Efficacy of Fractional Laser on Steroid Receptors in GSM Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
- Age 40–65 years;
- Absence of menstruation for at least 12 months;
- Presence of symptoms attributable to GSM (i.e., vaginal dryness/dyspareunia);
- Women who underwent a conventional colposcopic examination and a Frost index consistent with atrophy;
- Women who had not undergone hormone replacement in the past 12 months.
2.3.2. Exclusion Criteria
- Acute or recurrent urogenital infection or inflammation;
- Preneoplastic lesions;
- Pelvic prolapse;
- Pregnancy;
- Immunosuppression;
- Severe chronic disease;
- Previous pelvic surgery;
- Use of hormone therapy for <1 year;
- Previous pelvic radiotherapy.
2.4. Laser Application
2.5. Biopsy
2.6. Immunohistochemistry
2.7. Statistical Analyses
- Student’s t-test was used for independent samples [15] to compare the groups (estrogen and laser) based on the current age, body mass index (BMI), number of normal deliveries, and increased thickness of the vaginal epithelium;
- Pearson’s chi-squared and Fisher’s exact test or its extension [16] were used to compare groups (estrogen and laser) based on marital status, schooling, hypertension, osteoporosis, depression, increased thickness of the vaginal epithelium, estrogen levels, and progesterone receptor levels;
- The Mann–Whitney U-test [17] was used to compare groups (estrogen and laser) based on age at the beginning of menopause;
- The Wilcoxon rank-sum test [17] was used to compare levels (scores) of estrogen and progesterone receptors between time periods (initial and final);
- A repeated-measures analysis of variance [18] was used to compare the thickness of the vaginal epithelium of the groups (estrogen and laser) and time periods (initial and final);
- Spearman’s correlation coefficient [17] was used to quantify the correlation of the vaginal epithelial thickness with estrogen and progesterone receptor levels. An alpha significance level of 5% was used for all conclusions obtained through inferential analyses. The data were analyzed using Excel 2010 for Windows (Microsoft, Redmond, WA, USA) for proper storage of the information. Statistical analyses were performed using SPSS Statistics Version 24 (IBM, Armonk, NY, USA) and R Version 3.6.3 (R Foundation, Vienna, Austria) [19].
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monterrosa-Castro, A.; Portela-Buevas, K. Manejo de la atrofia vulvovaginal posmenopausica. Rev. Chil. Ginecol. Obstet. 2014, 79, 489–501. [Google Scholar] [CrossRef]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef]
