Abstract
The implementation of bioreactor systems for the production of bacterial inoculants as biofertilizers has become very important in recent decades. However, it is essential to know the bacterial growth optimal conditions to optimize the production and efficiency of bioinoculants. The aim of this work was to identify the best nutriment and mixing conditions to improve the specific cell growth rates (µ) of two PGPB (plant growth-promoting bacteria) rhizobial strains at the bioreactor level. For this purpose, the strains Sinorhizobium mexicanum ITTG-R7T and Sinorhizobium chiapanecum ITTG-S70T were previously reactivated in a PY-Ca2+ (peptone casein, yeast extract, and calcium) culture medium. Afterward, a master cell bank (MCB) was made in order to maintain the viability and quality of the strains. The kinetic characterization of each bacterial strain was carried out in s shaken flask. Then, the effect of the carbon and nitrogen sources and mechanical agitation was evaluated through a factorial design and response surface methodology (RSM) for cell growth optimization, where µ was considered a response variable. The efficiency of biomass production was determined in a homemade bioreactor, taking into account the optimal conditions obtained during the experiment conducted at the shaken flask stage. In order to evaluate the biological quality of the product obtained in the bioreactor, the bacterial strains were inoculated in common bean (Phaseolus vulgaris var. Jamapa) plants under bioclimatic chamber conditions. The maximum cell growth rate in both PGPB strains was obtained using a Y-Ca2+ (yeast extract and calcium) medium and stirred at 200 and 300 rpm. Under these growth conditions, the Sinorhizobium strains exhibited a high nitrogen-fixing capacity, which had a significant (p < 0.05) impact on the growth of the test plants. The bioreactor system was found to be an efficient alternative for the large-scale production of PGPB rhizobial bacteria, which are intended for use as biofertilizers in agriculture.
1. Introduction
The continuous growth of the human population necessitates the production of an increasing amount of food to satisfy basic nutritional needs [1]. Consequently, there has been a significant rise in the systematic application of chemical fertilizers and other agro-inputs, such as fungicides, herbicides, and pesticides, to meet these societal demands. However, the indiscriminate use of toxic agrochemicals has resulted in severe disruptions to ecosystems and significant environmental problems, including soil degradation, erosion, poor soil health, and fertility loss, as well as water and air pollution [2]. As an emerging alternative, the use of biofertilizers formulated with plant growth-promoting bacteria (PGPBs) in agricultural systems has become a viable option to achieve sustainable ecological development and enhance crop fertility and productivity. Notably, the genera Rhizobium, Sinorhizobium, Mesorhizobium, Bradyrhizobium, Devosia, Methylobacterium, Burkholderia, and Cupriavidus stand out among the most beneficial microorganisms commonly utilized in biofertilizer formulations [3,4,5]. PGPB not only possesses nitrogen-fixing capabilities but also exhibits additional functional qualities that contribute to plant growth, including the production of phytohormones, siderophores, vitamins, and antibiotics [6,7].
Previous studies have primarily focused on the isolation and genomic analysis of native beneficial bacteria, leading to the identification of new rhizobial species [8]. The strains Sinorhizobium mexicanum ITTG-R7T and Sinorhizobium chiapanecum ITTG-S70T were isolated from nodules of the Acaciella angustissima shrubby legume [9,10]. These bacteria establish symbiotic relationships and form nodules in various legume species [10]. In field-level biofertilization experiments, these bacterial strains have demonstrated a positive impact on growth and fruit production in crops, as well as in other non-leguminous plant species [11]. These native bacteria exhibit desirable characteristics as plant growth-promoting (PGP) bacteria, including a high nitrogen fixation potential, phosphate solubilization, auxin synthesis, and siderophore production [12,13,14].
A crucial aspect of biofertilizer production is the cultivation and growth of microorganisms [15]. Typically, the process begins at the laboratory level using shake flasks and is later expanded to pilot-scale bioreactors for bacterial production. These processes necessitate the careful consideration of factors related to bacterial growth and the use of a specific culture medium [16]. However, as biofertilizer production progresses to the pilot bioreactor level or larger scales, costs significantly increase, requiring advanced technical infrastructure [17]. Biofertilizer biomass production liquid fermentation is most commonly used since this system is highly homogeneous and allows a rough control of critical process variables, such as temperature, pH, dissolved oxygen concentration, and agitation [18]. There are research works that are focused on the production of biofertilizers in bioreactors; these investigations have been developed in commercial brand bioreactors allowing for controlled conditions of temperature, pH, input and output flows, and sterility, as well as the measurement of variables, such as carbon dioxide concentration and oxygen [19,20]. Data regarding the large-scale production of Rhizobium/Sinorhizobium bacteria are scarce, and few studies have reported the production of these as biofertilizers in bioreactors. Ruiz-Valdiviezo et al. [21] reported the production of biofertilizers formulated with Rhizobium calliandrae LBP2-1T and S. mexicanum ITTG R7T in shaken flasks. These authors found that the carbon source and agitation played a crucial role in biomass production, resulting in concentrations above 109 CFU/mL. Furthermore, after 240 days of storage, both rhizobial strains exhibited a significant positive impact on plant growth, nodulation, and the total N content in inoculated common bean plants. On the other hand, Trujillo-Roldan et al. [22] scaled up a shake flask process to a 10 L laboratory-scale bioreactor and a 1000 L pilot-scale bioreactor for the production of the PGP bacterium, Azospirillum brasilense, in a liquid inoculant formulation. They reported a concentration of 3.5 to 7.5 × 108 CFU/mL obtained in shake flasks and the bioreactor. Interestingly, the bacterial inoculants had a positive impact on the maize crop’s yield even after they were stored for two years at room temperature. In this case, the optimal conditions for achieving the best growth and biomass production of the Azospirillum strain were found to be a volumetric mass transfer coefficient (KLa) of 31 h−1 and an agitation speed of 200 rpm. Furthermore, Gamboa-Suasnavart et al. [23] conducted a scale-up process from shake flasks to a bioreactor, taking into account the power input and the morphology of Streptomyces lividans to produce recombinant APA (45/47 kDa protein) from Mycobacterium tuberculosis. These researchers observed that the culture conditions in shake flasks influenced the morphology of filamentous S. lividans, as well as the productivity and O-mannosylation of the Ala-Pro-rich recombinant O-glycoprotein from M. tuberculosis. However, it should be noted that these studies highlight high equipment costs and complex operating conditions, which limit their applicability for research purposes or their adoption in agricultural sectors in rural areas.
