sEMG Spectral Analysis and Machine Learning Algorithms Are Able to Discriminate Biomechanical Risk Classes Associated with Manual Material Liftings
Abstract
:1. Introduction
2. Materials and Methods
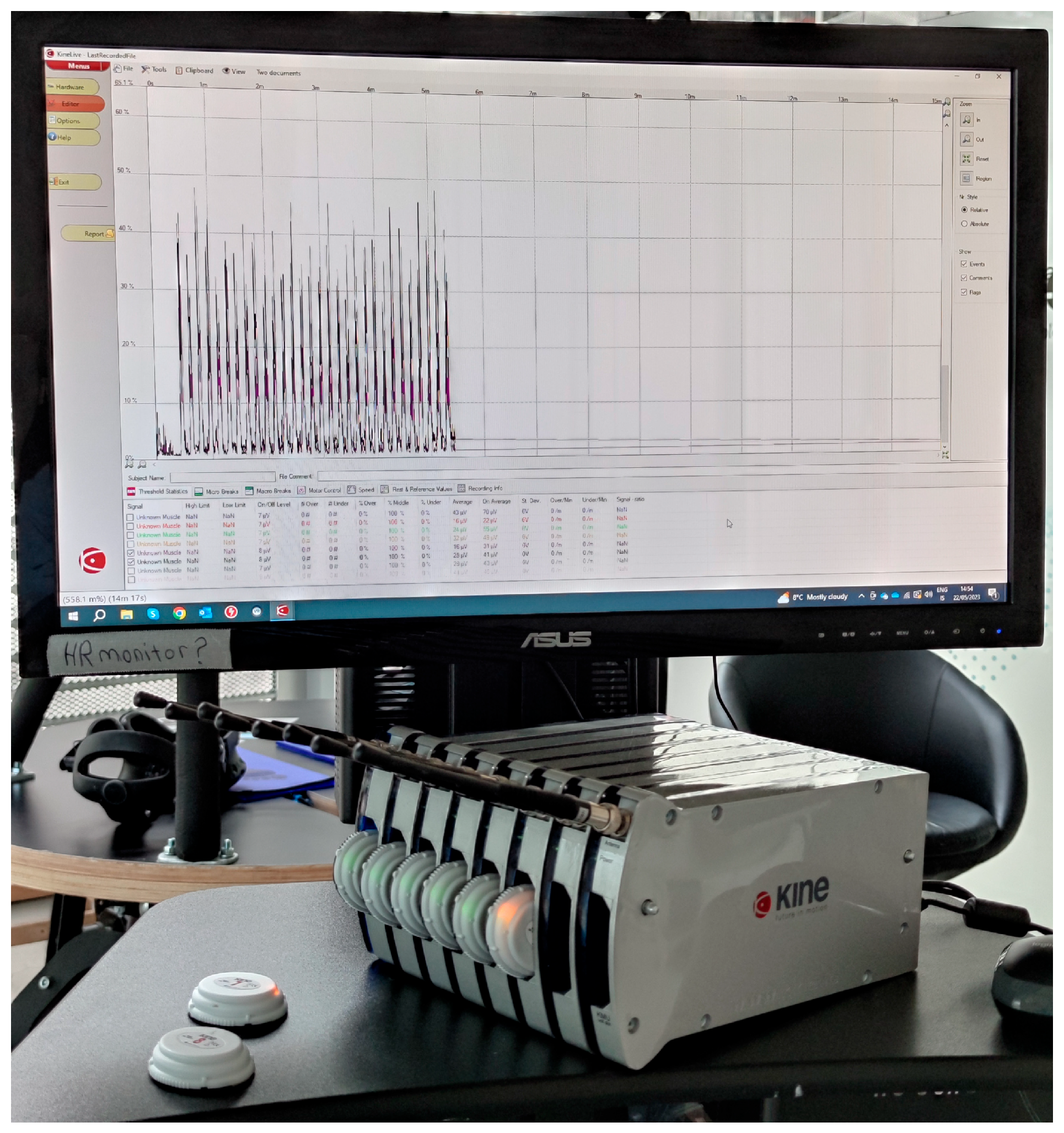
2.1. Wearable System for Surface Electomiography—The KineLive System
2.2. Revised NIOSH Lifting Equation
- LC: Load Constant 25/20 kg (males, <45/>45 years old, respectively), 20/15 kg (females, <45/>45 years old, respectively);
- HM: Horizontal Multiplier;
- VM: Vertical Multiplier;
- DM: Distance Multiplier;
- AM: Asymmetric Multiplier;
- FM: Frequency Multiplier;
- GM: Grab Multiplier.