- Gracia, C.R.; Freeman, E.W. Onset of the menopause transition: The earliest signs and symptoms. Obstet. Gynecol. Clin. N. Am. 2018, 45, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Cruz, V.L.; Steiner, M.L.; Pompei, L.M.; Strufaldi, R.; Fonseca, F.L.A.; Santiago, L.H.S.; Wajsfeld, T.; Fernandes, C.E. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018, 25, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Nappi, R.E.; Palacios, S.; Panay, N.; Particco, M.; Krychman, M.L. Vulvar and vaginal atrophy in four European countries: Evidence from the European REVIVE Survey. Climacteric 2016, 19, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.J. Treatment of vulvovaginal atrophy. JAMA 2019, 322, 1910–1911. [Google Scholar] [CrossRef] [PubMed]
- Pitsouni, E.; Grigoriadis, T.; Falagas, M.E.; Salvatore, S.; Athanasiou, S. Laser therapy for the genitourinary syndrome of menopause. A systematic review and meta-analysis. Maturitas 2017, 103, 78–88. [Google Scholar] [CrossRef]
- Nappi, R.E.; Particco, M.; Biglia, N.; Cagnacci, A.; Di Carlo, C.; Luisi, S.; Paoletti, A.M. Attitudes and perceptions towards vulvar and vaginal atrophy in Italian postmenopausal women: Evidence from the European REVIVE survey. Maturitas 2016, 91, 74–80. [Google Scholar] [CrossRef]
- Taylor, A.H.; Guzail, M.; Al-Azzawi, F. Differential expression of oestrogen receptor isoforms and androgen receptor in the normal vulva and vagina compared with vulval lichen sclerosus and chronic vaginitis. Br. J. Dermatol. 2008, 158, 319–328. [Google Scholar] [CrossRef]
- Athanasiou, S.; Pitsouni, E.; Antonopoulou, S.; Zacharakis, D.; Salvatore, S.; Falagas, M.E.; Grigoriadis, T. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric 2016, 19, 512–518. [Google Scholar] [CrossRef]
- Filippini, M.; Del Duca, E.; Negosanti, F.; Bonciani, D.; Negosanti, L.; Sannino, M.; Cannarozzo, G.; Nisticò, S.P. Fractional CO2 laser: From skin rejuvenation to vulvo-vaginal reshaping. Photomed. Laser Surg. 2017, 35, 171–175. [Google Scholar] [CrossRef]
- Salvatore, S.; Leone Roberti Maggiore, U.; Athanasiou, S.; Origoni, M.; Candiani, M.; Calligaro, A.; Zerbinati, N. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: An ex vivo study. Menopause 2015, 22, 845–849. [Google Scholar] [CrossRef]
- Filipinni, M.; Farinelli, M.; Preliminary Results after Four Years of MonaLisa Touch Treatments on Subjects with Genitourinary Syndrome of Menopause (GSM). May 2017. Available online: https://www.monalisatouch.com/wp-content/uploads/2017/06/SX2V2LR-Filippini-SMSHWP-May17-eng.pdf (accessed on 25 August 2020).
- Michalany, J. Técnica Histológica Em Anatomia Patológica: Com Instruções Para o Cirurgião, Enfermeira e Citotécnico, 3rd ed.; Pedagogica e Universitaria: São Paulo, Brazil, 1998. [Google Scholar]
