1. Introduction
Atherosclerosis is a progressive disease characterised by the accumulation of lipids, fibrous materials, and minerals in the arteries, leading to the formation of plaque. Plaque disruption exposes thrombogenic matrix proteins, e.g., von Willebrand factor (VWF), fibrin, fibrinogen, thrombospondin, vitronectin, fibronectin, and collagens, etc., which can recruit and activate circulating platelets. This leads to arterial thrombosis (AT) and may block arteries and cause blood circulation impairment [
1]. AT contributes to cardiovascular diseases (myocardial infarction and ischemic stroke) upon plaque disruption [
2,
3]. Globally, AT is one of the leading causes of morbidity and mortality [
4]. Recruitment of platelets in the plaque rupture site initiates thrombus formation and can obstruct blood flow [
5]. Due to this fact, platelets become an important target to prevent arterial blockage during plaque disruption in atherosclerosis [
6].
Currently, diagnosis of AT relies on several techniques, such as X-ray angiography, magnetic resonance angiography (MRA), or coronary computerised tomography angiography (CTA). However, most of them are highly invasive [
7]. Furthermore, a potential risk is associated with unwanted plaque rupture during measurement, as in the case of angiographic techniques [
8]. Hence, a suitable therapy is immediately recommended, followed by a detection. One of the most sensitive and reliable non-invasive diagnostic techniques is cardiac magnetic resonance imaging (CMRI). CMRI is a painless, accurate, and precise way to identify high-risk atherosclerotic plaque [
9,
10]. CMRI has been well-accepted among clinicians irrespective of the risks associated with the contrast agents used. Gadolinium is the most commonly used contrast agent for MRI but is associated with severe toxic effects as it cannot be metabolised in the body and is retained long after completion of the MRI scan [
11]. Super-Paramagnetic Iron Oxide Nanoparticles (SPIONs) are emerging as a potential alternative to minimise such toxic side effects owing to their promising metabolic profiles inside the body [
12], along with their high spatial resolution in magnetic imaging. We have rationally chosen SPIONs because of their safety index and clinical use. FDA has already approved several iron oxide nanoparticle-based systems, such as Venofer
®, Ferrlecit
®, INFed
®, Dexferrum
®, and Feraheme
®, etc., to name a few. However, similar to other nanosystems, bare SPIONs are potential activators of platelets in specific conditions [
13]. Thus, thrombus detection using safer SPION conjugates to avoid cardiovascular events is very much needed.
In this proof-of-concept study, we have described the development of a bio-compatible, SPION-based theranostic nanosystem (Tx@ReoPro) with dual properties (i) to act as a therapeutic agent against thrombus growth and (ii) to monitor the growth restriction. We have confirmed that Tx@ReoPro does not exhibit any pro-aggregatory effect on platelets but rather restricts thrombus growth effectively by inhibiting platelet aggregation. Our synthesised Tx@ReoPro comprises the model companion drug ReoPro (or Abciximab, a clinically approved antiplatelet drug that has been prescribed during percutaneous coronary interventions (PCI). ReoPro has the potential to regulate thrombus growth, and MRI-sensitive SPIONs function as contrast agents for MRI for real-time disease surveillance. To the best of our knowledge, this is the first-ever feasibility study reporting on a theranostic approach that includes both AT detection and therapy concurrently in an in vitro set-up.
2. Materials and Methods
2.1. SPION Synthesis by Thermal Decomposition Method
SPIONs were synthesised by the previously reported thermal decomposition method [
14]. Briefly, 1.667 g of iron acetylacetonate (Sigma-Aldrich, Saint Louis, MO, USA) was mixed with 25 mL benzyl ether (Sigma-Aldrich, Saint Louis, MO, USA) and 25 mL oleylamine (Sigma-Aldrich, Saint Louis, MO, USA). The mixture was then heated at 110 °C in the presence of nitrogen purging, which afterwards was increased to 310 °C and continued for 2 hrs in reflux condition in a rotating heating mantle. After cooling down to room temperature, 60 mL ethanol was added to it. The final suspension was then centrifuged at 3000×
g for 20 min, and the supernatant was discarded. The precipitate was allowed to air dry and was finally resuspended in 20 mL hexane (Sigma-Aldrich, Saint Louis, MO, USA). The hexane suspension was then centrifuged at 25,000×
g for 1 hr to pellet the larger particles, and the supernatant was collected for further modification.
