Ultra-Low Intensity Post-Pulse Affects Cellular Responses Caused by Nanosecond Pulsed Electric Fields
Abstract
:1. Introduction
2. Results
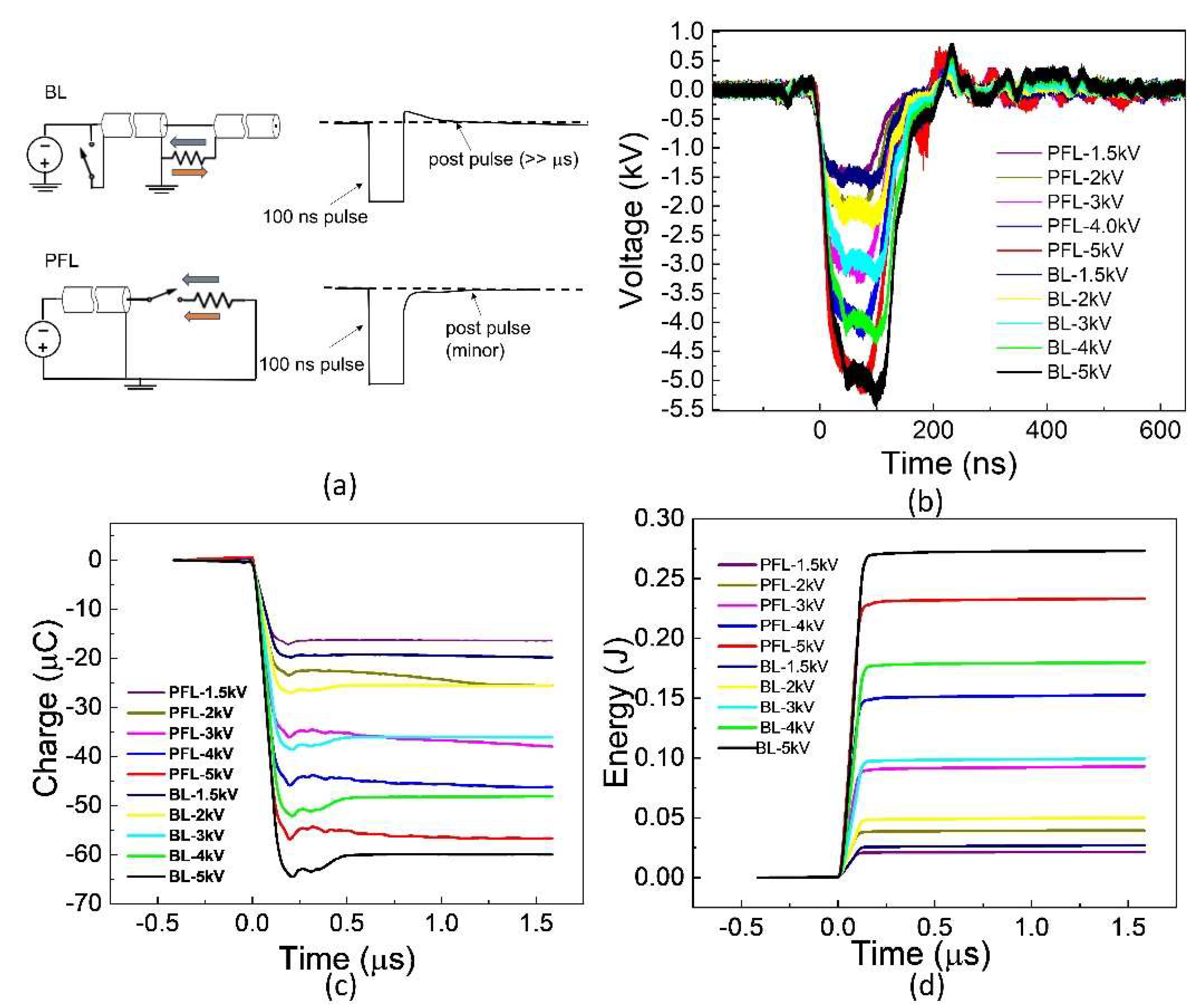
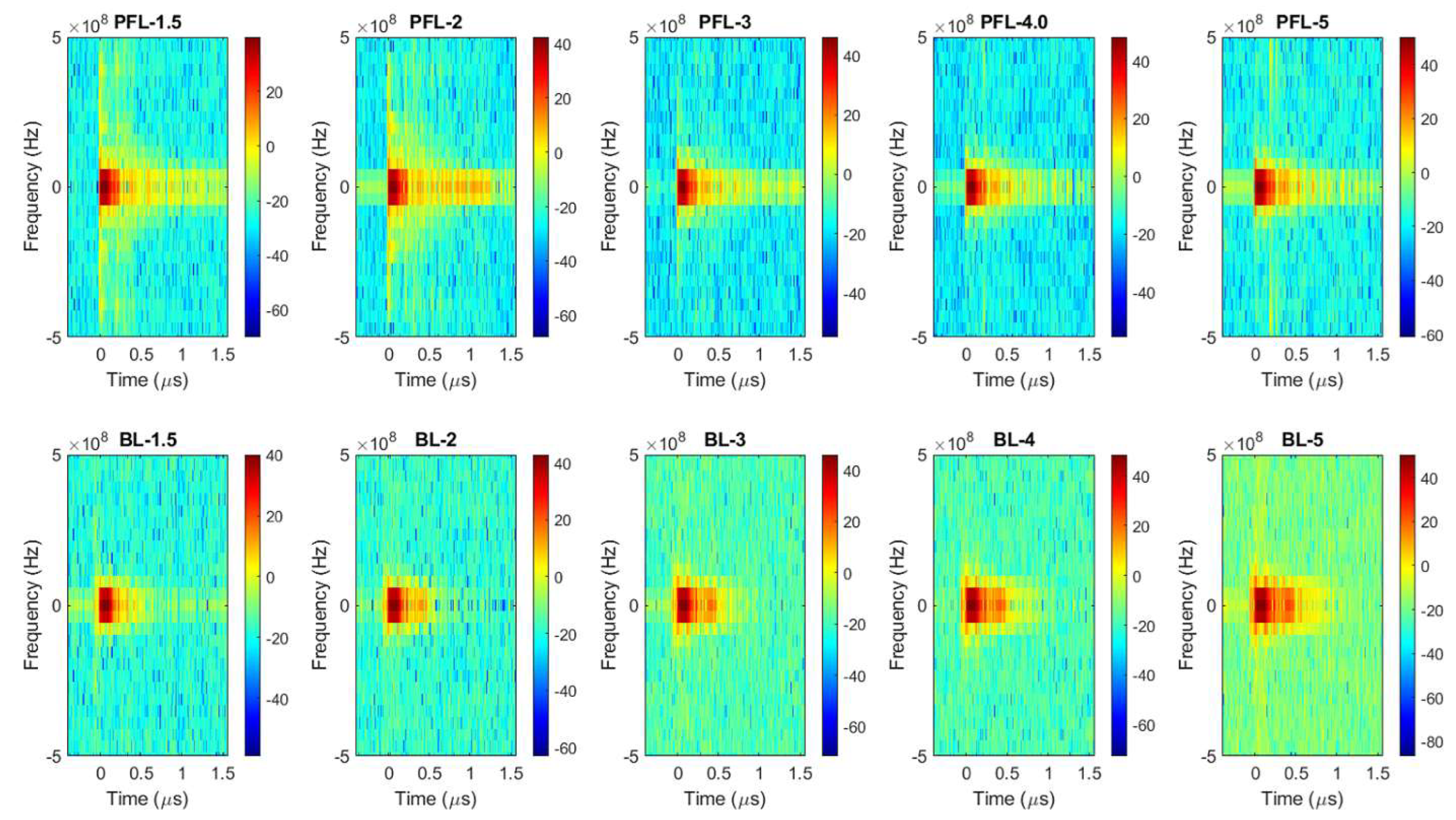
2.1. BL Had a Low-Intensity Post-Pulse Opposite to the Main Pulse, Contrary to PFL, despite Having the Same Main Pulse
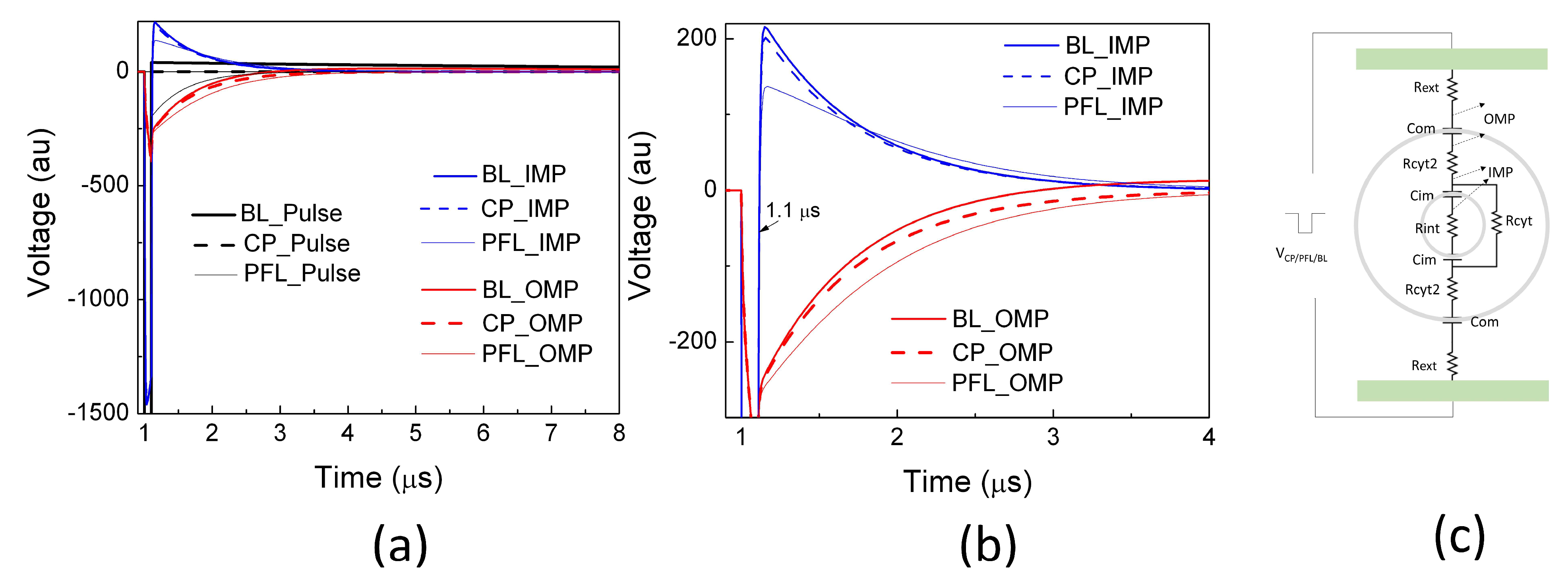
2.2. PFL Pulses Extended the Duration of OMP and Posed Less Change on IMP than BL Pulses
2.3. Effects of PFL and BL Pulsers on Cellular Plasma Membrane Responses
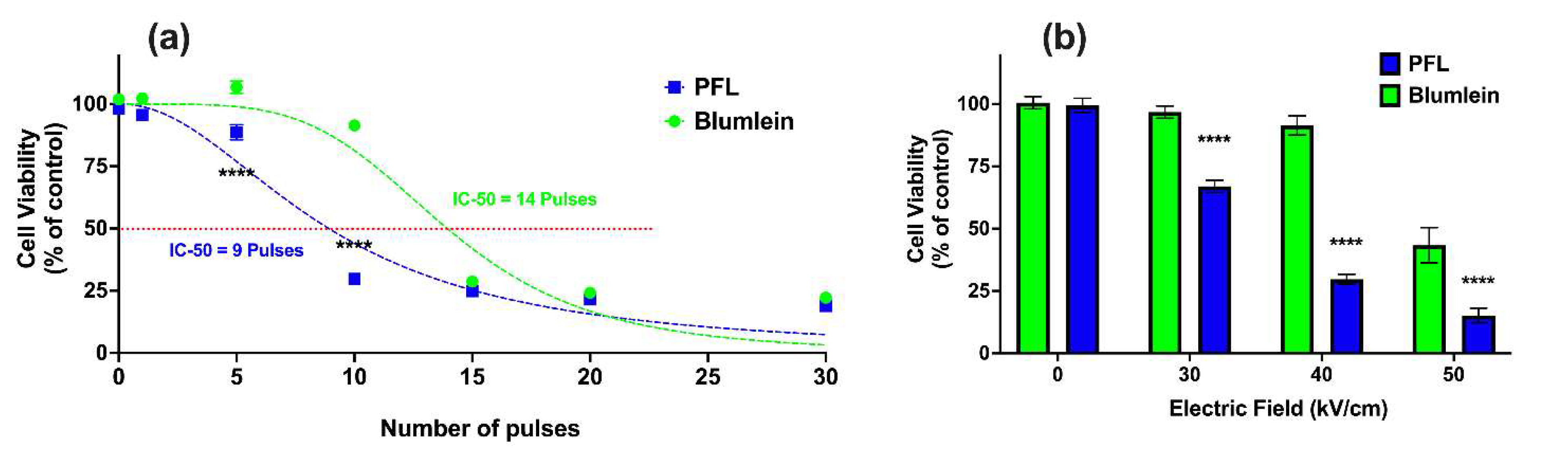
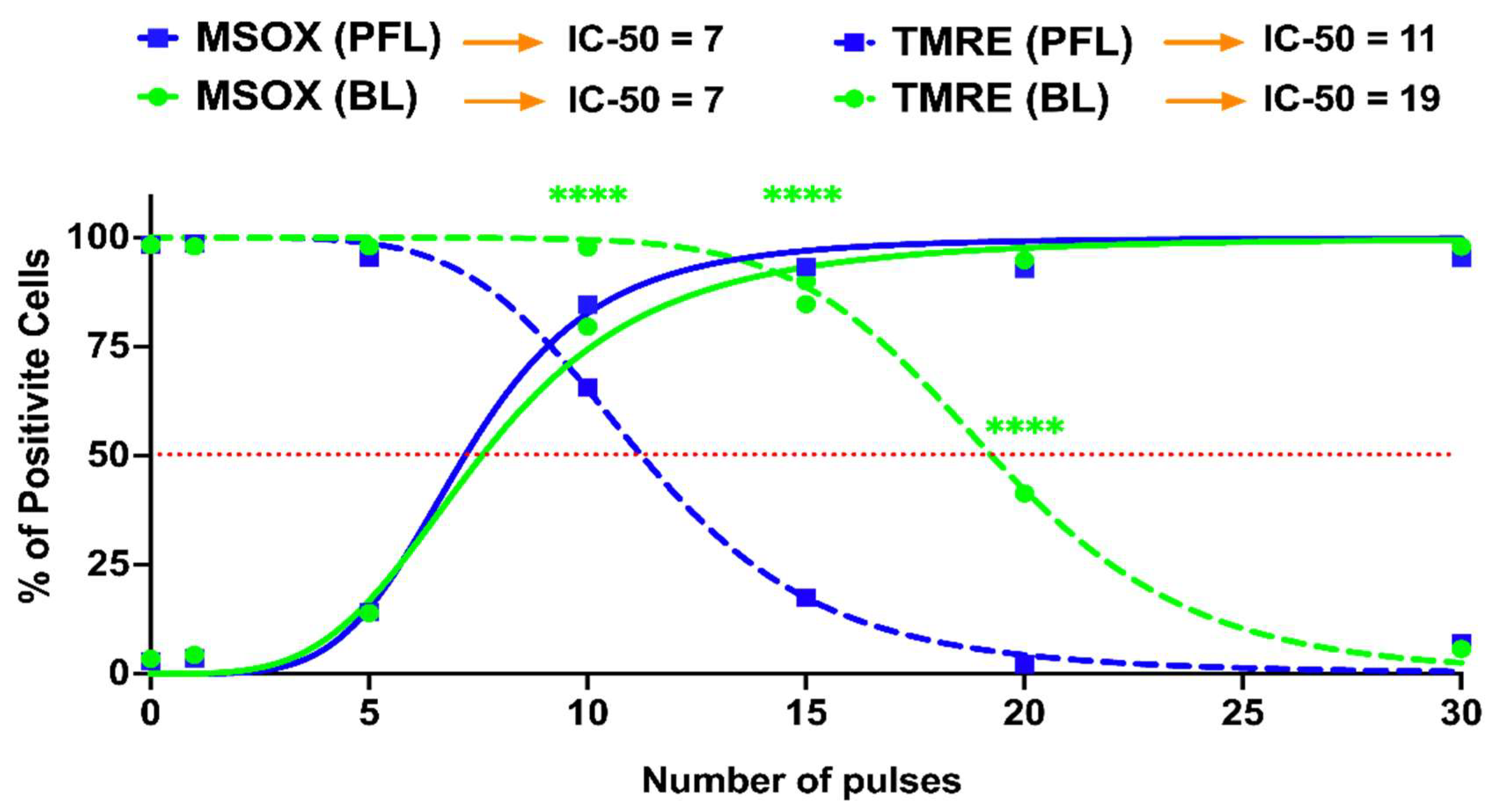
2.4. PFL Has a Lower IC 50 for Cell Death Induction than BL
2.5. Differential Loss of ΔΨm with Increases in mROS Production with the PFL and the BL Pulsers
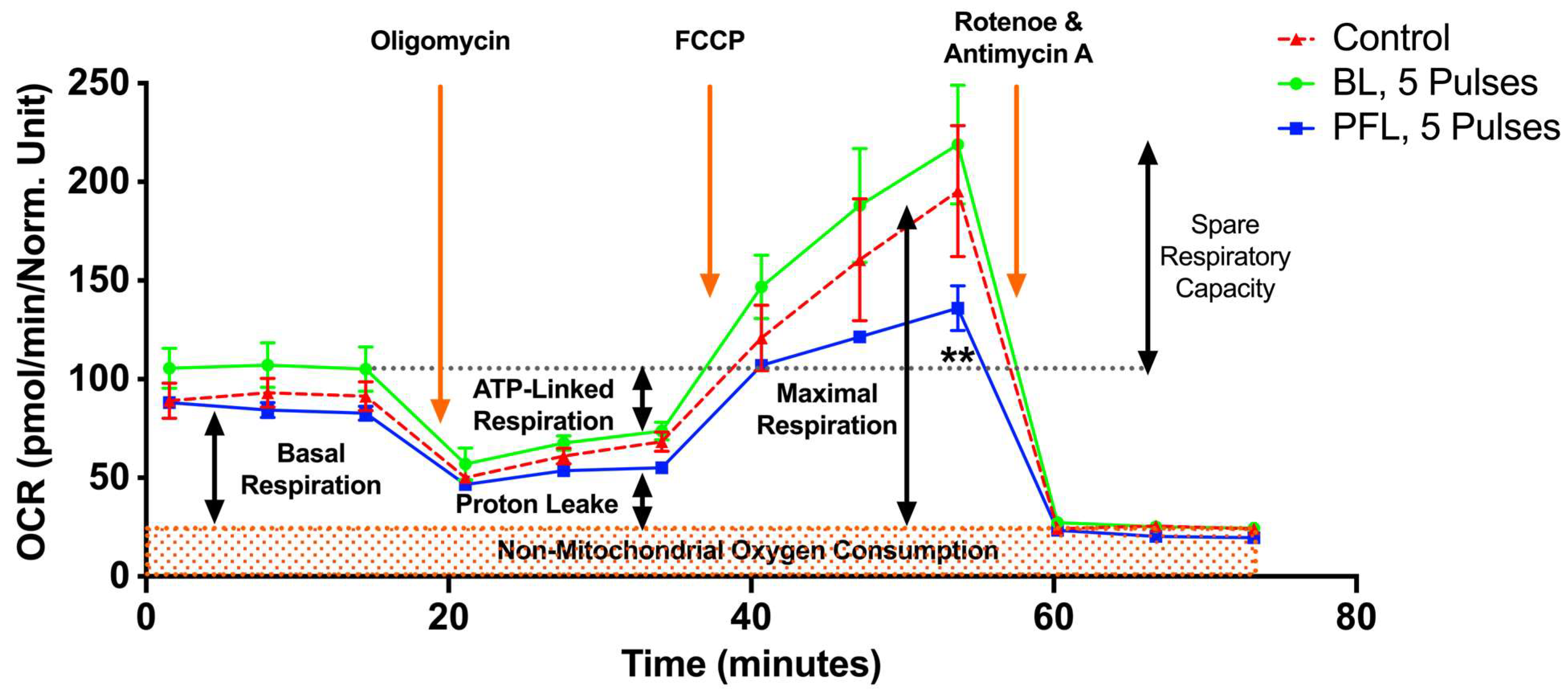
2.6. PFL but Not BL Caused a Decrease in Maximal OCR and Spare Respiratory Capacity (SRC)
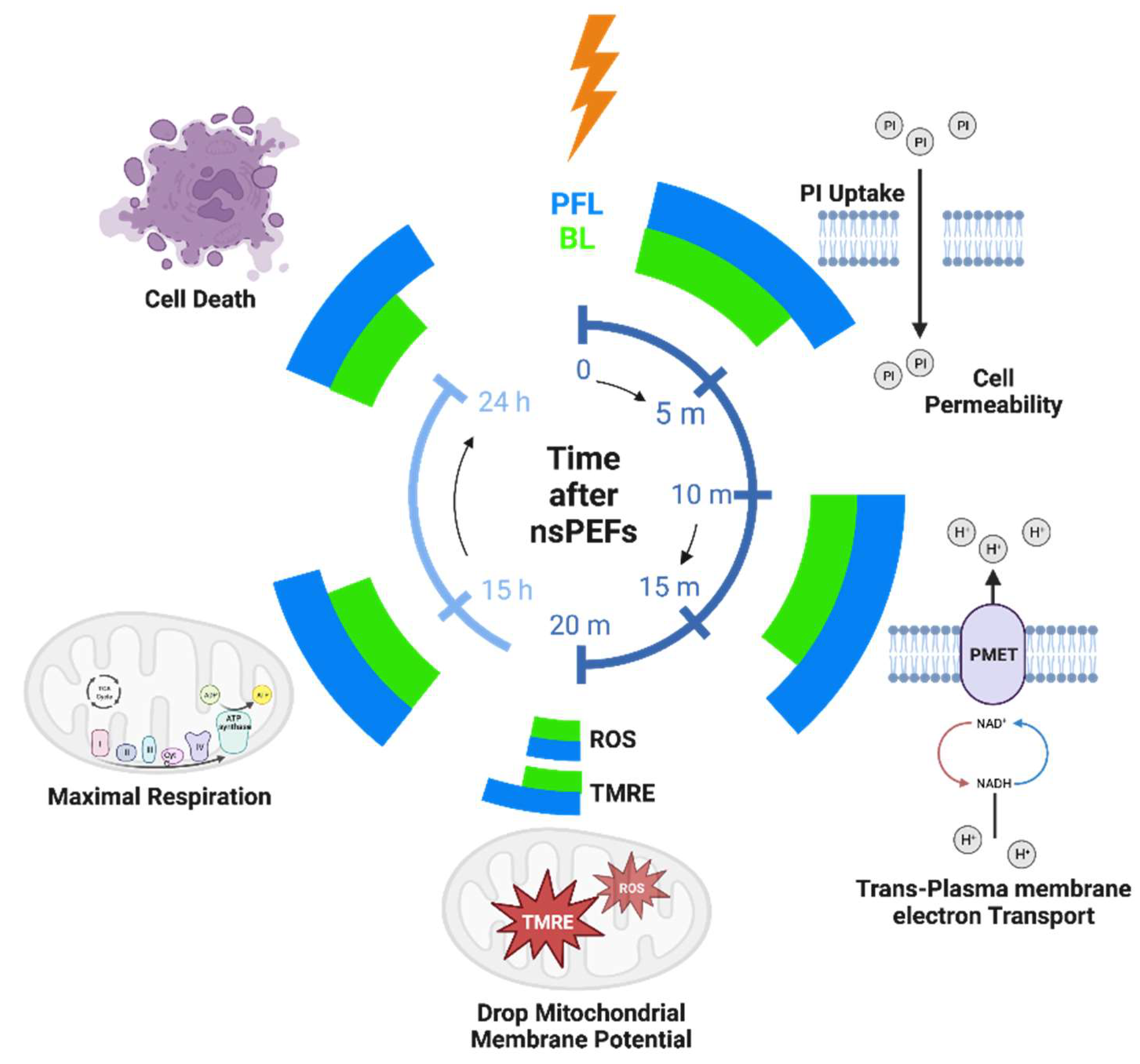
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Experimental Conditions and Protocols
Pulse generators and Cell Exposure System
5.2. Cell Culture
5.3. tPMET Rate Determination
5.4. Cell Viability Analysis
5.5. Flow Cytometry
5.6. Seahorse Assay
5.7. Statistics Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beebe, S.J.; Joshi, R.; Schoenbach, K.H.; Xiao, S. Ultrashort Electric Pulse Effects in Biology and Medicine; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Joshi, R.P.; Garner, A.L.; Sundararajan, R. Review of Developments in Bioelectrics as an Application of Pulsed Power Technology. IEEE Trans. Plasma Sci. 2023, 51, 1682–1717. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Kolb, J.F.; White, J.A.; Joshi, R.P.; Xiao, S.; Schoenbach, K.H. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2007, 28, 655–663. [Google Scholar] [CrossRef]