- Bussab, W.O.; Morettin, P.A. Estatística Básica, 5th ed.; Saraiva: São Paulo, Brazil, 2006. [Google Scholar]
- Agresti, A. Categorical Data Analysis; Wiley Interscience: New York, NY, USA, 1990. [Google Scholar]
- Siegel, S. Estatística Não-Paramétrica para Ciências do Comportamento, 2nd ed.; Artmed: Porto Alegre, Brazil, 2006. [Google Scholar]
- Netter, J.; Kutner, M.H.; Nachtsheim, C.J.; Li, W. Applied Linear Statistical Models, 4th ed.; McGraw-Hill Irwin: Boston, MA, USA, 1996. [Google Scholar]
- The R Foundation. The R Project for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 26 August 2020).
- Dória, O.; Giannini, A.; Buzzaccarini, G. Fractional CO2 laser for vulvo-vaginal atrophy in Gynecologic cancer patients: A valid Therapeutic Choice? A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 277, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, F.; Zhou, Y. Efficacy of CO2 laser treatment in post menopausal women with vulvo vaginal atrophy: A meta-analysis. Int. J. Gynecol. Obstet. 2022, 158, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, S.; Lami, A.; Baldassarre, M. Laser treatment alone or with estriol or moisturizers in postmenopausal women with vulvovaginal Atrophy. J. Sex. Med. 2022, 19, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Rahn, D.D.; Carberry, C.; Sanses, T.V.; Mamik, M.M.; Ward, R.M.; Meriwether, K.V.; Olivera, C.K.; Abed, H.; Balk, E.M.; Murphy, M.; et al. Vaginal estrogen for genitourinary syndrome of menopause: A systematic review. Obstet. Gynecol. 2014, 124, 1147–1156. [Google Scholar] [CrossRef]
- Portman, D.J.; Gass, M.L. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014, 21, 1063–1068. [Google Scholar] [CrossRef]
- Salvatore, S.; Nappi, R.E.; Zerbinati, N.; Calligaro, A.; Ferrero, S.; Origoni, M.; Candiani, M.; Leone Roberti Maggiore, U. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: A pilot study. Climacteric 2014, 17, 363–369. [Google Scholar] [CrossRef]
- Biehl, C.; Plotsker, O.; Mirkin, S. A systematic review of the efficacy and safety of vaginal estrogen products for the treatment of genitourinary syndrome of menopause. Menopause 2019, 26, 431–453. [Google Scholar] [CrossRef]
- Samuels, J.B.; Garcia, M.A. Treatment to external labia and vaginal canal with CO2 laser for symptoms of vulvovaginal atrophy in postmenopausal women. Aesthet. Surg. J. 2019, 39, 83–93. [Google Scholar] [CrossRef]
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause 2017, 24, 728–753. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Serati, M.; Origoni, M.; Candiani, M.; Iannitti, T.; Salvatore, S.; Marotta, F.; Calligaro, A. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med. Sci. 2015, 30, 429–436. [Google Scholar] [CrossRef]