2.2. Bio-Conjugation of Iron Nanoparticles
An amount of 200 mg polyethylene glycol dicarboxylic acid (HOOC-PEG-COOH, 2kd) (JenKem Technology, Allen TX, USA), 9 mL di-methyl formamide (DMF) (Sigma-Aldrich, Saint Louis, MO, USA), and 20 mL chloroform (Sigma-Aldrich, Saint Louis, MO, USA) were mixed in a reaction flask. Then, 50 mg NHS (N-Hydroxysuccinimide) and 50 mg EDC (1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide, (both from Thermo Fisher Scientific, Waltham, MA, USA) were dissolved separately in 330 μL di-methyl formamide (DMF) and added to the reaction flask. The mixture was then stirred at room temperature for 30 min. To that, 11 mg dopamine (Sigma-Aldrich, Saint Louis, MO, USA) dissolved in 330 μL DMF was added and stirred for 90 min. The above-mentioned oleylamine-coated iron oxide nanoparticles (8 to 10 mg of Fe3+ contained in 8 mL) were then added to the dopamine mixture and kept at room temperature with stirring conditions for 5 hrs. Hexane (Sigma-Aldrich, Saint Louis, MO, USA) was then added to the reaction mixture to make the final volume of 100 mL, which allowed for particles to settle down in the hexane medium. The precipitate was washed with hexane and air dried for 10–20 min. The pellet was resuspended in milliQ water, and the mixture was centrifuged at 800× g for 20 min to remove larger aggregates. The resultant supernatant was then centrifuged again at 800× g for 40 min with 10 kd nano-sep (Pall Pvt Ltd., Mumbai, India) to remove any unreacted PEGs. The final concentration of the SPION was 5 mg/mL.
2.3. Covalent Attachment of Drug to Particles
The SPIONs were suspended in 2-(N-morpholino) ethanesulfonic acid (MES) buffer (0.1 (M), pH 4.5) (Thermo Fisher Scientific, Waltham, MA, USA). 40 mM EDC (Thermo Fisher Scientific, Waltham, MA, USA) was then added to 4 mL of SPION suspension and stirred for 10 min. This was followed by an addition of 10 mM NHS (Thermo Fisher Scientific, Waltham, MA, USA). Both the EDC and NHS were dissolved in MES buffer. The mixture was agitated for 15 min. The solution was centrifuged at 800× g for 40 min to remove excess EDC and NHS. Abciximab (ReoPro, Parchem Chemicals, New York, NY, USA) was added to the particle suspension and stirred overnight. The final concentration of ReoPro was 2 mg/mL. The mixture was centrifuged at 800× g in nano sep (100 kd) for 40 min to remove excess ReoPro, whereafter ReoPro conjugated SPION (Tx@ReoPro) was suspended in PBS buffer (pH~7.3).
2.4. Optical Measurement
The absorbance spectrum of the SPION solution was measured using Thermo Scientific Evolution 300 UV-VIS (Waltham, MA, USA). The hydrodynamic diameter and zeta potential of SPIONs and Tx@ReoPro were measured using Malvern NanoZS [
3].
2.5. Transmission Electron Microscopy of Nanoparticles
Transmission electron microscopy (TEM) was performed at 75 kV for the characterisation of the size and morphology of the nanomaterial. A drop of SPION and Tx@ReoPro solution was placed on a 300 mesh copper grid coated with carbon and dried at room temperature prior to imaging using TEM (FEI Tecnai G2 Galadriel, Hillsboro, OR, USA).
2.6. Fourier Transform Infra-Red Spectroscopy (FTIR) for Conjugated Nanoparticles
PEG-coated SPIONs and Tx@ReoPro were lyophilised (12 Pa, −45 °C) in a lyophiliser (TOKYO RIKAKIKAI CO., LTD, Tokyo, Japan). Approximately 3–4 mg of the amorphous samples were mixed with ~200 mg of Potassium Bromide (KBr) in a mortar pestle. The mixture was pelleted out by applying ~12-ton pressure, and finally, the FT-IR spectrum of nano-conjugants was recorded in transmission mode (Thermo Scientific, Nicolet-6700, Waltham, MA, USA). Each spectrum was recorded after 256 scans and 4 cm−1 wave number resolution at room temperature.