In recent years, there has been a growing interest among local farmers in utilizing these native bacteria as biofertilizers for application in diverse strategic food crops in Mexico [24]. In response to the increasing demand for biofertilizers, local producers have resorted to the manual production of microbial inoculants. Due to limited knowledge and inadequate infrastructure, a wide array of products have been generated, comprising vermicompost, leachate, and fertilizers derived from livestock and poultry manure. Unfortunately, these products harbor a substantial bacterial pathogen load, which presents significant risks to human health and leads to considerable alterations in the soil microbiota [25,26]. Therefore, local farmers in Chiapas, Mexico, have initiated programs aimed at independently producing their own biofertilizers using artisanal methods, with the goal of achieving high nutritional quality and contaminant-free food production. However, they face significant challenges, as they lack detailed knowledge regarding the production and formulation of microbial inoculants. The objective of this study was to identify the best nutrimental and mixing conditions from shake flasks to a homemade laboratory bioreactor to achieve the efficient biomass production of native PGP bacterial strains S. mexicanum ITTG-R7T and S. chiapanecum ITTG-S70T.
2. Materials and Methods
2.1. Bacterial Strains
The native nitrogen-fixing strains S. mexicanum ITTG-R7T (DQ411930) and S. chiapanecum ITTG-S70T (EF457949) were employed in this study. These strains are novel Sinorhizobium species isolated from the shrubby legume Acaciella angustissima [10]. They are recognized for their capacity as plant growth-promoting bacteria (PGPB). Furthermore, they exhibit non-toxic and non-pathogenic characteristics. Dr. Reiner Rincón Rosales (from Tecnologico Nacional de México) generously provided these strains, which are preserved in the culture collection of the Genomic Ecology Laboratory at Tecnologico de Tuxtla Gutierrez, Chiapas, Mexico.
2.2. Initial Characterization of Bacterial Growth
The bacterial strains ITTG-R7T and ITTG-S70T were precultured in 500 mL Erlenmeyer flasks at 30 °C and 150 rpm, using 100 mL of a PY-Ca2+ culture medium. The PY-Ca2+ medium composition per liter included 5.0 g of casein peptone, 10 mL of 0.94 M CaCl2, and 3.0 g of yeast extract [11]. Subsequently, the strains were characterized for growth kinetics in 250 mL Erlenmeyer flasks containing 50 mL of the PY-Ca2+ medium at 30 °C and 120 rpm. Growth was measured by optical density (OD) at 600 nm using a Beckman Coulter DU730 spectrophotometer (Beckman Coulter, Inc, California, USA). Biomass production was determined based on fresh weight during bacterial growth. At the initial time (T0), 1 mL of inoculated culture medium was poured into a pre-weighed 1.5 mL Eppendorf tube (Eppendorf, Hamburg, Germany). The sample was centrifuged at 10,000 rpm for 1 min. The supernatant was discarded, and the difference in weight between the empty tube and the tube with the sample provided the weight of the fresh biomass. This process was performed in triplicate at different sampling times until the stationary phase. To maintain the stability and viability of the strains, a masterwork cell bank was created following the methodology suggested by Del Puerto et al. [27]. The bacteria were preserved in a mixture of 30% glycerol and 70% PY-Ca2+ broth and stored at −20 °C until use.
2.3. Experimental Design for Optimizing Native Sinorhizobium Strain Growth Conditions
A factorial design and response surface methodology (RSM) were used to identify the optimal nutritional and mixing conditions for S. mexicanum ITTG-R7T and S. chiapanecum ITTG-S70T. This experimental design employed a 32-factorial design. Two independent variables, namely culture medium (X1) and stirring (X2), were evaluated. The culture media used as test levels were as follows: Y-Ca2+ containing per liter: [10 mL of 0.94 M CaCl2 and 8.0 g of yeast extract at pH 6.8]; PY-Ca2+: [5.0 g of casein peptone, 10 mL of 0.94 M CaCl2, and 3.0 g of yeast extract at pH 6.8]; YEM: [10 g of mannitol, 0.5 g of K2HPO4, 0.2 g of MgSO4, 0.1 g of NaCl, 3.0 g of CaCO3, and 3.0 g of yeast extract at pH 6.8]. Each independent variable had three levels: a low level (−1), an intermediate level (0), and a high level (+1) (Table 1). Nine treatments in triplicate were evaluated, resulting in a total of 27 experimental units for each bacterial strain. The experimental unit consisted of a 250 mL Erlenmeyer flask containing 50 mL of the culture medium. During the cultivation of the strains, pH was maintained at 6.8, and the temperature was set at 30 °C in accordance with the recommendations suggested by Berovic [28].

Table 1.
Experimental design employed for optimization of bacterial growth conditions.
The response function (y) measured was the specific growth rate (μ). This value was related to the coded variables (Xi, i = 1 and 2) by a second-degree polynomial. The polynomial equation proposed for the two responses (y) was:
where y is the predicted response for the specific growth rate (μ), b0 = constant, b1 = culture medium, and b2 = stirring; b1 and b2 = linear coefficients; b11, and b22 = quadratic coefficients, and b12 = cross-product coefficients.
For the measurement of (μ), the OD was determined by UV-visible spectrophotometry at 600 nm. Data obtained in the experimental assays were analyzed by an analysis of variance ANOVA (p < 0.05) in order to determine differences between the treatments. The effect and regression coefficients of the individual linear, quadratic, and interaction terms were determined. The regression models were used to generate response surface plots.
2.4. Design and Construction of a Homemade Bioreactor
Based on the bacterial growth conditions determined during the optimization test at the flask level, a homemade bioreactor was designed and constructed. The bioreactor utilized a wide-mouth glass jar with a nominal capacity of 3.8 L (“6.10” diameter and “9.95” height). The design of various components, including the lid, clamp, motor base, aeration system, impeller, impeller shaft, baffles, and rotation system, was developed using Solidworks software (BIOVIA, Dassault Systèmes, Dearborn, MI, USA). The impeller chosen was of the Rushton type, with a diameter equal to 1/3 of the tank’s diameter, and four baffles were incorporated, with each baffle width being 1/12 of the tank’s diameter. The complete prototype design is currently undergoing a utility model registration process with the Mexican Institute of Industrial Property (IMPI) under registration number MX/u/2023/000016 (https://www.gob.mx/impi) (accessed on 16 January 2023).
2.5. Bacterial Growth Assay in Homemade Bioreactor
Initially, a sterilization test was conducted on the homemade bioreactor to assess its construction quality and ensure aseptic conditions for the cultivation of rhizobial bacteria. The equipment was autoclaved at 15 psi and 121 °C for 20 min. The cell biomass production efficiency in the homemade bioreactor was determined by fresh weight, considering an operating volume of 2.6 L for the Y-Ca2+ culture medium at 200 rpm and 0.3 vvm. During the cultivation of the strains, the pH was maintained at 6.8, and the temperature was 30 °C. At the initial time point (T0), 1 mL of the inoculated culture medium was transferred into a pre-weighed 1.5 mL Eppendorf tube. The sample was centrifuged at 10,000 rpm for 1 min, and the supernatant was discarded. The weight difference between the empty tube and the tube with the sample provided the fresh biomass weight. This process was carried out in triplicate at different sampling times until the stationary phase was reached.