- Lethaby, A.; Ayeleke, R.O.; Roberts, H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst. Rev. 2016, 2016, CD001500. [Google Scholar] [CrossRef] [PubMed]

| Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Estrogen | Laser | Total | ||||||
| (n = 12) | (n = 13) | (n = 25) | p | |||||
| Current age (years) | Average | 55.5 | 55.8 | 55.6 | 0.880 a | |||
| Median | 55.0 | 55.0 | 55.0 | |||||
| Minimum | 50.0 | 50.0 | 50.0 | |||||
| Maximum | 62.0 | 63.0 | 63.0 | |||||
| Standard deviation | 4.4 | 4.5 | 4.3 | |||||
| Body mass index (kg/m2) | Average | 27.3 | 28.2 | 27.8 | 0.591 a | |||
| Median | 26.7 | 26.5 | 26.6 | |||||
| Minimum | 22.0 | 22.1 | 22.0 | |||||
| Maximum | 33.7 | 37.9 | 37.9 | |||||
| Standard deviation | 3.4 | 4.7 | 4.1 | |||||
| Number of normal deliveries | 0 | 3 | 25.0% | 2 | 15.4% | 3 | 25.0% | 2 |
| 1 | - | - | 1 | 7.7% | - | - | 1 | |
| 2 | 5 | 41.7% | 5 | 38.5% | 5 | 41.7% | 5 | |
| 3 | 2 | 16.7% | 4 | 30.8% | 2 | 16.7% | 4 | |
| 4 | 1 | 8.3% | 1 | 7.7% | 1 | 8.3% | 1 | |
| 6 | 1 | 8.3% | - | - | 1 | 8.3% | - | |
| Marital status | Married | 10 | 83.3% | 11 | 84.6% | 10 | 83.3% | 11 |
| Single | - | - | 2 | 15.4% | - | - | 2 | |
| Widowed | 2 | 16.7% | - | - | 2 | 16.7% | - | |
| Schooling | Elementary incomplete | 3 | 25.0% | 4 | 30.8% | 3 | 25.0% | 4 |
| Elementary complete | 3 | 25.0% | - | - | 3 | 25.0% | - | |
| High school complete | 5 | 41.7% | 7 | 53.8% | 5 | 41.7% | 7 | |
| Higher education complete | 1 | 8.3% | 2 | 15.4% | 1 | 8.3% | 2 | |
| Systemic arterial hypertension | Yes | 6 | 50.0% | 5 | 38.5% | 6 | 50.0% | 5 |
| No | 6 | 50.0% | 8 | 61.5% | 6 | 50.0% | 8 | |
| Osteoporosis | Yes | - | - | 2 | 15.4% | - | - | 2 |
| No | 12 | 100.0% | 11 | 84.6% | 12 | 100.0% | 11 | |
| Depression | Yes | 4 | 33.3% | 2 | 15.4% | 4 | 33.3% | 2 |
| No | 8 | 66.7% | 11 | 84.6% | 8 | 66.7% | 11 | |
| Age at the onset of menopause (years) | Average | 44.5 | 47.8 | 46.2 | 0.366 b | |||
| Median | 47.5 | 49.0 | 49.0 | |||||
| Minimum | 30.0 | 41.0 | 30.0 | |||||
| Maximum | 51.0 | 54.0 | 54.0 | |||||
| Standard deviation | 7.0 | 4.0 | 5.8 | |||||
| Estrogen Group | Laser Group | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | End | Initial | End | Initial | End | |||||||
| Estrogen receptor | ||||||||||||
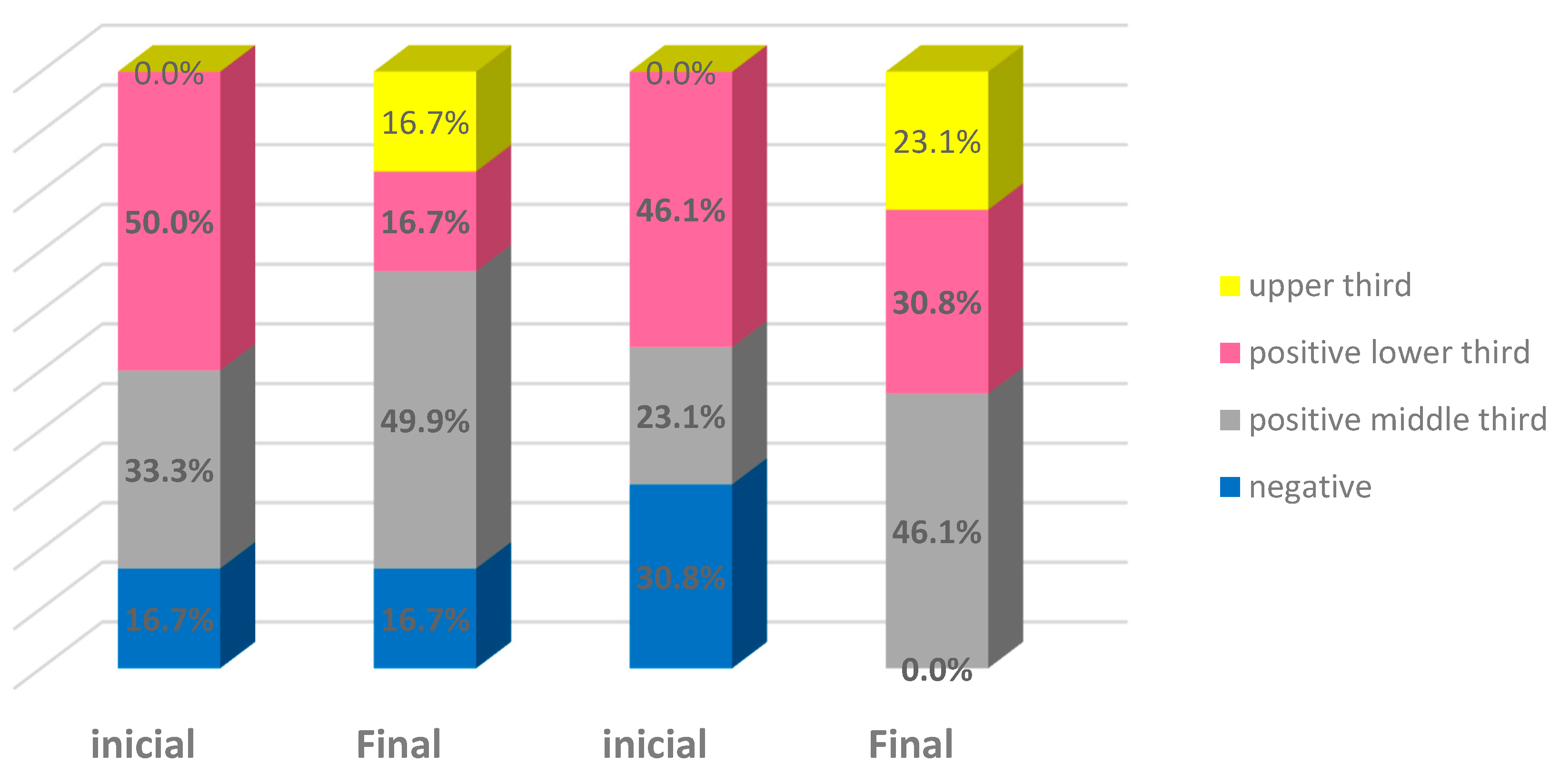
| Negative (score of 0) | 2 | 16.7% | 2 | 16.7% | 4 | 30.8% | - | - | 6 | 24.0% | 2 | 8.0% |
| Positive in lower layer (score of 1) | 6 | 50.0% | 2 | 16.7% | 6 | 46.2% | 4 | 30.8% | 12 | 48.0% | 6 | 24.0% |
| Positive up to the middle third (score of 2) | 4 | 33.3% | 6 | 50.0% | 3 | 23.1% | 6 | 46.2% | 7 | 28.0% | 12 | 48.0% |
| Positive up to the upper third (score of 3) | - | - | 2 | 16.7% | - | - | 3 | 23.1% | - | - | 5 | 20.0% |