2.3. Study Population
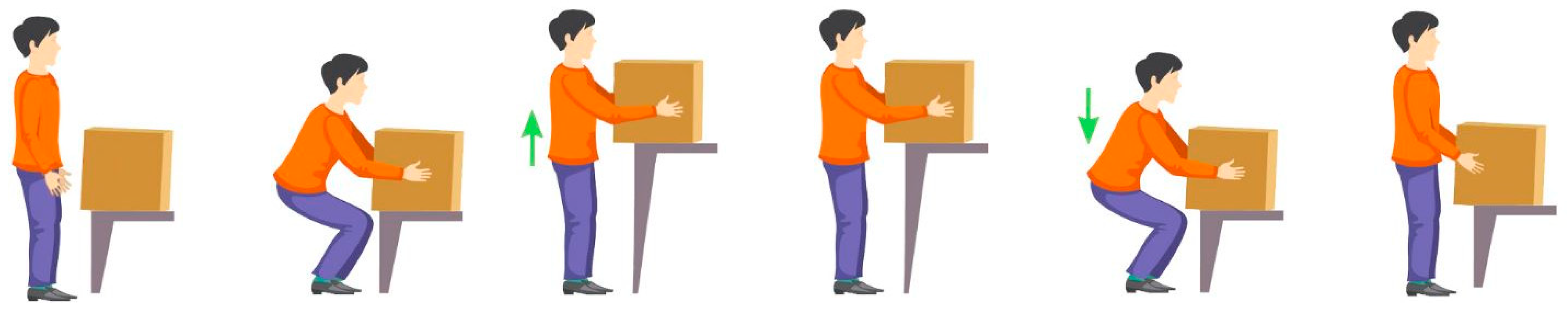
2.4. Study Protocol
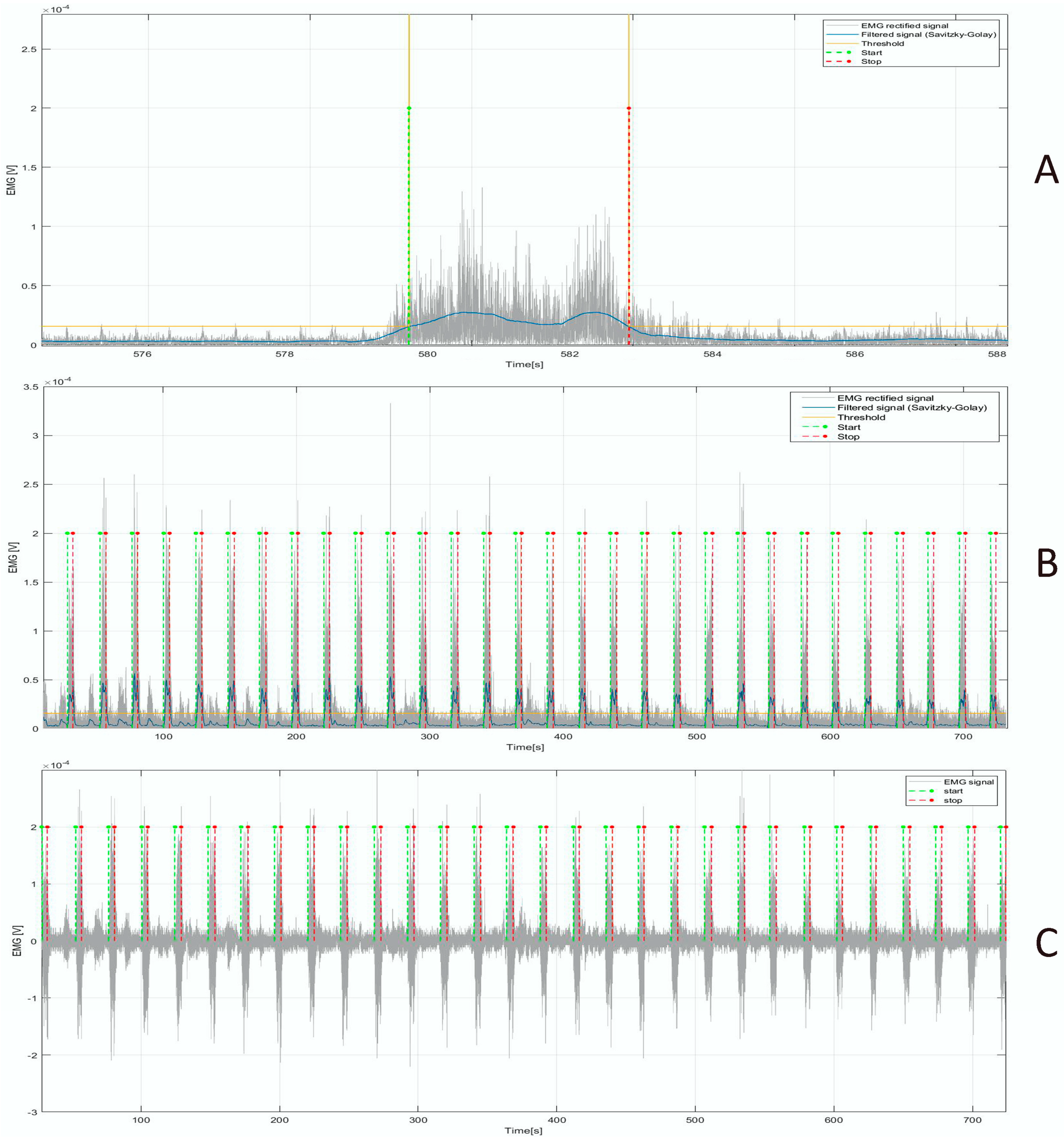
2.5. Digital Signal Processing and Feature Extraction
- Total power (Power) [V2]: the integral under the spectrum curve;
- Peak power (P_power) [V2/Hz]: the maximum value of the TPS;
- Median frequency (F_median) [Hz]: the frequency that divides the total power area into two equal parts;
- Mean frequency (F_mean) [Hz]: the mathematical mean of the spectrum curve;
- Peak frequency (F_peak) [Hz]: the frequency at which the P_power is attained;
- Kurtosis (adimensional): the standardized fourth moment of a distribution that represents a measure of the tailedness of a given distribution;
- Skewness (adimensional): the third standardized moment of a distribution that represents a measure of the asymmetry of a given distribution.