- Beebe, S.J.; Schoenbach, K.H. Nanosecond Pulsed Electric Fields: A New Stimulus to Activate Intracellular Signaling. J. Biomed. Biotechnol. 2005, 2005, 297–300. [Google Scholar] [CrossRef]
- Casciati, A.; Tanori, M.; Gianlorenzi, I.; Rampazzo, E.; Persano, L.; Viola, G.; Cani, A.; Bresolin, S.; Marino, C.; Mancuso, M.; et al. Effects of Ultra-Short Pulsed Electric Field Exposure on Glioblastoma Cells. Int. J. Mol. Sci. 2022, 23, 3001. [Google Scholar] [CrossRef]
- Beebe, S.; Fox, P.; Rec, L.; Somers, K.; Stark, R.; Schoenbach, K. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition. IEEE Trans. Plasma Sci. 2002, 30, 286–292. [Google Scholar] [CrossRef]
- McDaniel, A.; Freimark, B.; Navarro, C.; Von Rothstein, K.; Gonzalez, D.; Linder, K.; Nuccitelli, R. Nano-pulse stimulation™ therapy (NPS™) is superior to cryoablation in clearing murine melanoma tumors. Front. Oncol. 2023, 12, 948472. [Google Scholar] [CrossRef]
- Xie, F.; Varghese, F.; Pakhomov, A.G.; Semenov, I.; Xiao, S.; Philpott, J.; Zemlin, C. Ablation of Myocardial Tissue with Nanosecond Pulsed Electric Fields. PLoS ONE 2015, 10, e0144833. [Google Scholar] [CrossRef]
- Zemlin, C.; Xie, F.; Xiao, S. Optimal Parameters for the Ablation of Myocardial Tissue with Pulsed Electric Fields. In Proceedings of the 2013 American Heart Association Scientific Sessions, Dallas, TX, USA, 16–20 November 2013. [Google Scholar]
- Chen, R.; Sain, N.M.; Harlow, K.T.; Chen, Y.-J.; Shires, P.K.; Heller, R.; Beebe, S.J. A protective effect after clearance of orthotopic rat hepatocellular carcinoma by nanosecond pulsed electric fields. Eur. J. Cancer 2014, 50, 2705–2713. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Tran, K.; Lui, K.; Huynh, J.; Athos, B.; Kreis, M.; Nuccitelli, P.; De Fabo, E.C. Non-thermal Nanoelectroablation of UV-induced Murine Melanomas Stimulates an Immune Response. Pigment. Cell Melanoma Res. 2012, 25, 618–629. [Google Scholar] [CrossRef]