A prediction model for biomass production in the homemade bioreactor was developed using the least squares method. The non-linear GRG algorithm [29] was employed to obtain a polynomial equation for bacterial growth.
2.6. Bacterial Effectiveness through Plant Inoculation Assay
In order to assess the effectiveness of the bacterial strains produced in the homemade bioreactor, an inoculation test was conducted on common bean plants (Phaseolus vulgaris L.) cv. Jamapa (Figure S1). The seeds were subjected to a disinfection process involving a 5 min treatment with 70% ethanol, followed by a 15 min treatment with 25% sodium hypochlorite, and subsequent rinsing with sterile water [10]. The seeds were then germinated on plates containing an 0.8% water–agar medium until the radicle emerged. Erlenmeyer flasks containing vermiculite moistened with an N-free Fahraeus solution [0.1 g L−1 CaCl2; 0.12 g L−1 MgSO4·7H2O; 0.1 g L−1 KH2PO4; 0.15 g L−1 Na2HPO4·2H2O; 0.005 g L−1 Fe citrate; and trace amounts of Mn, Cu, Zn, B, Mo; pH 6.8] were used to grow the plants [30]. The inoculation assay employed a completely randomized design, evaluating four treatments with six replicates each. The first treatment (T1) and second treatment (T2) consisted of S. mexicanum ITTG-R7T and S. chiapanecum ITTG-S70T, respectively. The inoculants were obtained from the 3.8 L homemade bioreactor, cultivated in a Y-Ca2+ culture medium at 26 °C, and adjusted to a final concentration of 106 CFU ml−1. The third treatment (T3) involved plants treated with the triple 17 fertilizer (17% N, 17% P, and 17% K) as the positive control. The fourth treatment (T4) served as a negative control, consisting of non-inoculated plants. The plants were cultivated in controlled plant chamber conditions. After a 60-day period of cultivation, the plants were meticulously collected, and the following variables were determined: the plant’s total height (cm), dry weight (g), root weight (g), the number of nodules per plant, the total chlorophyll content, and total nitrogen content (mg per dry plant). All the variables were measured following the methodology described by Rincón-Molina et al. [31].
2.7. Statistical Analysis
The data obtained from the optimization of bacterial growth conditions and the inoculation test were analyzed using ANOVA at a significance level of alpha = 0.05, using the statistical software Statgraphics Centurion XV.2 (The Plains, VA, USA). Mean comparisons were conducted using the Tukey test (p < 0.05). Additionally, the main effects plots and response surface plots with contour areas were generated using Minitab V.16 software (State College, PA, USA).
3. Results
3.1. Bacterial Growth Characteristics
The specific growth rate (µ) of strain S. mexicanum ITTG-R7T was 0.1465 ± (0.02) h−1, and for strain S. chiapanecum ITTG-S70T was 0.1367 ± (0.01) h−1. In both, it was observed that the transition phase between the growth and the stationary phases occurred after 12 h of culture time (Figure 1). Biomass production before the stationary phase was 12.43 ± (1.26) g L−1 and 8.32 ± (0.50) g L−1, respectively. Additionally, for both strains, diauxic growth was observed during the log phase.
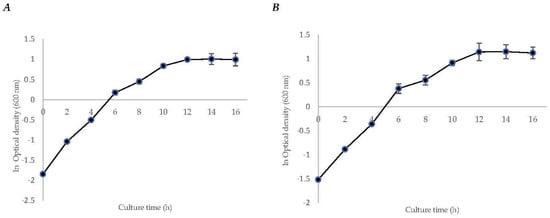
Figure 1.
Initial characterization kinetics of bacteria growth for strains of (A) S. mexicanum ITTG-R7T and (B) S. chiapanecum ITTG-S70T grown in shaken flasks.
3.2. Optimization of the Growth of Native Sinorhizobium Strains
The analysis of variance (ANOVA) of the growth rates revealed that factors X1 (culture medium) and X2 (stirring) had the most significant effect on the maximum growth rate (µ) of strain ITTG-R7T, with p-values < 0.0000 and <0.0002, respectively (Table 2). Furthermore, it was found that factor X1 (culture medium) had a significant effect (p < 0.0190) on the growth rate of strain ITTG-S70T, whereas factor X2 (stirring) did not exhibit a significant effect on µ. Furthermore, an interaction effect was observed between factors X1 (culture medium) and X2 (stirring), which significantly influenced (p < 0.0003) the growth of the strain ITTG-R7 T.

Table 2.
Significance level (p-value) of optimal growth conditions for bacterial strains.
The Pareto charts allowed us to determine the standardized effects of the evaluated factors on the maximum growth rate (Figure 2). In the case of strain ITTG R7T, it was evident that the factors, culture medium (X1) and agitation (X2), exerted a significant influence (p < 0.05) on the growth rate. Furthermore, the interaction [culture medium X agitation] had an effect on the variable under study (Figure 2A). For strain ITTG S70T, only agitation had an impact on the growth of this bacterial species (Figure 2B). Both graphs indicate that the culture medium had a negative influence on the range of bacterial growth, while agitation had a positive effect.
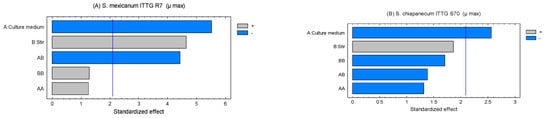
Figure 2.
Pareto charts of standardized effects on culture medium and stirring on maximum growth rate (µ) in native Sinorhizobium bacteria. (A) S. mexicanum ITTG-R7T and (B) S. chiapanecum ITTG-S70T.
The main effects plots provided a visual representation of the effects of factors X1 (culture medium) and X2 (stirring) on the growth of the bacterial strains (Figure 3). S. mexicanum ITTG R7T exhibited its highest growth rate when cultivated in the Y-Ca2+ medium (level −1.0) with a mechanical agitation of 300 rpm (level 1.0) (Figure 3A). On the other hand, the ITTG S70T strain achieved its maximum growth rate (µ) when cultured in the Y-Ca2+ medium (level −1.0) but with an agitation of 200 rpm (Figure 3B).
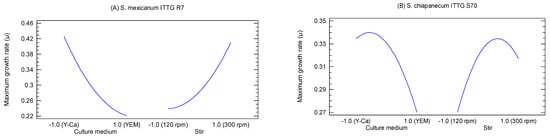
Figure 3.
Main effects plots on bacterial maximum growth rate (µ). (A) S. mexicanum ITTG-R7T and (B) ‘S. chiapanecum ITTG-S70T’.