2.6. Statistical Analysis
2.7. Machine Learning Analysis
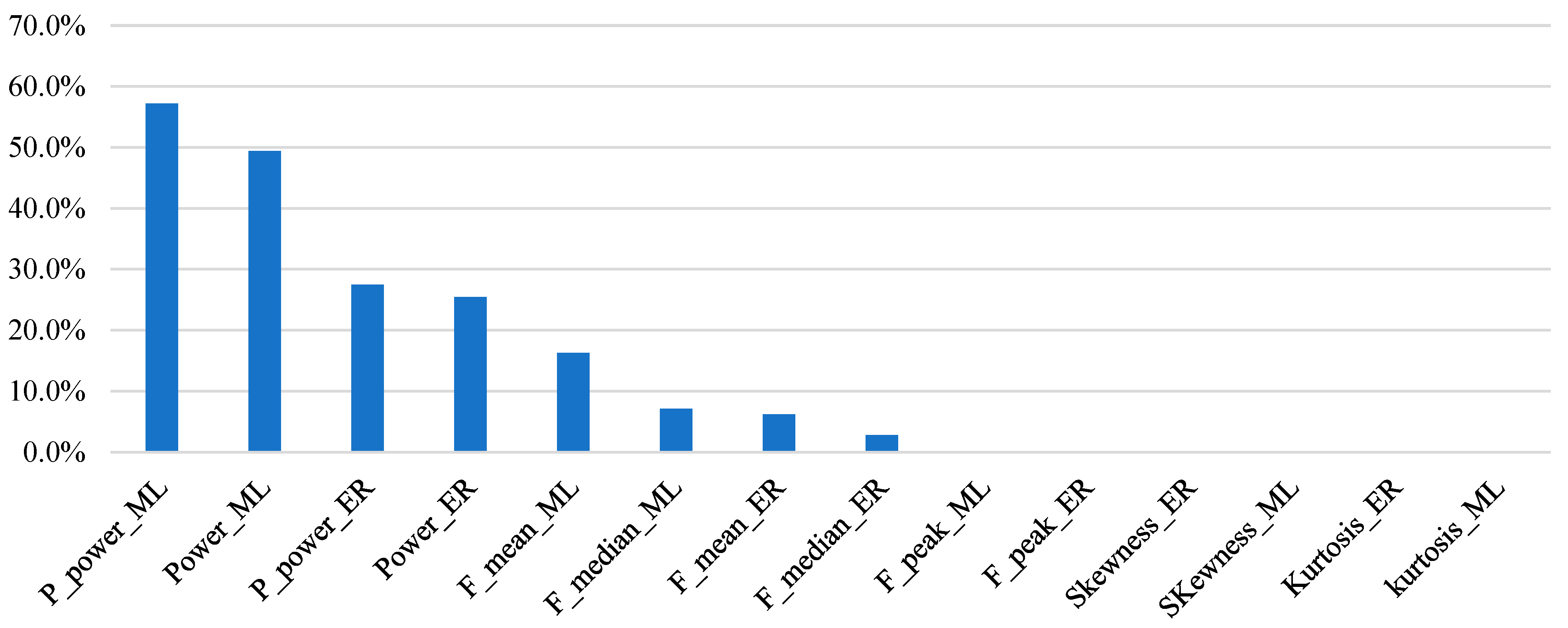
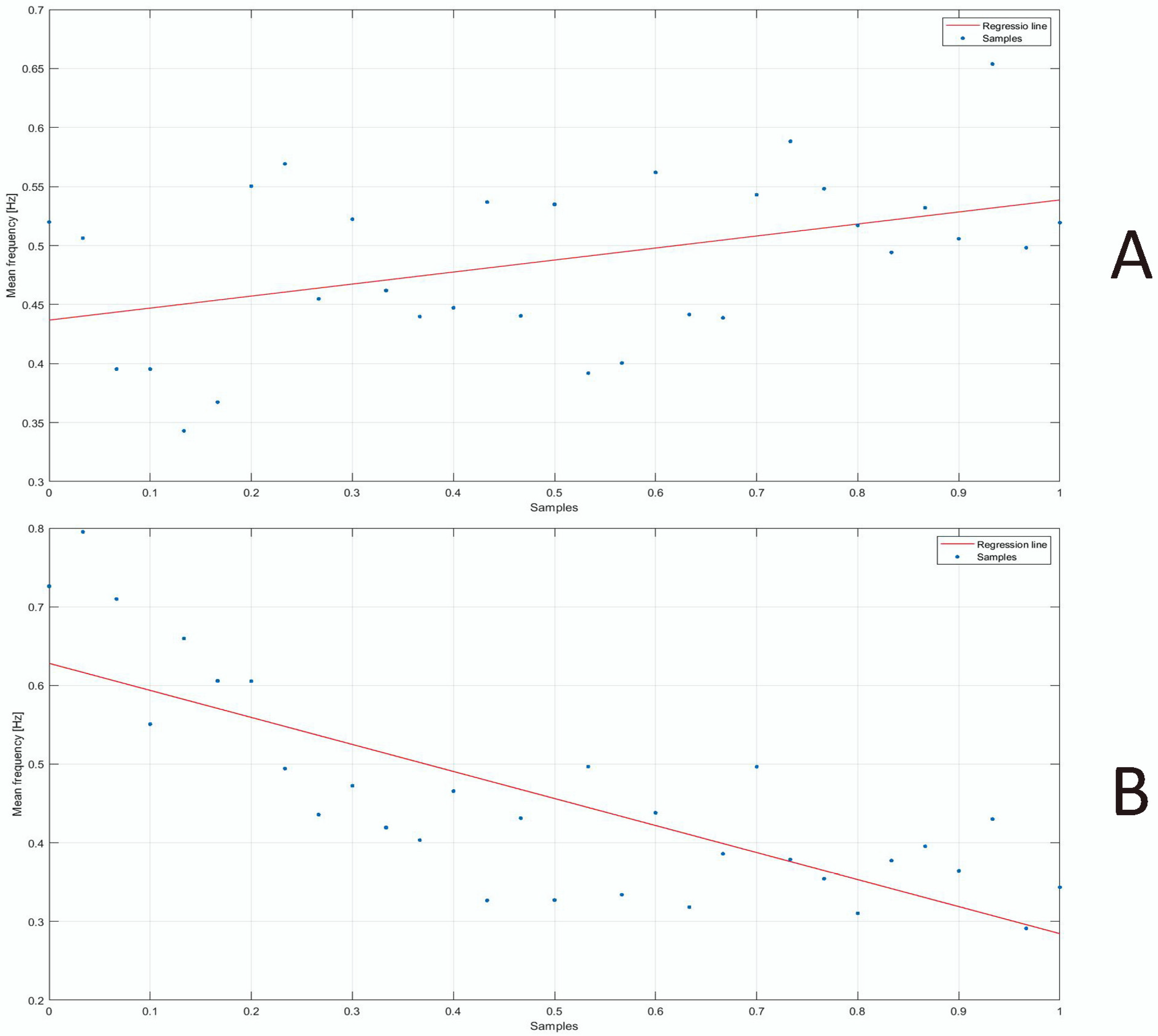
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radwin, R.G.; Marras, W.S.; Lavender, S.A. Biomechanical aspects of work-related musculoskeletal disorders. Theor. Issues Ergon. Sci. 2001, 2, 153–217. [Google Scholar] [CrossRef]
- Keyserling, W.M.; Brouwer, M.; Silverstein, B.A. A checklist for evaluating ergonomic risk factors resulting from awkward postures of the legs, trunk and neck. Int. J. Ind. Ergon. 1992, 9, 283–301. [Google Scholar] [CrossRef]
- Marras, W.S.; Lavender, S.A.; Leurgans, S.E.; Fathallah, F.A.; Ferguson, S.A.; Gary Allread, W.; Rajulu, S.L. Biomechanical risk factors for occupationally related low back disorders. Ergonomics 1995, 38, 377–410. [Google Scholar] [CrossRef] [PubMed]
- Punnett, L.; Fine, L.J.; Keyserling, W.M.; Herrin, G.D.; Chaffin, D.B. Back disorders and nonneutral trunk postures of automobile assembly workers. Scand. J. Work Environ. Health 1991, 17, 337–346. [Google Scholar] [CrossRef]
- Trask, C.; Mathiassen, S.E.; Wahlström, J.; Heiden, M.; Rezagholi, M. Modeling costs of exposure assessment methods in industrial environments. Work 2012, 41 (Suppl. S1), 6079–6086. [Google Scholar] [CrossRef]
- Buchholz, B.; Paquet, V.; Punnett, L.; Lee, D.; Moir, S. PATH: A work sampling-based approach to ergonomic job analysis for construction and other non-repetitive work. Appl. Ergon. 1996, 27, 177–187. [Google Scholar] [CrossRef]
- Karhu, O.; Kansi, P.; Kuorinka, I. Correcting working postures in industry: A practical method for analysis. Appl. Ergon. 1977, 8, 199–201. [Google Scholar] [CrossRef]
- Lynn, M.; Corlett, N. RULA: A survey method for the investigation of work-related upper limb disorders. Appl. Ergon. 1993, 24, 91–99. [Google Scholar]
- Hignett, S.; McAtamney, L. REBA: A survey method for the investigation of work-related upper limb disorders. Appl. Ergon. 2000, 31, 201–205. [Google Scholar] [CrossRef]
- Battevi, N.; Menoni, O.; Ricci, M.G.; Cairoli, S. MAPO index for risk assessment of patient manual handling in hospital wards: A validation study. Ergonomics 2006, 49, 671–687. [Google Scholar] [CrossRef]
- Walter, T.R.; Putz-Anderson, V.; Garg, A.; Lawrence, J. Revised NIOSH equation for the design and evaluation of manual lifting task. Ergonomics 1993, 36, 749–776. [Google Scholar]
- Takala, E.P.; Pehkonen, I.; Forsman, M.; Hansson, G.Å.; Mathiassen, S.E.; Neumann, W.P.; Sjøgaard, G.; Veiersted, K.B.; Westgaard, R.H.; Winkel, J. Systematic evaluation of observational methods assessing biomechanical exposures at work. Scand. J. Work Environ. Health 2010, 36, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Emodi, C.T. A general Computer-Based Methodology for Work Injury Analysis in a Production Assembly Line. Ph.D. Dissertation, University of Saskatchewan, Saskatoon, SK, Canada, 2007. [Google Scholar]
- Emodi, C.T.; Zhang, W.J.; Lang, S.Y.; Bi, Z.M. A Framework for Modeling and Analysis of Human Repetitive Operations in a Production/assembly Line (No. 2007-01-2500); SAE Technical Paper; SAE International: Pittsburgh, PA, USA, 2007. [Google Scholar]
- Donisi, L.; Amitrano, F.; Coccia, A.; Mercogliano, L.; Cesarelli, G.; D’Addio, G. Influence of the Backpack on School Children’s Gait: A Statistical and Machine Learning Approach. In Proceedings of the 8th European Medical and Biological Engineering Conference (EMBEC 2020), Portorož, Slovenia, 29 November–3 December 2020; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 682–688. [Google Scholar]
