases | Once daily; 0.0015–0.03% | MGD, skin pigmentation, conjunctival hyperemia, pseudo-dendritic keratitis, periorbitopathy, eyelid pigmentation, and hypertrichosis | ~25–35% |
| Alpha-adrenergic agonists (brimonidine, apraclonidine) [31,32] | Selective sympathetic agonists (α2); decrease AH production, and increase uveoscleral and trabecular meshwork (TM) outflow | 2–3 times daily; 0.1–0.5% | Allergic follicular conjunctivitis, contact dermatitis, blepharitis, and systemic hypotension | up to 26% |
| Carbonic anhydrase inhibitors (dorzolamide, brinzolamide), (oral: acetazolamide, methazolamide) [33,34] | Decrease AH production by inhibiting carbonic anhydrase enzyme in the ciliary processes | 2–4 times daily; 1–2% | Ocular surface irritation, reduction of basal tear secretion, and blepharitis | ~15–20% |
| Cholinergic agonists (pilocarpine, carbachol) [5,35,36,37] | Muscarinic receptor agonists; increase TM outflow | 4 times daily; 1–4% | MGD, blepharitis, pseudo-pemphigoid cicatrizing conjunctivitis, blurred vision, myopia, miosis, iris cysts, and retinal detachment | ~15–25% |
| Latanoprostene bunod (Vyzulta®) [38] | Induces TM expansion and vasodilation of episcleral veins, thereby increasing AH outflow | Once daily; 0.024% | Hyperemia, hypertrichosis, and eye irritation | ~35% |
| Rho Kinase inhibitors (netarsudil—Rhopressa®) [38,39] | Decrease episcleral venous pressure, increase TM outflow, and decrease AH production via inhibition of rho kinase enzyme | Once daily; 0.02% | Conjunctival hyperemia and hemorrhage, corneal edema, and SPK | ~25–30% |
| Dorzolamide and timolol maleate solution (combined) | Decrease AH production via a combination of carbonic anhydrase and beta-adrenergic receptor blockade | Twice daily; timolol 0.5%, dorzolamide 2% | Conjunctival goblet cell loss, MGD, SPK, pseudo-pemphigoid cicatrizing conjunctivitis, ocular surface irritation, reduction of basal tear secretion, and blepharitis | ~30–35% |
| Brimonidine tartrate and timolol maleate solution (combined) | Decrease AH production, increase uveoscleral outflow, and increase TM outflow via a combination of alpha and beta-adrenergic receptor blockade | Twice daily; timolol 0.5%, brimonidine 0.2% | Allergic follicular conjunctivitis, contact dermatitis, blepharitis, conjunctival goblet cell loss, MGD, SPK, and pseudo-pemphigoid cicatrizing conjunctivitis | ~30–35% |
| Netarsudil and latanoprost solution (Rocklatan®) | Decrease episcleral venous pressure, increase TM outflow, and decrease AH production via a combination of rho kinase inhibition and prostanoid receptor induction | Once daily; netarsudil 0.02%, latanoprost 0.005% | Hyperemia, conjunctival hemorrhage, MGD, lid pigmentation, pseudo-dendritic keratitis, periorbitopathy, and hypertrichosis | ~30–36% |
| Brimonidine and brinzolamide solution (combined) | Decrease AH production, and increase uveoscleral and TM outflow via inhibition of carbonic anhydrase and alpha-adrenergic receptors | 3 times daily; brimonidine 1%, brinzolamide 0.2% | Ocular surface irritation, reduction of basal tear secretion, blepharitis, allergic follicular conjunctivitis, and contact dermatitis | ~21–35% |
| Glaucoma Agents and Patient Characteristics | Study Methods | Study Results | Authors, Country, and Year |
|---|---|---|---|
| Newly diagnosed treatment-naïve POAG patients vs. those on topical anti-glaucoma medications | A prospective cohort study conducted on 120 eyes with POAG (60 on topical anti-glaucoma drops and 60 treatment-naïve eyes). | At 3, 6, and 12 months, the OSDI score, TBUT, Schirmer’s test, TMH, and TMD had significantly better values in the treatment-naïve group in comparison to the medicated group (p < 0.0001). | Srivastava et al. India, 2024 [40] |
| Patients with open-angle glaucoma or OHT on topical anti-glaucoma medications vs. healthy subjects | In this cross-sectional study, 75 patients were using topical anti-glaucoma medications and 65 were treatment-naïve subjects. OSDI, Schirmer’s test, TBUT, fluorescein staining, and CET were evaluated. | The treatment group had a significantly shorter TBUT, shorter Schirmer’s test, and greater fluorescein staining than those of the control group (p < 0.05). The mean CET of patients with glaucoma was significantly lower than that of controls in the central, paracentral, mid-peripheral, and peripheral zones (50.6 vs. 53.1 µm; p < 0.001). The number of medications and duration of treatment also affected the CET in all zones (p < 0.05). | Ye et al. China, 2022 [41] |
