The Impact of Rocuronium and Sugammadex on Length of Stay in Patients Undergoing Open Spine Surgery: A Propensity Score-Matched Analysis
Abstract
1. Introduction
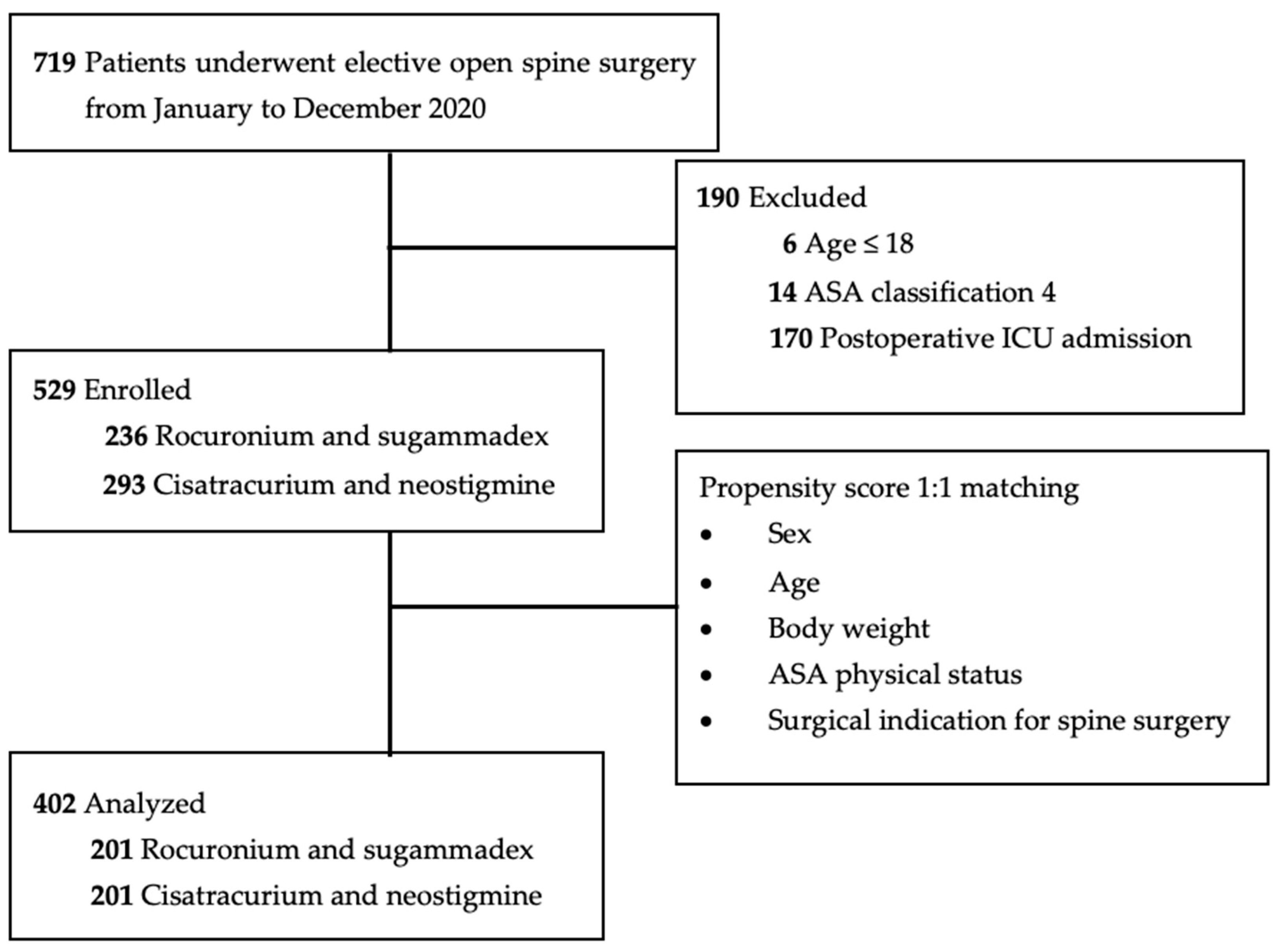
2. Materials and Methods
2.1. Data Collection and Study Design
2.2. Anesthesia Management
2.3. Primary and Secondary Outcomes
2.4. Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fearon, K.C.; Ljungqvist, O.; Von Meyenfeldt, M.; Revhaug, A.; Dejong, C.H.; Lassen, K.; Nygren, J.; Hausel, J.; Soop, M.; Andersen, J.; et al. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin. Nutr. 2005, 24, 466–477. [Google Scholar] [CrossRef]
- Debono, B.; Wainwright, T.W.; Wang, M.Y.; Sigmundsson, F.G.; Yang, M.M.H.; Smid-Nanninga, H.; Bonnal, A.; Le Huec, J.C.; Fawcett, W.J.; Ljungqvist, O.; et al. Consensus statement for perioperative care in lumbar spinal fusion: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Spine J. 2021, 21, 729–752. [Google Scholar] [CrossRef]
- Cedborg, A.I.; Sundman, E.; Boden, K.; Hedstrom, H.W.; Kuylenstierna, R.; Ekberg, O.; Eriksson, L.I. Pharyngeal function and breathing pattern during partial neuromuscular block in the elderly: Effects on airway protection. Anesthesiology 2014, 120, 312–325. [Google Scholar] [CrossRef]
- Cammu, G. Residual Neuromuscular Blockade and Postoperative Pulmonary Complications: What Does the Recent Evidence Demonstrate? Curr. Anesthesiol. Rep. 2020, 10, 131–136. [Google Scholar] [CrossRef]
- Hunter, J.M. Reversal of residual neuromuscular block: Complications associated with perioperative management of muscle relaxation. Br. J. Anaesth. 2017, 119, i53–i62. [Google Scholar] [CrossRef]
- Togioka, B.M.; Yanez, D.; Aziz, M.F.; Higgins, J.R.; Tekkali, P.; Treggiari, M.M. Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged surgery. Br. J. Anaesth. 2020, 124, 553–561. [Google Scholar] [CrossRef]
- Xiaobing, L.; Yan, J.; Wangping, Z.; Rufang, Z.; Jia, L.; Rong, W. Effects of sugammadex on postoperative respiratory management in children with congenital heart disease: A randomized controlled study. Biomed. Pharmacother. 2020, 127, 110180. [Google Scholar] [CrossRef]
- Oh, T.K.; Oh, A.Y.; Ryu, J.H.; Koo, B.W.; Song, I.A.; Nam, S.W.; Jee, H.J. Retrospective analysis of 30-day unplanned readmission after major abdominal surgery with reversal by sugammadex or neostigmine. Br. J. Anaesth. 2019, 122, 370–378. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Back, I.N. Palliative Medicine Handbook, 3rd ed.; BPM Books: Cardiff, UK, 2001. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Forsth, P.; Olafsson, G.; Carlsson, T.; Frost, A.; Borgstrom, F.; Fritzell, P.; Ohagen, P.; Michaelsson, K.; Sanden, B. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N. Engl. J. Med. 2016, 374, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.J.; Kim, K.J.; Jahng, T.A.; Kim, H.J. Minimally Invasive Robotic Versus Open Fluoroscopic-guided Spinal Instrumented Fusions: A Randomized Controlled Trial. Spine 2017, 42, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Yue, W.M.; Yeo, W.; Soeharno, H.; Tan, S.B. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur. Spine J. 2012, 21, 2265–2270. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Sivabalan, P.; Li, J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J. Clin. Neurosci. 2012, 19, 829–835. [Google Scholar] [CrossRef]
- Pierce, K.E.; Gerling, M.C.; Bortz, C.A.; Alas, H.; Brown, A.E.; Woo, D.; Vasquez-Montes, D.; Ayres, E.W.; Diebo, B.G.; Maglaras, C.; et al. Factors influencing length of stay following cervical spine surgery: A comparison of myelopathy and radiculopathy patients. J. Clin. Neurosci. 2019, 67, 109–113. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Chen, C.; Zhang, Y.; Wang, Z.; Wang, B.; Yan, W.; Li, M.; Yuan, W.; Wang, Y. Randomized, controlled, multicenter, clinical trial comparing BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion in China. Spine 2012, 37, 433–438. [Google Scholar] [CrossRef]
- Visioni, A.; Shah, R.; Gabriel, E.; Attwood, K.; Kukar, M.; Nurkin, S. Enhanced Recovery After Surgery for Noncolorectal Surgery?: A Systematic Review and Meta-analysis of Major Abdominal Surgery. Ann. Surg. 2018, 267, 57–65. [Google Scholar] [CrossRef]
- Adogwa, O.; Martin, J.R.; Huang, K.; Verla, T.; Fatemi, P.; Thompson, P.; Cheng, J.; Kuchibhatla, M.; Lad, S.P.; Bagley, C.A.; et al. Preoperative serum albumin level as a predictor of postoperative complication after spine fusion. Spine 2014, 39, 1513–1519. [Google Scholar] [CrossRef]
- Seicean, A.; Seicean, S.; Alan, N.; Schiltz, N.K.; Rosenbaum, B.P.; Jones, P.K.; Kattan, M.W.; Neuhauser, D.; Weil, R.J. Preoperative anemia and perioperative outcomes in patients who undergo elective spine surgery. Spine 2013, 38, 1331–1341. [Google Scholar] [CrossRef]
- Lee, H.K.; Jang, Y.H.; Choi, K.W.; Lee, J.H. The effect of electrically heated humidifier on the body temperature and blood loss in spinal surgery under general anesthesia. Korean J. Anesthesiol. 2011, 61, 112–116. [Google Scholar] [CrossRef]
- Burgess, L.C.; Wainwright, T.W. What Is the Evidence for Early Mobilisation in Elective Spine Surgery? A Narrative Review. Healthcare 2019, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Yuk, F.J.; Maniya, A.Y.; Rasouli, J.J.; Dessy, A.M.; McCormick, P.J.; Choudhri, T.F. Factors Affecting Length of Stay Following Elective Anterior and Posterior Cervical Spine Surgery. Cureus 2017, 9, e1452. [Google Scholar] [CrossRef]