- Onofrejova, D.; Balazikova, M.; Glatz, J.; Kotianova, Z.; Vaskovicova, K. Ergonomic assessment of physical load in Slovak industry using wearable technologies. Appl. Sci. 2022, 12, 3607. [Google Scholar] [CrossRef]
- Chander, H.; Burch, R.F.; Talegaonkar, P.; Saucier, D.; Luczak, T.; Ball, J.E.; Turner, A.; Kodithuwakku Arachchige, S.N.K.; Carroll, W.; Smith, B.K.; et al. Wearable stretch sensors for human movement monitoring and fall detection in ergonomics. Int. J. Environ. Res. Public Health 2020, 17, 3554. [Google Scholar] [CrossRef] [PubMed]
- Lind, C.M.; Diaz-Olivares, J.A.; Lindecrantz, K.; Eklund, J. A wearable sensor system for physical ergonomics interventions using haptic feedback. Sensors 2020, 20, 6010. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.; Li, L.; Ma, L. Human work and status evaluation based on wearable sensors in human factors and ergonomics: A review. IEEE Trans. Hum.-Mach. Syst. 2018, 49, 72–84. [Google Scholar] [CrossRef]
- Donisi, L.; Cesarelli, G.; Pisani, N.; Ponsiglione, A.M.; Ricciardi, C.; Capodaglio, E. Wearable Sensors and Artificial Intelligence for Physical Ergonomics: A Systematic Review of Literature. Diagnostics 2022, 12, 3048. [Google Scholar] [CrossRef]
- Lopez-Nava, I.H.; Munoz-Melendez, A. Wearable inertial sensors for human motion analysis: A review. IEEE Sens. J. 2016, 16, 7821–7834. [Google Scholar] [CrossRef]
- Hafez, K. Input Variables for Manual Material Handling Assessment Methods Obtained Using Body Worn Sensors. J. Ergon. 2022, 12, 1–12. [Google Scholar]
- Chan, V.C.; Ross, G.B.; Clouthier, A.L.; Fischer, S.L.; Graham, R.B. The role of machine learning in the primary prevention of work-related musculoskeletal disorders: A scoping review. Appl. Ergon. 2022, 98, 103574. [Google Scholar] [CrossRef]
- Ranavolo, A.; Draicchio, F.; Varrecchia, T.; Silvetti, A.; Iavicoli, S. Wearable monitoring devices for biomechanical risk assessment at work: Current status and future challenges—A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 2001. [Google Scholar] [CrossRef] [PubMed]
- Stefana, E.; Marciano, F.; Rossi, D.; Cocca, P.; Tomasoni, G. Wearable devices for ergonomics: A systematic literature review. Sensors 2021, 21, 777. [Google Scholar] [CrossRef] [PubMed]
- Conforti, I.; Mileti, I.; Del Prete, Z.; Palermo, E. Measuring biomechanical risk in lifting load tasks through wearable system and machine-learning approach. Sensors 2020, 20, 1557. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.E.; Vea, L.A. Real-Time Human Sitting Position Recognition Using Wireless Sensors. In Proceedings of the 2020 2nd International Conference on Image, Video and Signal Processing, Singapore, 20–22 March 2020; pp. 133–137. [Google Scholar]
- Olsen, G.F.; Brilliant, S.S.; Primeaux, D.; Najarian, K. Signal processing and machine learning for real-time classification of ergonomic posture with unobtrusive on-body sensors; application in dental practice. In Proceedings of the 2009 ICME International Conference on Complex Medical Engineering, Tempe, AZ, USA, 9–11 April 2009; pp. 1–11. [Google Scholar]
- Antwi-Afari, M.F.; Li, H.; Yu, Y.; Kong, L. Wearable insole pressure system for automated detection and classification of awkward working postures in construction workers. Autom. Constr. 2018, 96, 433–441. [Google Scholar] [CrossRef]
- Zhang, L.; Diraneyya, M.M.; Ryu, J.; Haas, C.T.; Abdel-Rahman, E. Automated monitoring of physical fatigue using jerk. In Proceedings of the International Symposium on Automation and Robotics in Construction (ISARC), Banff, AB, Canada, 21–24 May 2019; Volume 36, pp. 989–997. [Google Scholar]