2.7. Magnetic Force Microscopy
Magnetic force microscopic images of SPION and Tx@ReoPro nanoparticles were obtained with the Veeco VI Innova model (Bruker Axs Pvt. Ltd., Mumbai, India) using MESP probes (Co/Cr coated). The frequency range used was from 70–80 kHz; the spring constant(k) was 2.8 N/m; the radius of curvature was 20 nm at 0.5 Hz scan rate with 256 × 256 resolution.
2.8. Light Transmission Aggregometry
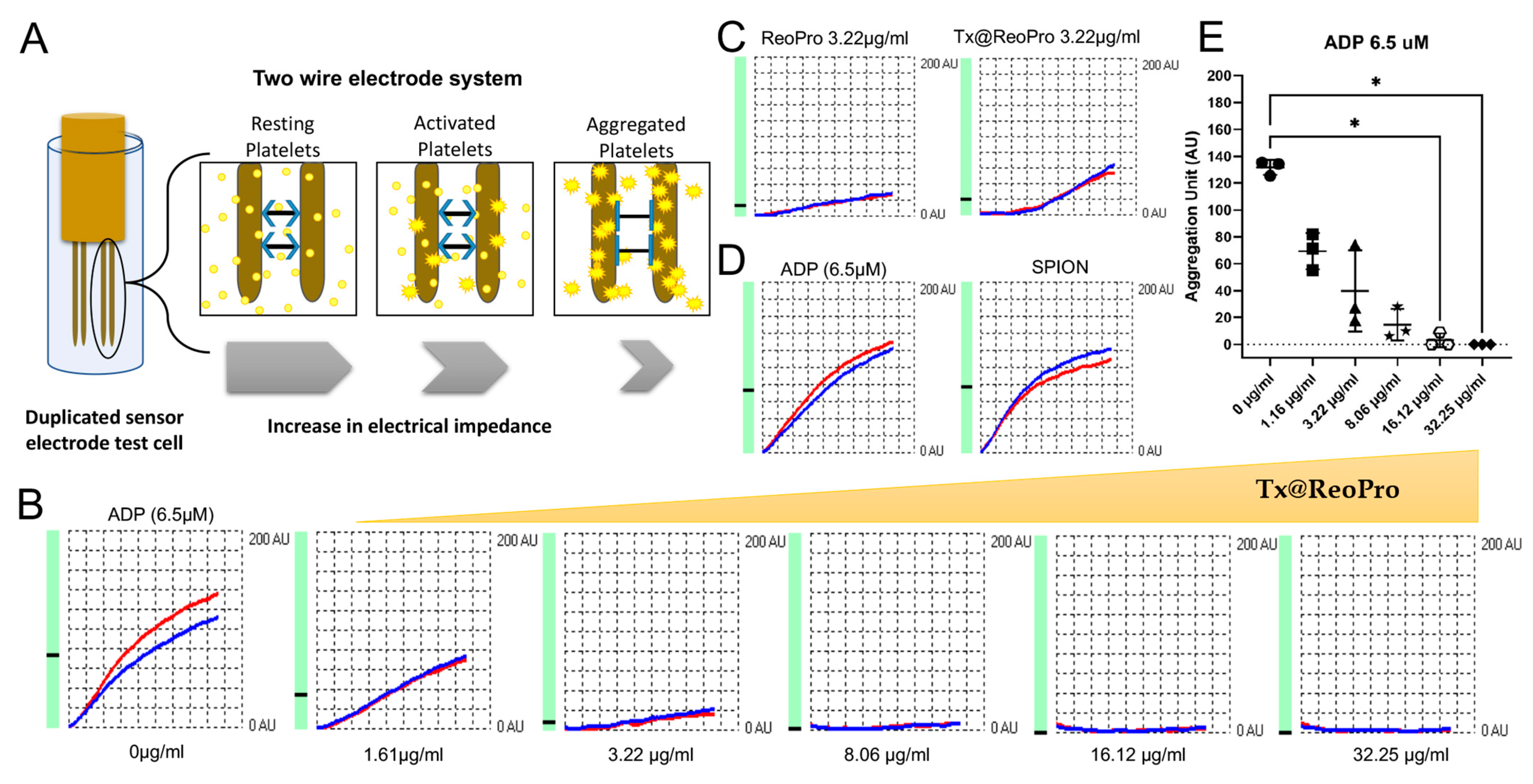
For platelet function studies using light transmission aggregometry, 9.5 mL blood was mixed in 3.2% sodium citrate anticoagulant (final ratio 9:1 whole blood/citrate). Platelet-rich plasma (PRP) was obtained after centrifuging blood at 200× g for 10 min. PPP (platelet-poor plasma) was obtained by centrifugation of blood at 1500× g for 10 min and served as respective blank. The PRP was incubated with PEG-coated SPIONs and Tx@ReoPro (with respective concentrations, mentioned in the figure legend) at 37 °C for 15 min. Platelet aggregation was initiated by ~6 μM ADP and recorded using a Chronolog optical aggregometer (model 700, Havertown, PA, USA).