- Guo, S.; Jing, Y.; Burcus, N.I.; Lassiter, B.P.; Tanaz, R.; Heller, R.; Beebe, S.J. Nano-pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. Int. J. Cancer 2018, 142, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Burcus, N.I.; Hornef, J.; Jing, Y.; Jiang, C.; Heller, R.; Beebe, S.J. Nano-Pulse Stimulation for the Treatment of Pancreatic Cancer and the Changes in Immune Profile. Cancers 2018, 10, 217. [Google Scholar] [CrossRef]
- Romeo, S.; Sarti, M.; Scarfì, M.R.; Zeni, L. Modified Blumlein Pulse-Forming Networks for Bioelectrical Applications. J. Membr. Biol. 2010, 236, 55–60. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, S.; White, J.A.; Kolb, J.F.; Stacey, M.; Schoenbach, K.H. Compact, nanosecond, high repetition rate, pulse generator for bioelectric studies. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 863–870. [Google Scholar] [CrossRef]
- Kenaan, M.; El Amari, S.; Silve, A.; Merla, C.; Mir, L.M.; Couderc, V.; Arnaud-Cormos, D.; Leveque, P. Characterization of a 50-Ω Exposure Setup for High-Voltage Nanosecond Pulsed Electric Field Bioexperiments. IEEE Trans. Biomed. Eng. 2011, 58, 207–214. [Google Scholar] [CrossRef]
- Xiao, S.; Zhou, C.; Yang, E.; Rajulapati, S.R. Nanosecond bipolar pulse generators for bioelectrics. Bioelectrochemistry 2018, 123, 77–87. [Google Scholar] [CrossRef]