- Donisi, L.; Cesarelli, G.; Coccia, A.; Panigazzi, M.; Capodaglio, E.M.; D’Addio, G. Work-related risk assessment according to the revised NIOSH lifting equation: A preliminary study using a wearable inertial sensor and machine learning. Sensors 2021, 21, 2593. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G.; Certa, A.; Abusohyon, I.; Longo, F.; Padovano, A. Machine Learning approach towards real time assessment of hand-arm vibration risk. IFAC-PapersOnLine 2021, 54, 1187–1192. [Google Scholar] [CrossRef]
- Zhao, J.; Obonyo, E. Applying incremental Deep Neural Networks-based posture recognition model for ergonomics risk assessment in construction. Adv. Eng. Inform. 2021, 50, 101374. [Google Scholar] [CrossRef]
- Umer, W.; Li, H.; Yantao, Y.; Antwi-Afari, M.F.; Anwer, S.; Luo, X. Physical exertion modeling for construction tasks using combined cardiorespiratory and thermoregulatory measures. Autom. Constr. 2020, 112, 103079. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.; Yang, X.; Umer, W. Estimating construction workers’ physical workload by fusing computer vision and smart insole technologies. In Proceedings of the International Symposium on Automation and Robotics in Construction (ISARC), Berlin, Germany, 20–25 July 2018; Volume 35, pp. 1–8. [Google Scholar]
- Mudiyanselage, S.E.; Nguyen, P.H.D.; Rajabi, M.S.; Akhavian, R. Automated Workers’ Ergonomic Risk Assessment in Manual Material Handling Using SEMG Wearable Sensors and Machine Learning. Electronics 2021, 10, 2558. [Google Scholar] [CrossRef]
- Donisi, L.; Capodaglio, E.; Pagano, G.; Amitrano, F.; Cesarelli, M.; Panigazzi, M.; D’Addio, G. Feasibility of Tree-based Machine Learning algorithms fed with surface electromyographic features to discriminate risk classes according to NIOSH. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 1–6. [Google Scholar]
- Jacob, D.; Unnsteinsdóttir Kristensen, I.S.; Aubonnet, R.; Recenti, M.; Donisi, L.; Ricciardi, C.; Svansson, H.Á.R.; Agnarsdóttir, S.; Colacino, A.; Jónsdóttir, M.K.; et al. Towards defining biomarkers to evaluate concussions using virtual reality and a moving platform (BioVRSea). Sci. Rep. 2022, 12, 8996. [Google Scholar] [CrossRef]
- Kristinsdóttir, S.; Þóra Þórisdóttir, A.; Björk Halldórsdóttir, L.; Magnúsdóttir, G.; Ingólfsdóttir, B.; Ingvarsson, P.E.; Helgason, Þ. A novel reflex analysis of healthy and spinal cord-injured individuals. In Current Directions in Biomedical Engineering; De Gruyter: Berlin, Germany, 2022; Volume 8, pp. 745–748. [Google Scholar]
- Lu, M.L.; Waters, T.R.; Krieg, E.; Werren, D. Efficacy of the revised NIOSH lifting equation to predict risk of low-back pain associated with manual lifting: A one-year prospective study. Hum. Factors 2014, 56, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Waters, T.R.; Baron, S.L.; Piacitelli, L.A.; Anderson, V.P.; Skov, T.; Haring-Sweeney, M.; Wall, D.K.; Fine, L.J. Evaluation of the revised NIOSH lifting equation: A cross-sectional epidemiologic study. Spine 1999, 24, 386–394. [Google Scholar] [CrossRef]
- Spector, J.T.; Lieblich, M.; Bao, S.; McQuade, K.; Hughes, M. Automation of workplace lifting hazard assessment for musculoskeletal injury prevention. Ann. Occup. Environ. Med. 2014, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Press, W.H.; Teukolsky, S.A. Savitzky-Golay smoothing filters. Comput. Phys. 1990, 4, 669–672. [Google Scholar] [CrossRef]
- Murthy, S.K. Automatic construction of decision trees from data: A multi-disciplinary survey. Data Min. Knowl. Discov. 1998, 2, 345–389. [Google Scholar] [CrossRef]
- Sheng, P.; Chen, L.; Tian, J. Learning-based road crack detection using gradient boost decision tree. In Proceedings of the 2018 13th IEEE Conference on Industrial Electronics and Applications (ICIEA), Wuhan, China, 31 May–2 June 2018; pp. 1228–1232. [Google Scholar]