- Hristovska, A.M.; Duch, P.; Allingstrup, M.; Afshari, A. The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A Cochrane systematic review with meta-analysis and trial sequential analysis. Anaesthesia 2018, 73, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Kang, S.W.; Kim, H.K.; Kim, H.S.; Kim, H. Effect of sugammadex on chest radiographic abnormality in the early postoperative period after video-assisted thoracoscopic lobectomy. Turk. J. Med. Sci. 2020, 50, 1236–1246. [Google Scholar] [CrossRef]
- Wu, E.B.; Huang, S.C.; Lu, H.I.; Illias, A.M.; Wang, P.M.; Huang, C.J.; Shih, T.H.; Chin, J.C.; Wu, S.C. Use of rocuronium and sugammadex for video-assisted thoracoscopic surgery is associated with reduced duration of chest tube drainage: A propensity score-matched analysis. Br. J. Anaesth. 2023, 130, e119–e127. [Google Scholar] [CrossRef] [PubMed]
- Abad-Gurumeta, A.; Ripolles-Melchor, J.; Casans-Frances, R.; Espinosa, A.; Martinez-Hurtado, E.; Fernandez-Perez, C.; Ramirez, J.M.; Lopez-Timoneda, F.; Calvo-Vecino, J.M. Evidence Anaesthesia Review, G. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia 2015, 70, 1441–1452. [Google Scholar] [CrossRef]
- Kheterpal, S.; Vaughn, M.T.; Dubovoy, T.Z.; Shah, N.J.; Bash, L.D.; Colquhoun, D.A.; Shanks, A.M.; Mathis, M.R.; Soto, R.G.; Bardia, A.; et al. Sugammadex versus Neostigmine for Reversal of Neuromuscular Blockade and Postoperative Pulmonary Complications (STRONGER): A Multicenter Matched Cohort Analysis. Anesthesiology 2020, 132, 1371–1381. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS((R))) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef]
- Stenberg, E.; Dos Reis Falcao, L.F.; O’Kane, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J. Surg. 2022, 46, 729–751. [Google Scholar] [CrossRef]
- Song, S.W.; Yoo, K.Y.; Ro, Y.S.; Pyeon, T.; Bae, H.B.; Kim, J. Sugammadex is associated with shorter hospital length of stay after open lobectomy for lung cancer: A retrospective observational study. J. Cardiothorac. Surg. 2021, 16, 45. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br. J. Anaesth. 2017, 118, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Mazo, V. Postoperative pulmonary complications. Minerva Anestesiol. 2010, 76, 138–143. [Google Scholar] [PubMed]
- Epstein, N.E. A review article on the benefits of early mobilization following spinal surgery and other medical/surgical procedures. Surg. Neurol. Int. 2014, 5, S66–S73. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.B.; Clark, L.; Glassman, S.D.; Shields, C.B. Decreasing hospital length of stay following lumbar fusion utilizing multidisciplinary committee meetings involving surgeons and other caretakers. Surg. Neurol. Int. 2017, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.M.; Rice, L.R.; Anderson, K.K.; McMahon, J.K.; Connelly, L.M.; Norvell, D.C. Factors affecting hospital length of stay following anterior cervical discectomy and fusion. Evid. Based Spine Care J. 2011, 2, 11–18. [Google Scholar] [CrossRef]