- Ma, J.; Yu, L.; Ren, L.; Liao, Y.; Dong, S.; Yao, C. High Voltage Nanosecond Pulse Generator based on Inductive Energy Storage with Adjustable Pulse Width. In Proceedings of the 2022 IEEE International Conference on High Voltage Engineering and Applications (ICHVE), Chongqing, China, 25–29 September 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Butkus, P.; Murauskas, A.; Tolvaišienė, S.; Novickij, V. Concepts and Capabilities of In-House Built Nanosecond Pulsed Electric Field (nsPEF) Generators for Electroporation: State of Art. Appl. Sci. 2020, 10, 4244. [Google Scholar] [CrossRef]
- Merla, C.; El Amari, S.; Kenaan, M.; Liberti, M.; Apollonio, F.; Arnaud-Cormos, D.; Couderc, V.; Leveque, P. A 10-Ω High-Voltage Nanosecond Pulse Generator. IEEE Trans. Microw. Theory Tech. 2010, 58, 4079–4085. [Google Scholar] [CrossRef]
- Kandratsyeu, A.; Sabaleuski, U.; Redondo, L.; Pakhomov, A.G. Four Channel 6.5 kV, 65 A, 100 ns–100 µs Generator with Advanced Control of Pulse and Burst Protocols for Biomedical and Biotechnological Applications. Appl. Sci. 2021, 11, 11782. [Google Scholar] [CrossRef]
- Garner, A.L.; Caiafa, A.; Jiang, Y.; Klopman, S.; Morton, C.; Torres, A.S.; Loveless, A.M.; Neculaes, V.B. Design, characterization and experimental validation of a compact, flexible pulsed power architecture for ex vivo platelet activation. PLoS ONE 2017, 12, e0181214. [Google Scholar] [CrossRef]
- Urabe, G.; Sato, T.; Nakamura, G.; Kobashigawa, Y.; Morioka, H.; Katsuki, S. 1.2 MV/cm pulsed electric fields promote transthyretin aggregate degradation. Sci. Rep. 2020, 10, 12003. [Google Scholar] [CrossRef]
- Yao, C.; Dong, S.; Zhao, Y.; Mi, Y.; Li, C. A Novel Configuration of Modular Bipolar Pulse Generator Topology Based on Marx Generator with Double Power Charging. IEEE Trans. Plasma Sci. 2016, 44, 1872–1878. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, X.; Guo, F.; Dong, S.; Mi, Y.; Sun, C. FPGA-Controlled All-Solid-State Nanosecond Pulse Generator for Biological Applications. IEEE Trans. Plasma Sci. 2012, 40, 2366–2372. [Google Scholar] [CrossRef]