2.9. Whole Blood Impedance Aggregometry
Whole blood platelet aggregation was studied using a Multiplate® analyser (Roche Diagnostic GmbH, Mannheim, Germany). For this study, venous blood from volunteers was collected in hirudin tubes and allowed to rest for 30 min. Blood was then incubated with PEG-coated SPIONs and Tx@ReoPro (from 1.61 μg/mL to 32.25 μg/mL) at room temperature for 10 min. By that time, 300 μL NaCl was added to the disposable test cells placed in the Multiplate® instrument. Then, 300 μL of hirudinised blood treated with PEG-coated SPIONs and Tx@ReoPro was added to the test cell and incubated for 3 min at 37 °C under stirring conditions. After that, 20 μL of ADP (Multiplate® ADPtest, final concentration 6.5 μM) was added to initiate platelet aggregation. The aggregations were monitored for 6 min. All the reagents used in this experiment were from Roche Diagnostic GmbH, Mannheim, Germany.
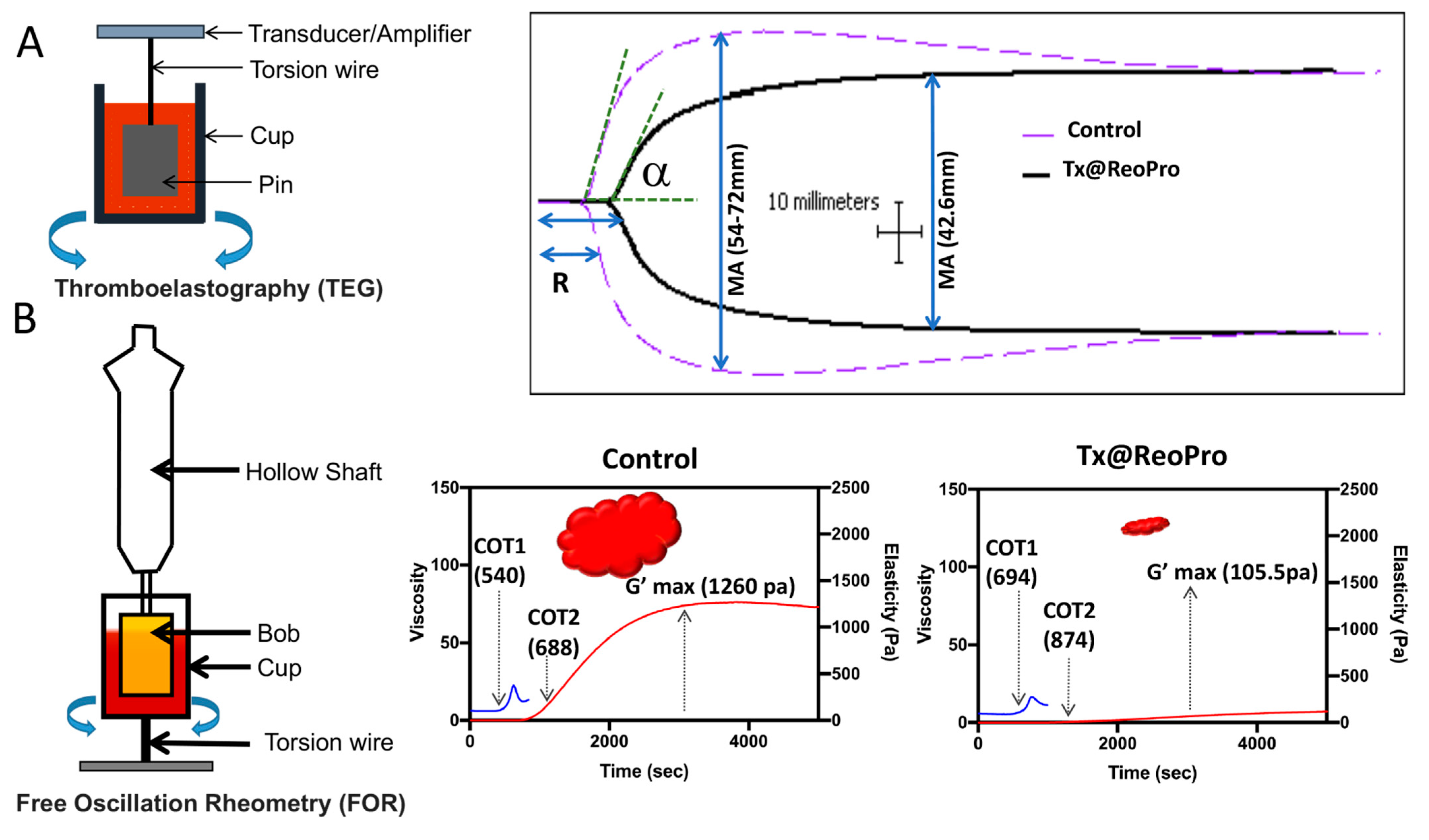
2.10. Free Oscillation Rheometry (FOR)
Viscoelastic whole blood coagulation measurements were performed by Free Oscillation Rheometry—FOR (ReoRox G2, MediRox AB, Nyköping, Sweden). For this study, citrated blood was incubated with PEG-coated SPIONs and Tx@ReoPro (final concentration 80 μg/mL) at room temperature for 20 min. A 50 μL ReoTRAP reagent (from ReoRox kit) and 25 μL 0.5 M CaCl