- Weinberger, K.Q.; Saul, L.K. Distance metric learning for large margin nearest neighbor classification. J. Mach. Learn. Res. 2009, 10, 207–244. [Google Scholar]
- Al-Aidaroos, K.M.; Bakar, A.A.; Othman, Z. Naive Bayes variants in classification learning. In Proceedings of the 2010 International Conference on Information Retrieval & Knowledge Management (CAMP), Shah Alam, Malaysia, 17–18 March 2010; pp. 276–281. [Google Scholar]
- Kotsiantis, S.B.; Zaharakis, I.; Pintelas, P. Supervised machine learning: A review of classification techniques. Emerg. Artif. Intell. Appl. Comput. Eng. 2007, 160, 3–24. [Google Scholar]
- Stoltzfus, J.C. Logistic regression: A brief primer. Acad. Emerg. Med. 2011, 18, 1099–1104. [Google Scholar] [CrossRef]
- Anguita, D.; Ghelardoni, L.; Ghio, A.; Oneto, L.; Ridella, S. The ‘K’ in K-fold Cross Validation. In Proceedings of the European Symposium on Artificial Neural Networks, Computational Intelligence and Machine Learning (ESANN), Bruges, Belgium, 25–27 April 2012; pp. 441–446. [Google Scholar]
- Sechidis, K.; Tsoumakas, G.; Vlahavas, I. On the stratification of multi-label data. In Machine Learning and Knowledge Discovery in Databases, Proceedings of the European Conference, ECML PKDD 2011, Athens, Greece, 5–9 September 2011; Proceedings, Part III 22; Springer: Berlin/Heidelberg, Germany, 2011; pp. 145–158. [Google Scholar]
- Hossin, M.; Sulaiman, M.N. A review on evaluation metrics for data classification evaluations. Int. J. Data Min. Knowl. Manag. Process 2015, 5, 1. [Google Scholar]
- Lei, S. A feature selection method based on information gain and genetic algorithm. In Proceedings of the 2012 International Conference on Computer Science and Electronics Engineering, Hangzhou, China, 23–25 March 2012; Volume 2, pp. 355–358. [Google Scholar]
- Petrofsky, J.; Laymon, M. Muscle temperature and EMG amplitude and frequency during isometric exercise. Aviat. Space Environ. Med. 2005, 76, 1024–1030. [Google Scholar]
- Klein, A.B.; Snyder-Mackler, L.; Roy, S.H.; DeLuca, C.J. Comparison of spinal mobility and isometric trunk extensor forces with electromyographic spectral analysis in identifying low back pain. Phys. Ther. 1991, 71, 445–454. [Google Scholar] [CrossRef]
- Roy, S.H.; De Luca, C.J.; Casavant, D.A. Lumbar muscle fatigue and chronic lower back pain. Spine 1989, 14, 992–1001. [Google Scholar] [CrossRef]
- Kramer, M.; Ebert, V.; Kinzl, L.; Dehner, C.; Elbel, M.; Hartwig, E. Surface electromyography of the paravertebral muscles in patients with chronic low back pain. Arch. Phys. Med. Rehabil. 2005, 86, 31–36. [Google Scholar] [CrossRef]
- D’Addio, G.; Donisi, L.; Cesarelli, G.; Amitrano, F.; Coccia, A.; La Rovere, M.T.; Ricciardi, C. Extracting features from Poincare plots to distinguish congestive heart failure patients according to NYHA classes. Bioengineering 2021, 8, 138. [Google Scholar] [CrossRef]
- Vispute, S.; Saini, M.L. Performance Analysis of Soil Health Classifiers Using Data Analytics Tools and Techniques for Best Model and Tool Selection. Int. J. Online Biomed. Eng. 2022, 18, 169–189. [Google Scholar] [CrossRef]
- Fillbrunn, A.; Dietz, C.; Pfeuffer, J.; Rahn, R.; Landrum, G.A.; Berthold, M.R. KNIME for reproducible cross-domain analysis of life science data. J. Biotechnol. 