- Beebe, S.J.; Chen, Y.-J.; Sain, N.M.; Schoenbach, K.H.; Xiao, S. Transient Features in Nanosecond Pulsed Electric Fields Differentially Modulate Mitochondria and Viability. PLoS ONE 2012, 7, e51349. [Google Scholar] [CrossRef]
- Novickij, V.; Rembiałkowska, N.; Szlasa, W.; Kulbacka, J. Does the shape of the electric pulse matter in electroporation? Front. Oncol. 2022, 12, 958128. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Semenov, I.; Xiao, S.; Pakhomova, O.N.; Gregory, B.; Schoenbach, K.H.; Ullery, J.C.; Beier, H.T.; Rajulapati, S.R.; Ibey, B.L. Cancellation of cellular responses to nanoelectroporation by reversing the stimulus polarity. Cell. Mol. Life Sci. 2014, 71, 4431–4441. [Google Scholar] [CrossRef]
- Bagalkot, T.R.; Leblanc, N.; Craviso, G.L. Stimulation or Cancellation of Ca2+ Influx by Bipolar Nanosecond Pulsed Electric Fields in Adrenal Chromaffin Cells Can Be Achieved by Tuning Pulse Waveform. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Valdez, C.M.; Barnes, R.A.; Roth, C.C.; Moen, E.K.; Throckmorton, G.A.; Ibey, B.L. Asymmetrical bipolar nanosecond electric pulse widths modify bipolar cancellation. Sci. Rep. 2017, 7, 16372. [Google Scholar] [CrossRef] [PubMed]
- Kolb, J.F.; Kono, S.; Schoenbach, K.H. Nanosecond pulsed electric field generators for the study of subcellular effects. Bioelectromagnetics 2006, 27, 172–187. [Google Scholar] [CrossRef]
- Sözer, E.B.; Pakhomov, A.G.; Semenov, I.; Casciola, M.; Kim, V.; Vernier, P.T.; Zemlin, C.W. Analysis of electrostimulation and electroporation by high repetition rate bursts of nanosecond stimuli. Bioelectrochemistry 2021, 140, 107811. [Google Scholar] [CrossRef]