2 (from ReoRox Kit) were mixed with 1 mL of that treated blood using a disposable 1 mL syringe by gently pipetting up and down. The blood (1 mL) was then added to the reaction chamber, whereby the measurement of viscosity and elasticity was started automatically through the ReoRox G2 software [
15].
2.11. Thromboelastography (TEG)
Analysis of whole blood coagulation was performed by the addition of 1 mL whole blood to TEG kaolin cuvettes (Haemoscope, Skokie, IL, USA). The sample was mixed, and 2 aliquots of 450 μL each of blood were added to Tx@ReoPro (final concentration 80 μg/mL) and PEG-coated SPION (equivalent amount of Tx@ReoPro) separately. From each mixture, 360 μL of the treated blood was added to the TEG assay cups. Then, TEG tracings were recorded to follow the whole blood coagulation process.
2.12. Flow Cytometry
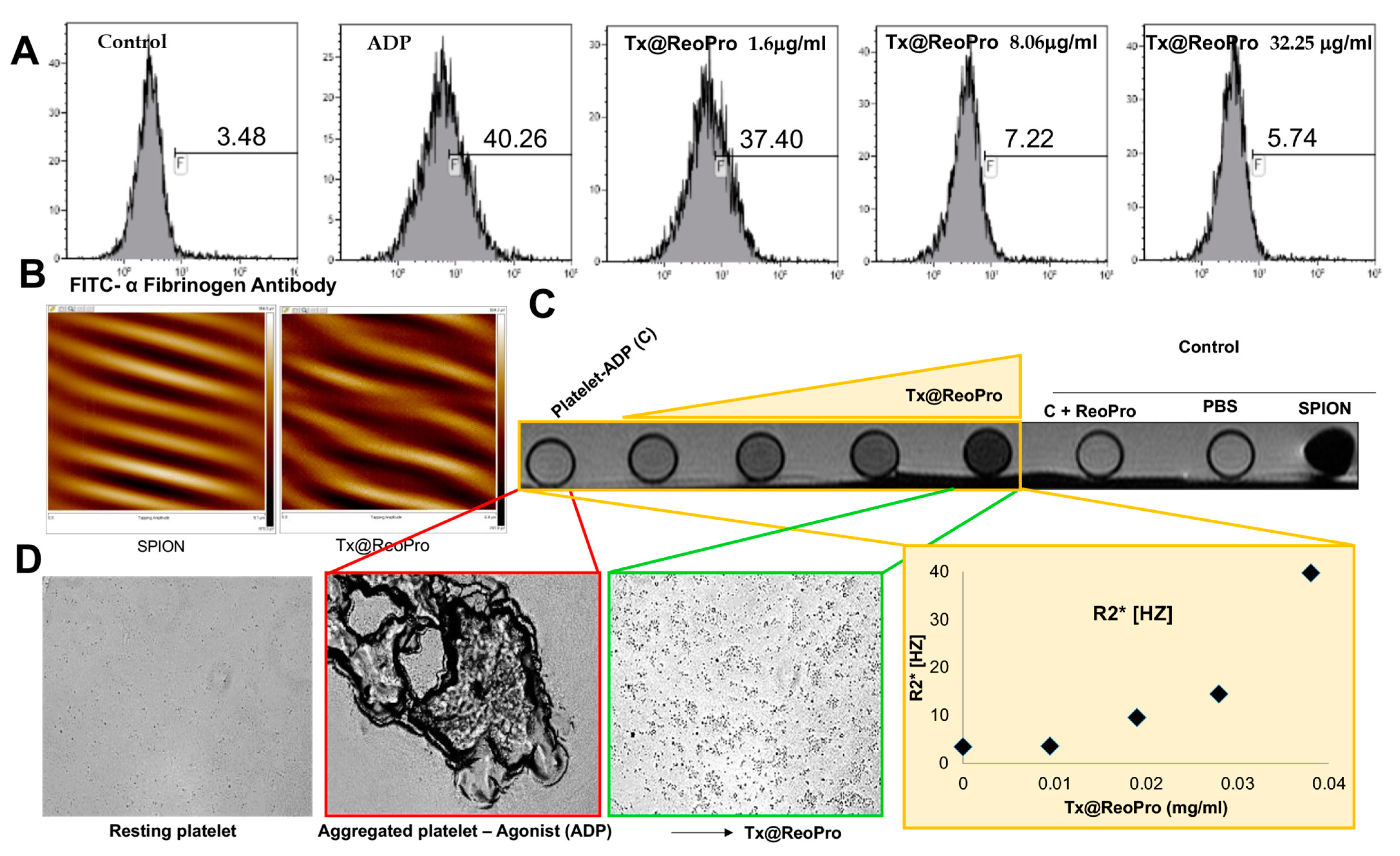
For flow cytometry experiments, hirudinised whole blood was incubated with varying concentrations of Tx@ReoPro (1.16 μg/mL, 8.06 μg/mL and 32.25 μg/mL) at room temperature for 10 min. After this incubation, 5 μL of treated blood was incubated with 5 μL of FITC conjugated α-fibrinogen antibody (Diapensia HB, Linkoping, Sweden, diluted 1:10) in 50 μL final volume of HEPES buffer (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 5.6 mM glucose, 1 g/L BSA, 20 mM 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), pH 7.4). The platelets were activated with 10 μM ADP at room temperature for 10 min. The activation was stopped using 600 μL HEPES buffer, and the samples were analysed by flow cytometry (Gallios; Beckman Coulter, Brea, CA, USA). For the negative control, HEPES with 10 mM EDTA was used instead of HEPES.