Table 3 presents the growth range (µ) of the strains S. mexicanum ITTG R7T and S. chiapanecum ITTG S70T under optimal culture medium and shaking conditions. The ITTG R7T strain exhibited the highest growth rate [µ = 0.7324 ± (0.030) h−1] when cultivated in the Y-Ca2+ medium at 300 rpm. Similarly, the ITTG S70T strain demonstrated a greater optimal growth range (µ = 0.3830 ± (0.054) h−1) when the Y-Ca2+ culture medium was utilized as a carbon and nitrogen source and maintained under constant stirring at 300 rpm. By contrast, both Sinorhizobium strains displayed a low growth rate (µ) when cultivated in the YEM medium at an agitation of 120 rpm.

Table 3.
Growth ranges (µ) achieved by indigenous Sinorhizobium strains in the optimization experiments using an experimentally calculated optical density (OD).
The optimal culture conditions were determined by fitting the experimental data to a regression model that incorporated the variables X1 (culture medium) and X2 (stir). The equations for optimizing the response variable (µ) of the strains S. mexicanum ITTG-R7T and S. chiapanecum ITTG-S70T were obtained under optimal culture conditions, which involved using a culture medium (CM) and stirring (S) while maintaining a constant temperature of 30 °C and a pH of 6.8.
For the strain S. mexicanum ITTG-R7T, the growth optimization equation was:
and for strain S. chiapanecum ITTG-S70T’, the growth optimization equation was:
The surface plot describes the behavior of the maximum growth range (µ) across the experimental region (Figure 4). Notably, the points where the surface reaches higher values correspond precisely to optimal treatments or conditions where the Sinorhizobium strains exhibit robust growth. Examining Figure 4A, we observed that the response surface for the ITTG R7T strain indicated its favorable growth when the culture medium was at a lower level (−1), specifically the Y-Ca2+ medium, and agitation was at a higher level (+1), which corresponded to 300 rpm. Similarly, from Figure 4B, we can determine that the optimal growth range for the ITTG S70T strain occurred when it was cultivated in the Y-Ca2+ medium at a lower intermediate level (0) of agitation, corresponding to 200 rpm. Therefore, it is evident that the culture medium played a critical role in achieving the optimal growth of the native bacterial strains while agitation could be adjusted within the range of 200 to 300 rpm.
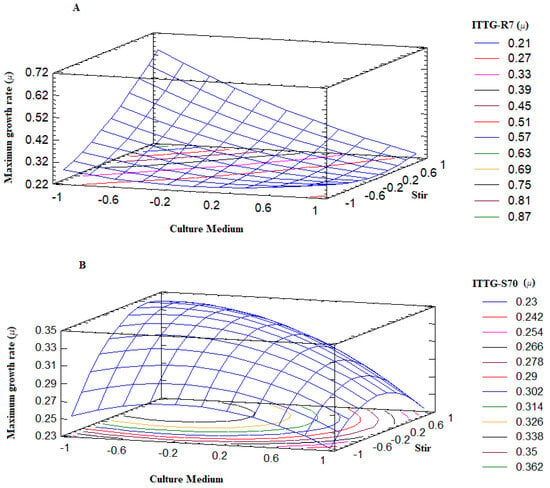
Figure 4.
Response surface plots on bacterial maximum growth rate. (A) S. mexicanum ITTG-R7T and (B) S. chiapanecum ITTG-S70T.
3.3. Production Efficiency in Homemade Bioreactor
A homemade stirred-tank bioreactor was fabricated from the reconversion of a borosilicate glass jar with a 3.8 L capacity (nominal volume). The lid, clamp, and motor base were 3D-printed in Phrozen TR300 resin. The aeration system, including the Rushton impeller, the impeller shaft, baffles, and the rotation system, were made of stainless steel (Figure 5). Sterilization tests performed on the assembled system and loaded with a culture medium showed that it maintained sterile conditions during the evaluation period (5 days).
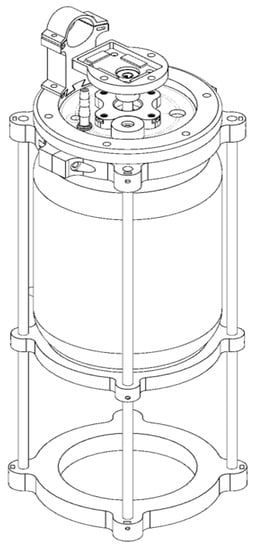
Figure 5.
Prototype of a homemade stirred-tank bioreactor registered with the Mexican Institute of Industrial Property (IMPI), registration number MX/u/2023/000016 (https://www.gob.mx/impi) (accessed on 16 January 2023).
To analyze the biomass production efficiency of the 3.8 L homemade bioreactor, the growth kinetics of the bacterial strains S. mexicanum ITTG-R7T (Figure 6A) and S. chiapanecum ITTG-S70T (Figure 6B) were obtained following the optimal conditions previously determined in the main effects plots. A maximum sampling time of 12 h was chosen for both growth kinetics in order to ensure consistency and standardization in the sample collection. A production of 18.53 ± (1.97) g L−1 and a biomass productivity of 1.54 ± (0.16) g L−1 h−1 for ITTG-R7T were reached after 12 h of growth in the Y-Ca2+ medium and 300 rpm. By contrast, a biomass concentration of 18.67 ± 0.40 g L−1 and a specific growth rate of 1.56 ± 0.04 g L−1 h−1 were achieved after 12 h of growth in the Y-Ca2+ medium at 200 rpm for ITTG-S70T. The prediction model that accurately described biomass production (BIOM) as a function of culture time (x) had an R2 value of 1.0.
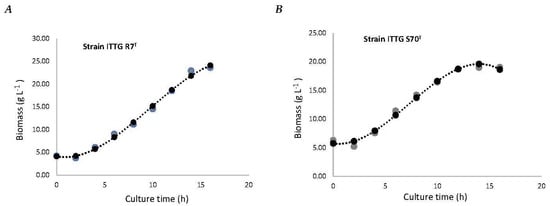
Figure 6.
Biomass production of bacterial strains: (A) S. mexicanum ITTG-R7T and (B) S. chiapanecum ITTG-S70T in Y-Ca2+ medium. Grey dots represent the measurements of the experiment and black dots are the mathematical model adjustment.
For the strain S. mexicanum ITTG-R7T (Figure 6A):
and, for strain S. chiapanecum ITTG-S70T (Figure 6B),
The prediction models for biomass production demonstrated a perfect fit with an R2 value of 1.0. This high level of accuracy confirmed the reliability of the models in estimating biomass production based on culture time. These results emphasized the significance of the prediction model in accurately predicting biomass production for the studied native Sinorhizobium strains.