| Total | 12 | 100.0% | 12 | 100.0% | 13 | 100.0% | 13 | 100.0% | 25 | 100.0% | 25 | 100.0% |
| Progesterone receptor | ||||||||||||
| Negative (score of 0) | 12 | 100.0% | 10 | 83.3% | 10 | 76.9% | 10 | 76.9% | 22 | 88.0% | 20 | 80.0% |
| Positive in lower layer (score of 1) | - | - | 2 | 16.7% | 3 | 23.1% | 2 | 15.4% | 3 | 12.0% | 4 | 16.0% |
| Positive up to the middle third (score of 2) | - | - | - | - | - | - | 1 | 7.7% | - | - | 1 | 4.0% |
| Estrogen Group | ||||
|---|---|---|---|---|
| Time | Correlated pairs | S * | CI ** | p |
| Initial | Thickness x estrogen | 0.118 | −0.489; 0.648 | 0.715 |
| Thickness x progesterone | - | - | - | |
| Final | Thickness x estrogen | −0.171 | −0.647; 0.490 | 0.596 |
| Thickness x progesterone | 0.468 | −0.145; 0.821 | 0.125 | |
| Laser group | ||||
| Time | Correlated pairs | S * | CI ** | p |
| Initial | Thickness x estrogen | 0.217 | −0.379; 0.689 | 0.477 |
| Thickness x progesterone | −0.100 | −0.617; 0.477 | 0.745 | |
| Final | Thickness x estrogen | 0.331 | −0.269; 0.746 | 0.270 |
| Thickness x progesterone | −0.203 | −0.678; 0.392 | 0.506 | |
| Estrogen + laser | ||||
| Time | Correlated pairs | S * | CI ** | p |
| Initial | Thickness x estrogen | 0.208 | −0.089; 0.633 | 0.317 |
| Thickness x progesterone | −0.140 | −0.507; 0.270 | 0.504 | |
| Final | Thickness x estrogen | 0.150 | −0.261; 0.515 | 0.475 |
| Thickness x progesterone | −0.116 | −0.489; 0.293 | 0.581 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, S.C.; Heinke, T.; Dutra, P.F.S.P.; Carmo, A.; Salmeron, C.; Karoleski, L.; Focchi, G.; Speck, N.M.G.; Pennati, B.M.; Silva, I. Efficacy of Fractional Laser on Steroid Receptors in GSM Patients. Bioengineering 2023, 10, 1087. https://doi.org/10.3390/bioengineering10091087
Pinho SC, Heinke T, Dutra PFSP, Carmo A, Salmeron C, Karoleski L, Focchi G, Speck NMG, Pennati BM, Silva I. Efficacy of Fractional Laser on Steroid Receptors in GSM Patients. Bioengineering. 2023; 10(9):1087. https://doi.org/10.3390/bioengineering10091087
Chicago/Turabian StylePinho, Stella Catunda, Thais Heinke, Paula Fernanda Santos Pallone Dutra, Andreia Carmo, Camilla Salmeron, Luciana Karoleski, Gustavo Focchi, Neila Maria Góis Speck, Beatrice Marina Pennati, and Ivaldo Silva. 2023. "Efficacy of Fractional Laser on Steroid Receptors in GSM Patients" Bioengineering 10, no. 9: 1087. https://doi.org/10.3390/bioengineering10091087
APA StylePinho, S. C., Heinke, T., Dutra, P. F. S. P., Carmo, A., Salmeron, C., Karoleski, L., Focchi, G., Speck, N. M. G., Pennati, B. M., & Silva, I. (2023). Efficacy of Fractional Laser on Steroid Receptors in GSM Patients. Bioengineering, 10(9), 1087. https://doi.org/10.3390/bioengineering10091087