- Berridge, M.; Tan, A. Trans-plasma membrane electron transport: A cellular assay for NADH-and NADPH-oxidase based on extracellular, superoxide-mediated reduction of the sulfonated tetrazolium salt WST-1. Protoplasma 1998, 205, 74–82. [Google Scholar] [CrossRef]
- Herst, P.M.; Berridge, M.V. Plasma membrane electron transport: A new target for cancer drug development. Curr. Mol. Med. 2006, 6, 895–904. [Google Scholar] [CrossRef]
- Ly, J.D.; Lawen, A. Transplasma membrane electron transport: Enzymes involved and biological function. Redox Rep. 2003, 8, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Bowman, A.M.; Ibey, B.L.; Andre, F.M.; Pakhomova, O.N.; Schoenbach, K.H. Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem. Biophys. Res. Commun. 2009, 385, 181–186. [Google Scholar] [CrossRef]
- Beebe, S.J.; Lassiter, B.P.; Guo, S. Nanopulse Stimulation (NPS) Induces Tumor Ablation and Immunity in Orthotopic 4T1 Mouse Breast Cancer: A Review. Cancers 2018, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, B.P.; Guo, S.; Beebe, S.J. Nano-Pulse Stimulation Ablates Orthotopic Rat Hepatocellular Carcinoma and Induces Innate and Adaptive Memory Immune Mechanisms that Prevent Recurrence. Cancers 2018, 10, 69. [Google Scholar] [CrossRef]
- Bernardi, P.; Rasola, A.; Forte, M.; Lippe, G.; Wang, D.Z.; Jones, A.W.; Wang, W.Z.; Wang, M.; Korthuis, R.J. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015, 95, 1111–1155. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Pinton, P. The Mitochondrial Permeability Transition Pore and Cancer: Molecular Mechanisms Involved in Cell Death. Front. Oncol. 2014, 4, 302. [Google Scholar] [CrossRef]