In terms of the biomass production yield under optimal growth conditions, significant differences (p < 0.05) were observed among the indigenous bacterial strains when different culture media were employed (Table 4). The strain S. mexicanum ITTG-R7T exhibited higher biomass production (18.53 ± 1.97 g L−1) when cultivated in the Y-Ca2+ medium, while a lower biomass yield (12.43 ± 1.26 g L−1) was obtained in the PY-Ca2+ medium, representing a 49.0% variation between the culture media. Similarly, the strain S. chiapanecum ITTG-S70T demonstrated a substantial biomass accumulation (18.67 ± 0.40 g L−1) when grown in the Y-Ca2+ medium, resulting in a 124.4% difference in biomass production compared to its performance in the PY-Ca2+ medium. These results underscore the significance of selecting the appropriate culture medium to attain an optimal biomass yield in native Sinorhizobium strains. The Y-Ca2+ medium proved to be more conducive to biomass production, highlighting the potential for enhancing growth and productivity by optimizing the growth conditions.

Table 4.
Biomass production yield for S. mexicanum ITTG-R7T and S. chiapanecum ITTG-S70T in the optimal and reference culture media.
On the other hand, the cost analysis (Table 5) of the culture media used for the biomass production of strains S. mexicanum ITTG R7T and S. chiapanecum ITTG-S70T determined that the Y-Ca2+ culture medium was 49% more cost-effective compared to the YEM medium, and 36% more cost-effective compared to the PY-Ca2+ medium per liter of the culture medium formulated for the homemade bioreactor.

Table 5.
Cost analysis of PY-Ca2+, YEM, and modified media (Y-Ca2+) for S. mexicanum ITTG-R7T and S. chiapanecum ITTG-S70T cultures.
3.4. Efficacy of Native Sinorhizobium Strains in Promoting Plant Growth
The results of the inoculation test using the S. mexicanum ITTG-R7T and S. chiapanecum ITTG-S70T strains cultured in the homemade bioreactor demonstrated the retention of their biological qualities, including high infectivity and effectiveness as plant growth-promoting bacteria (Table 6). The ITTG-R7T strain exhibited superior effects on various growth parameters, including the total height, total weight, root weight, the number of nodules, and total nitrogen content (p < 0.05), outperforming both the negative control (non-inoculated plants) and plants treated with an NPK triple 17 fertilizer. Additionally, the ITTG-S70T strain significantly increased chlorophyll levels (3.2 mg g−1) compared to other treatments (p < 0.05). Both strains effectively induced nodulation, with ITTG-R7T demonstrating the highest number of nodules per plant (51 nodules). These findings confirm the potential of native Sinorhizobium strains as biofertilizers, maintaining their biological attributes, infectivity, and plant growth-promoting capabilities when cultivated in the homemade bioreactor.

Table 6.
Growth parameters for P. vulgaris plants inoculated with S. mexicanum ITTG-R7T and S. chiapanecum ITTG-S70T.
4. Discussion
In recent years, the demand for eco-friendly agricultural practices has increased to mitigate the environmental impact of chemical fertilizers [32]. Sustainable agriculture necessitates strategies that enhance food production safety, landscape quality, and fertility conditions while gaining social acceptance [33]. Beneficial microorganisms as biofertilizers offer a sustainable and environmentally friendly approach to optimizing crop growth and productivity. However, scaling up biofertilizer production presents challenges, including attaining high cell concentrations, ensuring product safety by eliminating contaminants, and reducing production costs [34]. Overcoming these challenges is crucial for the widespread adoption of biofertilizers as efficient and viable solutions in modern agriculture [35].
This study presents, for the first time, the growth kinetics, optimal conditions, and production of native Sinorhizobium strains using a novel homemade bioreactor system. These strains exhibited rapid growth and high exopolysaccharide production. The bioreactor, designed for the Sinorhizobium bacteria, enabled high cell concentrations and efficient biofertilizer production. This approach provides a scalable method for producing larger quantities of bioinoculants compared to flask-level systems. These results could contribute to the development of sustainable, cost-effective, and user-friendly biofertilizer production methods.
According to the response surface methodology, it was determined that the Y-Ca2+ culture medium and 200 rpm stirring rate were the most suitable for the growth of the native Sinorhizobium strains. These results indicate that the addition of the yeast extract and soluble Ca2+ provided the essential nutrients for the growth of this bacterial species. Techniques for the isolation and cultivation of nitrogen-fixing bacteria have been reported by different authors [36,37,38]. The most common culture medium for growing bacterial species within the genus Sinorhizobium is YEM, which contains mannitol as a carbon source [39,40], and PY, which contains casein peptone [41,42]. Here, we report that both strains are able to grow in a culture medium with CaCl2 and yeast extract. This result completely differs from the works mentioned above, where the authors proposed cultural media that were complex in composition. The yeast extract is the most commonly used component in media to produce microbial biomass [43,44,45], and it is the main component in the culture medium presented in this work. The chemical components of the yeast extract are ill-defined due to extreme variations between the raw materials used in manufacturing processes. However, it is broadly known that the composition of the yeast extract mainly consisted of amino acids, peptides, growth factors (vitamins like thiamine, riboflavin, pantothenic acid, pyridoxine, niacin, cyanocobalamin, and other hydrosoluble vitamins of the B complex), trace elements, carbohydrates, and others [46,47]. The concentration of the yeast extract tested in this study allowed us to assume that this raw material acted as a source of both nitrogen and growth factors; this statement matches with the scientific reports that mention the exigent vitamin requirements in rhizobial strains [36,48]. Toledo et al. [42] demonstrated the successful isolation and cultivation of the Sinorhizobium americanum strain using the PY medium, which served as a specific and effective culture medium. Similarly, Rincón-Rosales et al. [8] achieved pure isolates of native Rhizobium strains with a high nitrogen fixation capacity using this identical culture medium. These studies highlight the reliability and efficiency of the PY medium for the isolation and cultivation of the Sinorhizobium and Rhizobium strains, respectively. In addition, it has been reported that rhizobial strains have various metabolic strategies to process nitrogen, highlighting the ubiquitous glutamine synthetase (GS) enzyme system for obtaining energy, and promoting the synthesis of important biomolecules from yeast extract glutamic acid [49]. This allowed us to realize the ability of these strains to grow in a culture medium with a nitrogen source that was rich in amino acids without requiring a carbon source such as mannitol.
On the other hand, the addition of CaCl2 to the culture medium (Y-Ca2+) played a key role in preventing toxicity from some components present in the yeast extract. Divalent cations, especially Ca2+, have been shown to prevent toxicity caused mainly by glycine and sodium chloride [50]. Additionally, the addition of CaCl2 improves the protein channels of exclusion in the bacterial cell membrane and influences the production of metabolites, such as EPS, that contribute to alleviating toxicity due to high salinity concentrations.
The findings in this work contribute directly to obtaining bacterial biomass using a culture medium with components that are easily accessible in raw materials, the preparation of which is simple and fast. Additionally, we demonstrated that when culturing Sinorhizobum strains, it is possible to exclude the most expensive ingredient of YEM broth (mannitol); this modification allowed for an over 35% reduction in the cost with the modified medium (Y-Ca2+) compared to YEM and PY, as shown in Table 4. This highlights the significance of the Y-Ca2+ medium, not only for its efficiency in promoting growth and biomass production but also for its cost-effectiveness, especially in the context of a homemade bioreactor setup. Consequently, the use of the Y-Ca2+ medium presents an appealing and suitable option for the large-scale production of these rhizobial bacteria.