2017, 261, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Balderston, S.; Taulbee, J.J.; Celaya, E.; Fung, K.; Jiao, A.; Smith, K.; Hajian, R.; Gasiunas, G.; Kutanovas, S.; Kim, D.; et al. Discrimination of single-point mutations in unamplified genomic DNA via Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 2021, 5, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, C.; Ponsiglione, A.M.; Scala, A.; Borrelli, A.; Misasi, M.; Romano, G.; Russo, G.; Triassi, M.; Improta, G. Machine learning and regression analysis to model the length of hospital stay in patients with femur fracture. Bioengineering 2022, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Özkan, S.B.; Apaydin, S.M.F.; Özkan, Y.; Düzdar, I. Comparison of open source data mining tools: Naive Bayes algorithm example. In Proceedings of the 2019 Scientific Meeting on Electrical-Electronics & Biomedical Engineering and Computer Science (EBBT), Istanbul, Turkey, 24–26 April 2019; pp. 1–4. [Google Scholar]
- Kamen, G.; Gabriel, D.A. Essentials of Electromyography; Human Kinetics Publishers: Champaign, IL, USA, 2009. [Google Scholar]
- Donisi, L.; Cesarelli, G.; Capodaglio, E.; Panigazzi, M.; D’Addio, G.; Cesarelli, M.; Amato, F. A Logistic Regression Model for Biomechanical Risk Classification in Lifting Tasks. Diagnostics 2022, 12, 2624. [Google Scholar] [CrossRef]
- Varrecchia, T.; De Marchis, C.; Rinaldi, M.; Draicchio, F.; Serrao, M.; Schmid, M.; Conforto, S.; Ranavolo, A. Lifting activity assessment using surface electromyographic features and neural networks. Int. J. Ind. Ergon. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Varrecchia, T.; De Marchis, C.; Draicchio, F.; Schmid, M.; Conforto, S.; Ranavolo, A. Lifting Activity Assessment Using Kinematic Features and Neural Networks. Appl. Sci. 2020, 10, 1989. [Google Scholar] [CrossRef]
- Ranavolo, A.; Varrecchia, T.; Iavicoli, S.; Marchesi, A.; Rinaldi, M.; Serrao, M.; Conforto, S.; Cesarelli, M.; Draicchio, F. Surface electromyography for risk assessment in work activities designed using the “revised NIOSH lifting equation”. Int. J. Ind. Ergon. 2018, 68, 34–45. [Google Scholar] [CrossRef]
- Lindstrom, L.; Kadefors, R.; Petersen, I. An electromyographic index for localized muscle fatigue. J. Appl. Physiol. 1977, 43, 750–754. [Google Scholar] [CrossRef]
- Van Boxtel, A.; Goudswaard, P.; Van der Molen, G.M.; Van Den Bosch, W.E. Changes in electromyogram power spectra of facial and jaw-elevator muscles during fatigue. J. Appl. Physiol. 1983, 54, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, M.; Schindler-Ivens, S.; Williams, D.M.; Chandran, R.; Sharma, S.S. EMG frequency content changes with increasing force and during fatigue in the quadriceps femoris muscle of men and women. J. Electromyogr. Kinesiol. 2003, 13, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.N.; Cresswell, A.G.; Thorstensson, A. Electromyographic responses of the human triceps surae and force tremor during sustained sub-maximal isometric plantar flexion. Acta Physiol. Scand. 1994, 152, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Lin, Y.; Cheng, L.; Yang, G.; Liu, L.; Zhang, W.J. A socially inspired framework for human state inference using expert opinion integration. IEEE/ASME Trans. Mechatron. 2011, 16, 874–878. [Google Scholar] [CrossRef]


| Characteristics | |
|---|---|
| Age (years) | 24.50 ± 3.25 |
| Height (cm) | 181.75 ± 4.23 |
| Weight (kg) | 80.38 ± 8.52 |
| Body Mass Index (kg/m2) | 24.40 ± 3.28 |
| Trial 1 (LI < 1, LI = 0.5) | Trial 2 (LI > 1, LI = 1.3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Vertical Displacement (cm) | Frequency (lifts/min) | Weight Lifted (kg) | Vertical Displacement (cm) | Frequency (lifts/min) | Weight Lifted (kg) | |||
| M & F | M & F | M | F | M & F | M | F | M | F |
| 50–120 | 2.5 | 7 | 5 | 50–120 | 6 | 4 | 15 | 10 |
| Features | NO-RISK Mean ± Std | RISK Mean ± Std | p-Value |
|---|---|---|---|
| Power_ER | 8.316 × 10−9 ± 7.216 × 10−9 | 1.084 × 10−8 ± 6.612 × 10−9 | <0.001 |
| P_power_ER | 1.205 × 10−10 ± 7.835 × 10−11 | 1.891 × 10−10 ± 1.037 × 10−10 | <0.001 |