| Glaucoma patients on topical anti-glaucoma medications vs. healthy controls | 94 patients with glaucoma on topical medications (study group) and 94 patients in the treatment-naïve control group were assessed using OSDI, TBUT, lissamine green staining, and Schirmer’s test. | OSDI scores were significantly higher in the study group (72.4%) vs. controls (44.6%). Similarly, the study group had decreased tear production (84% vs. 53%, respectively), abnormal TBUT (67.1% vs. 47.8%), and positive lissamine green staining (36.2% vs. 31.8%) compared to the control group. | Pai and Reddy India, 2018 [42] |
| Patients with POAG or OHT on topical anti-glaucoma medications vs. healthy controls | 211 eyes of patients with POAG or OHT on topical medication were recruited. Controls consisted of 51 eyes. Outcome measures were fluorescein corneal staining score, TMH, TBUT, and OSDI. | Compared to controls, significantly higher OSDI (10.24 vs. 2.5; p < 0.001) and corneal staining (≥1: 64.93% vs. 32.61%; p < 0.001) scores were recorded in the medication group. No significant differences in TBUT and TMH were observed between groups. | Pérez-Bartolomé et al. Spain, 2017 [43] |
| Glaucoma patients on topical anti-glaucoma medications vs. OHT patients or relatives of glaucoma patients not on topical medications | In this cross-sectional study, 109 participants (79 on topical medications and 30 controls) were evaluated via OSDI, Schirmer’s test, TBUT, and fluorescein staining. | The medication group had significantly shorter TBUT (6.0 vs. 9.5 s; p < 0.03), greater fluorescein staining (1.0 vs. 0; p < 0.001), and higher impression cytology grade than the control group (1.0 vs. 0.6; p < 0.001). | Cvenkel et al. Slovenia, 2015 [44] |
| Patients with POAG on topical anti-glaucoma medications vs. healthy controls | Age-matched patients were assigned to 2 groups: the glaucoma group (31 patients) and the treatment-naïve control group (30 patients). Each patient was assessed with OSDI, conjunctival/corneal staining, and TBUT. | OSDI scores of the glaucoma group positively correlated to the amount and duration of drops used. The glaucoma group had a higher mean OSDI score than the control group (18.97 vs. 6.25). Abnormal TBUT and staining scores were seen in the glaucoma group compared with the control group (68% vs. 17%). | Saade et al. USA, 2015 [45] |
| Patients with glaucoma or OHT on 0, 1, or ≥2 topical anti-glaucoma medications | 39 patients treated for glaucoma or OHT and 9 untreated patients were included in this study. Corneal sensitivity was measured using the Cochet-Bonnet esthesiometer, Schirmer’s test, TBUT, corneal and conjunctival fluorescein staining, and OSDI. | Corneal sensitivity of patients treated with IOP-lowering medications was negatively correlated to the number of instillations of P drops (p < 0.001) and duration of treatment (p = 0.001). There was no significant difference in OSDI or Schirmer’s test scores between the groups. | Van Went et al. France, 2011 [46] |
| Patients with POAG, pseudoexfoliation glaucoma, pigment dispersion glaucoma, or OHT on topical anti-glaucoma medications | This prospective observational study assessed OSDI in 630 patients with POAG, pseudoexfoliation glaucoma, pigment dispersion glaucoma, or OHT who were on topical IOP-lowering medications. | 305 patients (48.4%) had an OSDI score indicating either mild, moderate, or severe OSD symptoms. Higher OSDI scores were observed in patients using multiple IOP-lowering medications (p = 0.0001). | Fechtner et al. USA, 2010 [47] |
| Patients using P vs. PF topical beta-blocker drops | In a multicenter cross-sectional survey in four European countries, ophthalmologists in private practice enrolled 9658 patients using P or PF beta-blocking eyedrops between 1997 and 2003. Subjective symptoms, conjunctival and palpebral signs, and SPK were assessed before and after a change in therapy. | Palpebral, conjunctival, and corneal signs were significantly more frequent (p < 0.0001) in the P-group than in the PF-group, such as pain or discomfort during instillation (48% vs. 19%), foreign body sensation (42% vs. 15%), stinging or burning (48% vs. 20%), and dry eye sensation (35% vs. 16%). A significant decrease (p < 0.0001) in all ocular symptoms was observed in patients who switched from P to PF eye drops. | Jaenen et al. Belgium, 2007 [48] |
| Patients with POAG or OHT using P vs. PF topical anti-glaucoma medications | This prospective epidemiological survey was carried out in 1999 by 249 ophthalmologists on 4107 patients. Ocular symptoms, conjunctiva, and cornea were assessed between P and PF eye drops. | All symptoms were more prevalent with P than with PF drops (p < 0.001): discomfort upon instillation (43% vs. 17%), burning-stinging (40% vs. 22%), foreign body sensation (31% vs. 14%), dry eye sensation (23% vs. 14%), and tearing (21% vs. 14%). An increased incidence (>2 times) and duration of ocular signs were seen with P eye drops, which decreased upon switching to PF drops (p < 0.001). | Pisella et al. France, 2002 [49] |