In this study, agitation played a critical role in the cultivation and production of rhizobial bacteria in a homemade bioreactor. Shaking at 200–300 rpm ensured the homogeneous mixing of the culture medium, providing sufficient nutrients and oxygen while facilitating waste product removal. Furthermore, optimal shaking conditions prevented cell aggregation, promoting bacterial growth and reproduction [16]. Therefore, the precise control of agitation is essential for maximizing bioreactor efficiency and bacterial cell production, as highlighted by previous studies [17,18]. In summary, proper agitation in the handmade bioreactor was essential for optimizing the growth and production of the native Sinorhizobium bacteria and ensuring the quality of the culture.
In addition, the inoculation of Phaseolus vulgaris plants with bacterial inoculants obtained from the homemade bioreactor confirmed the preserved biological functionality of the Sinorhizobium strains. It is crucial for the bacteria cultivated in the bioreactor to possess a high infectivity capacity, effectiveness, and cell viability, with a cell concentration exceeding 1 × 106 CFU/mL to ensure favorable impacts on plant growth. These strains displayed remarkable infectivity, the ability to form nodules, and exhibited effective nitrogen fixation. Remarkably, the Sinorhizobium strains showcased the superior growth and development of bean plants compared to alternative treatments, owing to their plant growth-promoting attributes and adaptability to leguminous plants [10,14,32]. The establishment of symbiotically efficient root nodules by bacterial strains resulted in enhanced plant height, total weight, and root weight, facilitated by the legume–rhizobial symbiosis that enhances biological nitrogen fixation. These findings underscore the potential of cultivating and producing nitrogen-fixing Sinorhizobium bacteria at scale utilizing a homemade bioreactor and lay the groundwork for modeling analogous processes for other bacterial species with biofertilizer potential.
Obtaining bioinoculants with plant growth-promoting properties, especially nitrogen fixation, through cost-effective and user-friendly production systems provides an alternative for farmers to address environmental pollution and economic challenges associated with chemical fertilizers. In this regard, careful attention should be given to the selection of specific bacterial strains for formulating and producing bioinoculant products. These strains must not only enhance crop growth but also ensure safety for humans, plants, soil, and the environment. Farmers can use autoclaves and appropriate equipment to sterilize the bioreactor. Providing training on equipment handling and maintenance is crucial. Although homemade bioreactors are user-friendly, farmers may benefit from guidance in growth media preparation and bacterial culture management. Offering technical advice is recommended. The cost of home bioreactors is justified considering the long-term benefits of producing cost-effective biofertilizers, reducing reliance on expensive chemical fertilizers, and promoting sustainable farming. Home bioreactors represent an economically viable and environmentally friendly approach for farmers, especially in vulnerable agricultural sectors. The production process of biofertilizers in homemade bioreactors should be economically viable, facilitating the adoption of these biotechnologies by various vulnerable agricultural sectors, particularly in Mexico, which aim to reduce excessive synthetic fertilizer application and dependence by embracing new technologies.
5. Conclusions
Bacterial production in the laboratory prototype bioreactor led to appropriate cell concentrations. This is the first report on the use of a homemade, economical, and efficient bioreactor designed in southern Mexico for the production of rhizobial biofertilizers from native strains. Thus, this study introduces a new approach for obtaining larger amounts of inoculants, contributing to the overall biotechnological approaches for biofertilizer production for use in conventional agriculture systems and industries. The results demonstrate that the elite nitrogen-fixing bacterial strains S. mexicanum ITTG-R7T and S. chiapanecum ITTG-S70T, cultivated in a Y-Ca2+ culture medium and at 200–300 rpm, were highly effective in promoting plant growth when used to inoculate bean plants. These findings have important implications for sustainable agriculture practices that aim to reduce reliance on synthetic fertilizers and promote the use of environmentally friendly options, such as bio-inoculants. The bioreactor system was found to be an efficient and low-cost alternative for the large-scale production of PGPB rhizobial bacteria intended for use as biofertilizers in agricultural crops.
6. Patents
The prototype bioreactor design was registered as a utility model with the Instituto Mexicano de la Propiedad Industrial (IMPI), registration number MX/u/2023/000016.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bioengineering10080960/s1, Figure S1: Inoculation test on common bean plants (Phaseolus vulgaris) treated with Sinorhizobium bacteria (inoculation treatments).
Author Contributions
R.R.-R. and L.A.M.-G. designed the study. C.I.R.-M., L.M.C.V.-C., J.J.V.-M. and V.M.R.-V. performed the laboratory experiments. A.G.-J. contributed the new reagents and analytical tools. F.A.R.-M., J.D.F.-F. and R.R.-R. performed the data analysis. C.I.R.-M., L.A.M.-G. and R.R.-R. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was obtained from Tecnológico Nacional de México (TECNM) No. 13529.22-P and 14094.22-P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that all relevant data supporting the findings of this study are included in the article.