| F_peak_ER | 48.611 ± 11.562 | 47.975 ± 11.226 | 0.523 |
| F_median_ER | 66.192 ± 10.160 | 64.776 ± 9.057 | <0.001 |
| F_mean_ER | 79.215 ± 11.387 | 77.833 ± 10.237 | <0.001 |
| Kurtosis_ER | 71.608 ± 37.604 | 74.134 ± 37.724 | 0.011 |
| Skewness_ER | 6.787 ± 1.361 | 6.954 ± 1.257 | 0.001 |
| Features | NO-RISK Mean ± Std | RISK Mean ± Std | p-Value |
|---|---|---|---|
| Power_ML | 1.095 × 10−8 ± 6.919 × 10−9 | 1.881 × 10−8 ± 1.326 × 10−8 | <0.001 |
| P_power_ML | 1.298 × 10−10 ± 8.016 × 10−11 | 2.896 × 10−10 ± 2.435 × 10−10 | <0.001 |
| F_peak_ML | 51.650 ± 16.828 | 48.935 ± 16.022 | 0.004 |
| F_median_ML | 78.600 ± 15.788 | 74.606 ± 17.650 | <0.001 |
| F_mean_ML | 97.406 ± 15.216 | 92.443 ± 17.717 | <0.001 |
| Kurtosis_ML | 66.686 ± 37.298 | 75.974 ± 48.0.69 | <0.001 |
| Skewness_ML | 6.347 ± 1.518 | 6.781 ± 1.828 | <0.001 |
| SVM | KNN | DT | GR Boost | LR | NB | |
|---|---|---|---|---|---|---|
| Accuracy | 0.961 | 0.907 | 0.715 | 0.943 | 0.85 | 0.671 |
| F-measure | 0.962 | 0.91 | 0.735 | 0.942 | 0.857 | 0.719 |
| Specificity | 0.955 | 0.866 | 0.642 | 0.963 | 0.801 | 0.500 |
| Sensitivity | 0.967 | 0.947 | 0.789 | 0.923 | 0.898 | 0.841 |
| Precision | 0.956 | 0.876 | 0.688 | 0.962 | 0.819 | 0.627 |
| Recall | 0.967 | 0.947 | 0.789 | 0.923 | 0.898 | 0.841 |
| AUCROC | 0.985 | 0.961 | 0.712 | 0.987 | 0.910 | 0.782 |
| NO-RISK | RISK | |
|---|---|---|
| NO-RISK | 238 | 8 |
| RISK | 11 | 235 |
| SVM | KNN | DT | GR Boost | LR | NB | |
|---|---|---|---|---|---|---|
| Accuracy | 0.848 ± 0.068 | 0.787 ± 0.102 | 0.522 ± 0.153 | 0.647 ± 0.189 | 0.785 ± 0.066 | 0.553 ± 0.067 |
| F-measure | 0.855 ± 0.059 | 0.728 ± 0.187 | 0.509 ± 0.212 | 0.687 ± 0.209 | 0.776 ± 0.082 | 0.518 ± 0.193 |
| Specificity | 0.806 ± 0.148 | 0.923 ± 0.084 | 0.448 ± 0.405 | 0.483 ± 0.398 | 0.794 ± 0.165 | 0.488 ± 0.476 |
| Sensitivity | 0.890 ± 0.095 | 0.650 ± 0.240 | 0.583 ± 0.330 | 0.812 ± 0.288 | 0.776 ± 0.168 | 0.618 ± 0.408 |
| Precision | 0.836 ± 0.102 | 0.909 ± 0.078 | 0.585 ± 0.226 | 0.638 ± 0.215 | 0.817 ± 0.118 | 0.653 ± 0.206 |
| Recall | 0.890 ± 0.095 | 0.650 ± 0.240 | 0.583 ± 0.330 | 0.812 ± 0.288 | 0.776 ± 0.168 | 0.618 ± 0.408 |
| AUCROC | 0.918 ± 0.106 | 0.907 ± 0.074 | 0.559 ± 0.200 | 0.735 ± 0.207 | 0.839 ± 0.087 | 0.674 ± 0.127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donisi, L.; Jacob, D.; Guerrini, L.; Prisco, G.; Esposito, F.; Cesarelli, M.; Amato, F.; Gargiulo, P. sEMG Spectral Analysis and Machine Learning Algorithms Are Able to Discriminate Biomechanical Risk Classes Associated with Manual Material Liftings. Bioengineering 2023, 10, 1103. https://doi.org/10.3390/bioengineering10091103
Donisi L, Jacob D, Guerrini L, Prisco G, Esposito F, Cesarelli M, Amato F, Gargiulo P. sEMG Spectral Analysis and Machine Learning Algorithms Are Able to Discriminate Biomechanical Risk Classes Associated with Manual Material Liftings. Bioengineering. 2023; 10(9):1103. https://doi.org/10.3390/bioengineering10091103
Chicago/Turabian StyleDonisi, Leandro, Deborah Jacob, Lorena Guerrini, Giuseppe Prisco, Fabrizio Esposito, Mario Cesarelli, Francesco Amato, and Paolo Gargiulo. 2023. "sEMG Spectral Analysis and Machine Learning Algorithms Are Able to Discriminate Biomechanical Risk Classes Associated with Manual Material Liftings" Bioengineering 10, no. 9: 1103. https://doi.org/10.3390/bioengineering10091103
APA StyleDonisi, L., Jacob, D., Guerrini, L., Prisco, G., Esposito, F., Cesarelli, M., Amato, F., & Gargiulo, P. (2023). sEMG Spectral Analysis and Machine Learning Algorithms Are Able to Discriminate Biomechanical Risk Classes Associated with Manual Material Liftings. Bioengineering, 10(9), 1103. https://doi.org/10.3390/bioengineering10091103