- Giorgio, V.; Burchell, V.; Schiavone, M.; Bassot, C.; Minervini, G.; Petronilli, V.; Argenton, F.; Forte, M.; Tosatto, S.; Lippe, G.; et al. Ca(2+) binding to F-ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017, 18, 1065–1076. [Google Scholar] [CrossRef]
- Handy, D.; Loscalzo, J. Redox Regulation of Mitochondrial Function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, B.V. Redox Regulation of the Mitochondrial Permeability Transition Pore. Biosci. Rep. 1997, 17, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Linard, D.; Kandlbinder, A.; Degand, H.; Morsomme, P.; Dietz, K.J.; Knoops, B. Redox characterization of human cyclophilin D: Identification of a new mammalian mitochondrial redox sensor? Arch. Biochem. Biophys. 2009, 491, 39–45. [Google Scholar] [CrossRef]
- Liu, S.; Feng, G.; Wang, G.L.; Liu, G.J. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur. J. Pharmacol. 2008, 584, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Casciola, M.; Xiao, S.; Apollonio, F.; Paffi, A.; Liberti, M.; Muratori, C.; Pakhomov, A.G. Cancellation of nerve excitation by the reversal of nanosecond stimulus polarity and its relevance to the gating time of sodium channels. Cell. Mol. Life Sci. 2019, 76, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Sadik, M.M.; Yu, M.; Zheng, M.; Zahn, J.D.; Shan, J.W.; Shreiber, D.I.; Lin, H. Scaling Relationship and Optimization of Double-Pulse Electroporation. Biophys. J. 2014, 106, 801–812. [Google Scholar] [CrossRef]
- Arena, C.B.; Sano, M.B.; Rylander, M.N.; Davalos, R.V. Theoretical Considerations of Tissue Electroporation With High-Frequency Bipolar Pulses. IEEE Trans. Biomed. Eng. 2010, 58, 1474–1482. [Google Scholar] [CrossRef]