Acknowledgments
We thank CONAHCYT for granting a postdoctoral scholarship to Clara Ivette Rincón-Molina. We thank the company 3R BIOTEC for allowing us to carry out the experiments and bioreactor design.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The challenge of feeding the world. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant Growth Promoting Rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Divjot, K.; Kusam, L.R.; Ajar, N.Y.; Neelam, Y.; Manish, K.; Vinod, K.; Pritesh, V.; Harcharan, S.D.; Anil, K.S. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotech. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Menéndez, E.; Rivera, L.P.; Marcos-García, M.; Martínez-Hidalgo, P.; Mateos, P.F.; Martínez-Molina, E.; Velázquez, M.A.; García-Fraile, P.; Rivas, R. Use of Rhizobium Leguminosarum as a Potential Biofertilizer for Lactuca Sativa and Daucus Carota Crops. J. Plant. Nutr. Soil Sci. 2013, 176, 876–882. [Google Scholar] [CrossRef]
- Gen-Jimenez, A.; Flores-Félix, J.D.; Rincón-Molina, C.I.; Manzano-Gomez, L.A.; Rogel, M.A.; Ruiz-Valdiviezo, V.M.; Rincón-Molina, F.A.; Rincón-Rosales, R. Enhance of tomato production and induction of changes on the organic profiles mediated by Rhizobium biofortification. Front. Microbiol. 2023, 14, 1235930. [Google Scholar] [CrossRef]
- Thilakarathna, M.; Raizada, M.A. Meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol. Biochem. 2017, 105, 177–196. [Google Scholar] [CrossRef]
- Buitrago, R.B.; de Bashan, L.E.G.; Pedraza, R.O. Bacterias Promotoras de Crecimiento Vegetal; En sistemas de agricultura sostenible; Corporación colombiana de investigación agropecuaria—Mosquera (Colombia): Agrosavia, Colombia, 2021; Volume 372, ISBN 978-958-740-500-2. [Google Scholar]
- Rincón-Rosales, R.; Villalobos-Escobedo, J.M.; Rogel, M.A.; Martínez, J.; Ormeño-Orrillo, E.; Martínez-Romero, E. Rhizobium calliandrae sp. nov., Rhizobium mayense sp. nov. and Rhizobium jaguaris sp. nov., rhizobial species nodulating the medicinal legume Calliandra grandiflora. Int. J. Syst. Evol. Microbiol. 2013, 63, 3423–3429. [Google Scholar] [CrossRef] [PubMed]
- Lloret, L.; Ormeño-Orrillo, E.; Rincón, R.; Martínez-Romero, J.; Rogel-Hernández, M.A.; Martínez-Romero, E. Ensifer mexicanus sp. nov. a new species nodulating Acacia angustissima (Mill.) Kuntze in Mexico. Syst. Appl. Microbiol. 2007, 30, 280–290. [Google Scholar] [CrossRef]
- Rincón-Rosales, R.; Lloret, L.; Ponce, E.; Martínez-Romero, E. Rhizobia with different symbiotic efficiencies nodulate Acaciella angustissima in Mexico, including Sinorhizobium chiapanecum sp. nov. which has common symbiotic genes with Sinorhizobium mexicanum. FEMS Microbiol. Ecol. 2009, 67, 103–117. [Google Scholar] [CrossRef]
- Rincón-Molina, C.I.; Martínez-Romero, E.; Manzano-Gómez, L.A.; Rincón-Rosales, R. Growth Promotion of Guava “Pear” (Psidium guajava cv.) by Sinorhizobium mexicanum in Southern Mexican Agricultural Fields. Sustainability 2022, 14, 12391. [Google Scholar] [CrossRef]
- Rincón-Rosales, R.; Ruiz-Valdiviezo, V.M.; Montes-Molina, J.A.; Gutiérrez-Miceli, F.A.; Dendooven, L. Aluminium tolerance in the tropical leguminous N2-fixing shrub Acaciella angustissima (Mill.) Britton & Rose inoculated with Sinorhizobium mexicanum. Gayana Bot. 2011, 68, 188–195. [Google Scholar] [CrossRef]
- Rincón-Rosales, R.; Rogel, M.A.; Guerrero, G.; Rincón-Molina, C.I.; López-López, A.; Manzano-Gómez, L.A.; Martínez-Romero, E. Genomic Data of Acaciella Nodule Ensifer mexicanus ITTG R7T. Microbiol. Resour. Announc. 2021, 10, e01251-20. [Google Scholar] [CrossRef]
- Rincón-Molina, C.I.; Martínez-Romero, E.; Aguirre-Noyola, J.L.; Manzano-Gómez, L.A.; Zenteno-Rojas, A.; Rogel, M.A.; Rincón-Molina, F.A.; Ruíz-Valdiviezo, V.M.; Rincón-Rosales, R. Bacterial Community with Plant Growth-Promoting Potential associated to pioneer plants from an active mexican volcanic complex. Microorganisms 2022, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Grageda-Cabrera, O.A.; Díaz-Franco, A.; Peña-Cabriales, J.J.; Vera-Nuñez, J.A. Impacto de los biofertilizantes en la agricultura. Rev. Mex. Cienc. Agric. 2012, 3, 1261–1274. Available online: https://www.redalyc.org/articulo.oa?id=263123222015 (accessed on 1 August 2023). [CrossRef][Green Version]
- Patiño-Vera, M.; Jiménez, B.; Balderas, K.; Ortiz, M.; Allende, R.; Carrillo, A.; Galindo, E. Pilot-scale production and liquid formulation of Rhodotorula minuta, a potential biocontrol agent of mango anthracnose. J. Appl. Microbiol. 2005, 99, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Tapia, F.; Vázquez-Ramírez, D.; Genzel, Y.; Reichl, U. Bioreactors for high cell density and continuous multi-stage cultivations: Options for process intensification in cell culture-based viral vaccine production. Appl. Microbiol. Biotechnol. 2016, 100, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Crater, J.S.; Lievense, J.C. Scale-up of industrial microbial processes. FEMS Microbiol. Lett. 2018, 365, 138. [Google Scholar] [CrossRef]
- Parra-Cota, F.I.; Peña-Cabriales, J.J.; de los Santos-Villalobos, S.; Martínez-Gallardo, N.A.; Délano-Frier, J.P. Burkholderia ambifaria and B. caribensis promote growth and increase yield in grain amaranth (Amaranthus cruentus and A. hypochondriacus) by improving plant nitrogen uptake. PLoS ONE 2014, 9, e88094. [Google Scholar] [CrossRef]
- Rojas-Padilla, J.; Chaparro-Encinas, L.A.; Robles-Montoya, R.I.; de los Santos Villalobos, S. Growth promotion on wheat (Triticum turgidum L. subsp. durum) by co-inoculation of native Bacillus strains isolated from the Yaqui Valley, Mexico. Nova Sci. 2020, 12, 24. [Google Scholar] [CrossRef]
- Ruíz-Valdiviezo, V.M.; Canseco, L.M.C.V.; Suárez, L.A.C.; Gutiérrez-Miceli, F.A.; Dendooven, L.; Rincón-Rosales, R. Symbiotic potential and survival of native rhizobia kept on different carriers. Braz. J. Microbiol. 2015, 46, 73542. [Google Scholar] [CrossRef]
- Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Gonzalez-Monterrubio, C.F.; Acevedo-Sánchez, E.V.; Martínez-Salinas, C.; García-Cabrera, R.I.; Gamboa-Suasnavart, R.A.; Marín-Palacio, L.D.; Villegas, J.; Blancas-Cabrera, A. Scale-up from shake flasks to pilot-scale production of the plant growth-promoting bacterium Azospirillum brasilense for preparing a liquid inoculant formulation. Appl. Microbiol. Biotechnol. 2013, 97, 9665–9674. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Suasnavart, R.A.; Marín-Palacio, L.D.; Martínez-Sotelo, J.A.; Espitia, C.; Servín-González, L.; Valdez-Cruz, N.A.; Trujillo-Roldán, M.A. Scale-up from shake flasks to bioreactor, based on power input and Streptomyces lividans morphology, for the production of recombinant APA (45/47 kDa protein) from Mycobacterium tuberculosis. World J. Microbiol. Biotechnol. 2013, 29, 1421–1429. [Google Scholar] [CrossRef]