2.13. In Vitro Magnetic Resonance Imaging
In a phantom experiment, the relaxation rates R2 [1/T2, s−1] and R2* [1/T2*, s−1] were measured in vials with platelets with increasing concentrations of Tx@ReoPro (0, 0.01, 0.02, 0.03, 0.04 mg/mL). Images were acquired with a clinical Philipsachieva1.5T. The R2 was measured using a turbo spin echo sequence with 10 echoes, TR 1 sec, flip angle 90°, and a 180° inversion pulse with a first TE of 20 ms and a delta TE of 28 ms. The R2* was measured using a gradient echo sequence with a 32 echo readout, 2 sec TR, and a flip angle of 30° with a first TE of 2.8 ms and a delta TE of 2.4 ms. The region of interest was manually placed in the centre of each vial, and the relaxation rates were measured by fitting the time series to a mono-exponential signal model using the lsqcurvefit in Matlab R2017a software (The MathWorks, Inc. Natick, MA, USA). To minimise the effect of stimulated echoes in the spin echo sequence, the first echo was removed when measuring R2.
4. Discussion
Platelets play a major role in arterial thrombosis and contribute to its severity. Real-time monitoring of arterial thrombosis, along with its growth restriction by targeting platelets, could, therefore, be a very interesting area of exploration for future applications in the clinical field.