| Category | Examples |
|---|---|
| Detergents | benzalkonium chloride (BAK) polidronium chloride (polyquaternium-1, Polyquad®) |
| Oxidative agents | stabilized oxychloro complex (SOC, Purite®) sodium perborate (GenAqua®) |
| Ionic tamponade agents | borate, sorbitol, propylene glycol, and zinc (SofZia®) |
| Product | Product Status | Mechanism of Action |
|---|---|---|
| Extraocular Drug Delivery Systems | ||
Gel-forming drops
| Preclinical | The higher viscosity gel-containing drops stay on the surface of the eyes for a longer period of time, thereby providing greater surface protection. |
| Ocular inserts | Bimatoprost Ocular Ring® is in Phase 2, and TODDD® is in Phase 1. | Ocular rings containing anti-glaucoma medications may be inserted in the upper and lower fornices for slow release, thickening the precorneal tear film and protecting the eye. |
| Passive Diffusion Contact Lenses (PDCLs) | Preclinical | Anti-glaucoma drug impregnated CLs release active ingredients through passive diffusion. |
Molecular Imprinted Contact Lenses (MICLs)
| Preclinical | During the fabrication of MICLs, molecular sites akin to drug receptor sites are embedded in the polymer, increasing loading and sustained release of anti-glaucoma drugs. |
| Punctal Plugs (PPs) | Evolute® is in Phase 2, and OTX-TP® is in Phase 3. | PPs block tear drainage and increase tear film contact time with the ocular surface. |
| Intraocular Drug Delivery Systems | ||
| Anterior Chamber (AC) Intracameral Implants (II) | Phase 2 or 3 | II are injected in the AC or anchored in the trabecular meshwork (TM) and slowly release medications over months. They are either biodegradable hydrogel or titanium implants. |
Subconjunctival Implants (SI)
| Phase 1 or 2a | SI impregnated with glaucoma drugs are injected subconjunctivally to provide slow drug release. |
| Innovative Technological Devices | ||
Intense Pulsed Light (IPL) Therapy
| Phase 4 | High intensity light pulses are directed around the eyes, which may destroy abnormal blood vessels and alter meibomian gland architecture and function. |
Thermal Pulsation Devices (TPD)
| Phase 4 | TPDs consist of disposable eyepieces which direct heat and pressure over the eyelids to liquefy and express meibomian gland secretions. |
| Photobiomodulation | Phase 2 | Photobiomodulation uses a mask to emit light over the face and eyelids. Blue light inhibits microbial growth while red light generates heat, promotes tissue repair, and decreases inflammation. |
| Other Emerging Therapies | ||
Nanoparticles
| Preclinical | Nanoparticles consisting of certain polymers, lipids, or metals may improve drug bioavailability, enabling slow release and reducing adverse effects. |
| Gene Therapy | Preclinical | Ocular gene therapy can target the TM to increase AH outflow and offer neuroprotection by limiting retinal ganglion cell (RGC) loss. |
| Stem Cell Applications | Preclinical | Stem cells can be used to improve TM structure and function, promote RGC survival, and improve corneal barrier dysfunction. |
Umbilical Cord Blood Serum (CBS) Eye Drops
| Phase 2 | CBS drops contain high levels of growth factors and anti-inflammatory cytokines. |
Acupuncture
| Phase 3 | Acupuncture may downregulate proinflammatory cytokines and increase the release of acetylcholine in the lacrimal glands, promoting tear secretion. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemer, Ö.E.; Mekala, P.; Dave, B.; Kooner, K.S. Managing Ocular Surface Disease in Glaucoma Treatment: A Systematic Review. Bioengineering 2024, 11, 1010. https://doi.org/10.3390/bioengineering11101010
Kemer ÖE, Mekala P, Dave B, Kooner KS. Managing Ocular Surface Disease in Glaucoma Treatment: A Systematic Review. Bioengineering. 2024; 11(10):1010. https://doi.org/10.3390/bioengineering11101010
Chicago/Turabian StyleKemer, Özlem Evren, Priya Mekala, Bhoomi Dave, and Karanjit Singh Kooner. 2024. "Managing Ocular Surface Disease in Glaucoma Treatment: A Systematic Review" Bioengineering 11, no. 10: 1010. https://doi.org/10.3390/bioengineering11101010
APA StyleKemer, Ö. E., Mekala, P., Dave, B., & Kooner, K. S. (2024). Managing Ocular Surface Disease in Glaucoma Treatment: A Systematic Review. Bioengineering, 11(10), 1010. https://doi.org/10.3390/bioengineering11101010