- Ramírez-Puebla, S.T.; Ormeño-Orrillo, E.; Rogel, M.A.; López-Guerrero, M.G.; López-López, A.; Martínez-Romero, J.; Negrete-Yankelevich, S.; Martínez-Romero, E. La diversidad de los rizobios nativos de México a la luz de la genómica. Rev. Mex. Biodivers. 2019, 90, e902681. [Google Scholar] [CrossRef]
- Bocatti, C.R.; Ferreira, E.; Ribeiro, R.A.; de Oliveira Chueire, L.M.; Delamuta, J.R.M.; Kobayashi, R.K.T.; Nogueira, M.A. Microbiological quality analysis of inoculants based on Bradyrhizobium spp. and Azospirillum brasilense produced “on farm” reveals high contamination with non-target microorganisms. Braz. J. Microbiol. 2022, 53, 267–280. [Google Scholar] [CrossRef]
- Vassileva, M.; Mocali, S.; Canfora, L.; Malusá, E.; García del Moral, L.F.; Martos, V.; Flor-Peregrin, E.; Vassilev, N. Safety level of microorganism-bearing products applied in soil-plant systems. Front. Plant Sci. 2002, 13, 862875. [Google Scholar] [CrossRef]
- Del Puerto, C.A.; Iglesias, E.; Morales, T.; Baños, N.; Nocedo, M.D.; Carnota, G.; Martínez, R. Organización y manejo de la colección de cepas de referencia del Instituto Finlay. Vaccimonitor 2009, 18, 20–24. [Google Scholar]
- Berovic, M. Sterilisation in Biotechnology. Biotechnol. Annu. Rev. 2005, 11, 257–279. [Google Scholar]
- Stuart, S.; Leon, L. Solving Large Sparse Nonlinear Programs Using GRG. ORSA J. Comput. 1992, 4, 2–15. [Google Scholar] [CrossRef]
- Fahraeus, G. The infection of clover root hair by nodule bacteria studied by a single glass slide technique. J. Gen. Microbiol. 1957, 16, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Molina, C.I.; Martínez-Romero, E.; Ruiz-Valdiviezo, V.M.; Velázquez, E.; Ruiz-Lau, N.; Rogel-Hernández, M.A.; Villalobos-Maldonado, J.J.; Reiner, R.-R. Plant growth-promoting potential of bacteria associated to pioneer plants from an active volcanic site of Chiapas (Mexico). Appl. Soil Ecol. 2020, 146, 103390. [Google Scholar] [CrossRef]
- Begom, F.M.; Ahmed, M.G.U.; Sultana, R.; Akter, F. Impact of Rhizobium biofertilizer on agronomical performance of lentil (BARI Masur-6) in Bangladesh. Arch. Agric. Environ. Sci. 2021, 6, 114–120. [Google Scholar] [CrossRef]
- Saha, L.; Bauddh, K. Sustainable agricultural approaches for enhanced crop productivity, better soil health, and improved ecosystem services. In Ecological and Practical Applications for Sustainable Agriculture; Springer: Singapore, 2020; pp. 1–23. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; López, A.; Martos, V.; Reyes, A.; Maksimovic, Y.; Eichler-Löbermann, B.; Malusa, E. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 2015, 99, 4983–4996. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Baldani, J.I.; Reis, V.M.; Videira, S.S.; Boddey, L.H.; Baldani, V.L.D. The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: A practical guide for microbiologists. Plant Soil 2014, 384, 413–431. [Google Scholar] [CrossRef]
- Smercina, D.N.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. To fix or not to fix: Controls on free-living nitrogen fixation in the rhizosphere. Appl. Environ. Microbiol. 2019, 85, e02546-18. [Google Scholar] [CrossRef]
- Kawaka, F. Characterization of symbiotic and nitrogen fixing bacteria. AMB Express 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Elboutahiri, N.; Thami-Alami, I.; Udupa, S.M. Phenotypic and genetic diversity in Sinorhizobium meliloti and S. medicae from drought and salt affected regions of Morocco. BMC Microbiol. 2010, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guanzon, I.M.; Mason, M.L.T.; Juico, P.P.; Fiegalan, F.T. Isolation of three genera of microorganisms in lahar-laden soils of Sta. Rita, Pampanga, Philippines through the 16s rRNA gene sequence analysis. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2023, 73, 1–12. [Google Scholar] [CrossRef]
- Wang, E.T.; Tan, Z.Y.; Willems, A.; Fernández-López, M.; Reinhold-Hurek, B.; Martínez-Romero, E. Sinorhizobium morelense sp. nov., a Leucaena leucocephala-associated bacterium that is highly resistant to multiple antibiotics. Int. J. Syst. Evol. Microbiol. 2002, 52, 1687–1693. [Google Scholar] [CrossRef]
- Toledo, I.; Lloret, L.; Martínez-Romero, E. Sinorhizobium americanus sp. nov., a new Sinorhizobium species nodulating native Acacia spp. in Mexico. Syst. Appl. Microbiol. 2003, 26, 54–64. [Google Scholar] [CrossRef]
- Zarei, O.; Dastmalchi, S.; Hamzeh-Mivehroud, M. A simple and rapid protocol for producing yeast extract from Saccharomyces cerevisiae suitable for preparing bacterial culture media. IJPRI 2016, 15, 907. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Ngu, L.H.; Ong, D.E.L.; Nissom, P.M. Low-cost cultivation of Sporosarcina pasteurii strain in food-grade yeast extract medium for microbially induced carbonate precipitation (MICP) application. Biocatal. Agric. Biotech. 2019, 17, 247–255. [Google Scholar] [CrossRef]
- Hayek, S.A.; Gyawali, R.; Aljaloud, S.O.; Krastanov, A.; Ibrahim, S.A. Cultivation media for lactic acid bacteria used in dairy products. J. Dairy Res. 2019, 86, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Reddy, J.; Buckland, B.; Greasham, R. Toward consistent and productive complex media for industrial fermentations: Studies on yeast extract for a recombinant yeast fermentation process. Biotechnol. Bioeng. 2003, 82, 640–652. [Google Scholar] [CrossRef]
- Lee, J.S.; Little, B.J. Electrochemical and chemical complications resulting from yeast extract addition to stimulate microbial growth. Corrosion 2015, 71, 1434–1440. [Google Scholar] [CrossRef]
- Watson, R.J.; Heys, R.; Martin, T.; Savard, M. Sinorhizobium meliloti cells require biotin and either cobalt or methionine for growth. Appl. Environ. Microbiol. 2001, 67, 3767–3770. [Google Scholar] [CrossRef][Green Version]
- Yin, H.; Yang, F.; He, X.; Du, X.; Mu, P.; Ma, W. Advances in the functional study of glutamine synthetase in plant abiotic stress tolerance response. Crop. J. 2022, 10, 917–923. [Google Scholar] [CrossRef]
- Huang, B.; Qin, P.; Xu, Z.; Zhu, R.; Meng, Y. Effects of CaCl2 on viscosity of culture broth, and on activities of enzymes around the 2-oxoglutarate branch, in Bacillus subtilis CGMCC 2108 producing poly-(γ-glutamic acid). Bioresour. Technol. 2011, 102, 3595–3598. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).