In this feasibility study, we have shown a novel theranostic nanotool, Tx@ReoPro, that can efficiently bind to platelets and restrict platelet aggregation and in vitro thrombus growth. Synthesised PEGylated SPIONs were small in size, with uniform diameter, and could easily be conjugated with the antiplatelet drug ReoPro (
Figure 1). The PEGylated SPIONs did not show any effect on platelet aggregation or fibrinogen binding to platelets (
Figure 2,
Figures S3 and S6 in Supplementary Materials), indicating that the core module does not affect platelet function. The PEG, an FDA-approved drug ligand [
22], not only served as a second protective bio-layer on the nanoparticle but also expanded functional space on the surface to accommodate higher numbers of drug molecules per particle. The model drug that has been used here is ReoPro, which is a monoclonal antibody that blocks the platelet fibrinogen receptor GPIIbIIIa. As a result, fibrinogen cannot bind to GPIIbIIIa receptors on the platelet’s surface, resulting in inhibition of platelet aggregation and further clot formation [
15,
23]. ReoPro had been in clinical use in patients with myocardial infarction or unstable angina who went for percutaneous coronary intervention (PCI). ReoPro administration was reported to reduce major adverse cardiac events (MACE) among such patients [
24]. We have observed that Tx@ReoPro was highly stable and could be in aqueous suspension (PBS) for around 6 months. The functional validation of the developed nano-system-based theranostic module was performed using clinically relevant state-of-the-art methods. To confirm the functional activity of the drug after conjugation with the nanoparticle, platelet aggregometry was conducted using both impedance and light transmission-based methods (
Figure 2,
Figures S2B and S3 in Supplementary Materials). Results showed that Tx@ReoPro inhibits platelet aggregation in a dose-dependent manner (
Figure 2B,E and
Figure S2B in Supplementary Material). A complete inhibition of aggregation was observed in light transmission platelet aggregometry at a dose of 80 μg/mL Tx@ReoPro, whereas for impedance-based whole-blood platelet aggregometry it was 32 μg/mL of Tx@ReoPro. As the blood sample processing and detection principles were different for these different aggregometry, the effective inhibitory concentrations were also found to be different.
How inhibition of platelet function by Tx@ReoPro affects the viscoelasticity during clot formation is an important area of concern. In our study, we used TEG and FOR to show that in the presence of Tx@ReoPro, clot strength or elasticity decreased remarkably (
Figure 3). To elaborate further, a decrease in the MA in the TEG experiment was also found with Tx@ReoPro. MA represents the maximal strength of the fibrin clot and is dependent on fibrin–platelet interactions via GPIIbIIIa, where the influence of platelet function is 80% and the influence of the fibrin network is 20% [
19]. Therefore, ReoPro-induced inhibition in fibrinogen binding to platelets is perfectly reflected in the decrease in MA. Furthermore, G’max in the FOR system showed a significant reduction, which further proved the active functionality of Tx@ReoPro. This confirms that the conjugated Tx@ReoPro nanotool can reduce clot strength, which was the prime aim of this study. Specific inhibition of GPIIbIIIa is important to study to confirm that target (GpIIbIIIa) specific binding of ReoPro is conserved after nano-conjugation. Flow cytometric data showed that Tx@ReoPro can inhibit fibrinogen binding to activated platelets and proves that Tx@ReoPro binds specifically to GpIIbIIIa (
Figure 4).
From the above discussion, it is evident that in an in vitro condition, Tx@ReoPro can efficiently inhibit platelet function and clot formation by target-specific inhibition of the platelet fibrinogen receptor, GPIIbIIIa. Along with the therapeutic aspects (platelet function and clot inhibition), Tx@ReoPro has the potential to aid in diagnosis/monitoring with prompt identification of the site of thrombus formation, making it a potential theranostic nanotool for arterial thrombosis. MFM and MRI images have shown that Tx@ReoPro is a magnetically active molecule and that the magnetic properties were retained even after conjugation (
Figure 4 and
Figure S4 in Supplementary Material). The MRI results, along with respective bright field microscopy images, confirmed that Tx@ReoPro could be used for thrombus detection through MRI and could restrict platelet aggregation and, therefore, potentially reduce thrombus build-up (
Figure 4).
In a clinical set-up during PCI, the patients for abciximab therapy received intravenous abciximab as a bolus dose of 0.25 mg/kg body weight (~20 mg to start) followed by a continuous infusion of 0.125 gm/kg/min up to a maximal dose of 10 μg/min for 12 h, amounting to 7.2 mg within a timeframe of 12 h with obvious individual variation [
25]. Therefore, to check the comparable amount of drug, in this study, Tx@ReoPro was used from 1 to 80 μg/mL to see its antiplatelet effect.