- Sano, M.B.; DeWitt, M.R.; Teeter, S.D.; Xing, L. Optimization of a single insertion electrode array for the creation of clinically relevant ablations using high-frequency irreversible electroporation. Comput. Biol. Med. 2018, 95, 107–117. [Google Scholar] [CrossRef]
- Boye, C.; Christensen, K.; Asadipour, K.; DeClemente, S.; Francis, M.; Bulysheva, A. Gene electrotransfer of FGF2 enhances collagen scaffold biocompatibility. Bioelectrochemistry 2021, 144, 107980. [Google Scholar] [CrossRef]
- Ford, W.E.; Ren, W.; Blackmore, P.F.; Schoenbach, K.H.; Beebe, S.J. Nanosecond pulsed electric fields stimulate apoptosis without release of pro-apoptotic factors from mitochondria in B16f10 melanoma. Arch. Biochem. Biophys. 2010, 497, 82–89. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asadipour, K.; Zhou, C.; Yi, V.; Beebe, S.J.; Xiao, S. Ultra-Low Intensity Post-Pulse Affects Cellular Responses Caused by Nanosecond Pulsed Electric Fields. Bioengineering 2023, 10, 1069. https://doi.org/10.3390/bioengineering10091069
Asadipour K, Zhou C, Yi V, Beebe SJ, Xiao S. Ultra-Low Intensity Post-Pulse Affects Cellular Responses Caused by Nanosecond Pulsed Electric Fields. Bioengineering. 2023; 10(9):1069. https://doi.org/10.3390/bioengineering10091069
Chicago/Turabian StyleAsadipour, Kamal, Carol Zhou, Vincent Yi, Stephen J. Beebe, and Shu Xiao. 2023. "Ultra-Low Intensity Post-Pulse Affects Cellular Responses Caused by Nanosecond Pulsed Electric Fields" Bioengineering 10, no. 9: 1069. https://doi.org/10.3390/bioengineering10091069
APA StyleAsadipour, K., Zhou, C., Yi, V., Beebe, S. J., & Xiao, S. (2023). Ultra-Low Intensity Post-Pulse Affects Cellular Responses Caused by Nanosecond Pulsed Electric Fields. Bioengineering, 10(9), 1069. https://doi.org/10.3390/bioengineering10091069