- Wang, M.Y.; Cummock, M.D.; Yu, Y.; Trivedi, R.A. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J. Neurosurg. Spine 2010, 12, 694–699. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Bernardo, W.M.; Nunes, V.M. Benefit of general anesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta-analysis. Braz. J. Anesthesiol. 2017, 67, 72–84. [Google Scholar] [CrossRef]
- Punjasawadwong, Y.; Phongchiewboon, A.; Bunchungmongkol, N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst. Rev. 2014, 2014, CD003843. [Google Scholar] [CrossRef]
- Vivien, B.; Di Maria, S.; Ouattara, A.; Langeron, O.; Coriat, P.; Riou, B. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology 2003, 99, 9–17. [Google Scholar] [CrossRef]
- Garcia, P.S.; Kreuzer, M.; Hight, D.; Sleigh, J.W. Effects of noxious stimulation on the electroencephalogram during general anaesthesia: A narrative review and approach to analgesic titration. Br. J. Anaesth. 2021, 126, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Kreuzer, M.; Hight, D.; Gaskell, A.; Devari, P.; Singh, D.; Taylor, N.B.; Whalin, M.K.; Lee, S.; Sleigh, J.W.; et al. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: An early sign of postoperative complications. Br. J. Anaesth. 2019, 122, 622–634. [Google Scholar] [CrossRef]
- Bocskai, T.; Kovacs, M.; Szakacs, Z.; Gede, N.; Hegyi, P.; Varga, G.; Pap, I.; Toth, I.; Revesz, P.; Szanyi, I.; et al. Is the bispectral index monitoring protective against postoperative cognitive decline? A systematic review with meta-analysis. PLoS ONE 2020, 15, e0229018. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Yang, T.X.; Lee, S.W.K. Effect of Anaesthesia Depth on Postoperative Delirium and Postoperative Cognitive Dysfunction in High-Risk Patients: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e30120. [Google Scholar] [CrossRef]
- Luo, C.; Zou, W. Cerebral monitoring of anaesthesia on reducing cognitive dysfunction and postoperative delirium: A systematic review. J. Int. Med. Res. 2018, 46, 4100–4110. [Google Scholar] [CrossRef] [PubMed]
- Evered, L.A.; Chan, M.T.V.; Han, R.; Chu, M.H.M.; Cheng, B.P.; Scott, D.A.; Pryor, K.O.; Sessler, D.I.; Veselis, R.; Frampton, C.; et al. Anaesthetic depth and delirium after major surgery: A randomised clinical trial. Br. J. Anaesth. 2021, 127, 704–712. [Google Scholar] [CrossRef]
- Sarin, P.; Urman, R.D.; Ohno-Machado, L. An improved model for predicting postoperative nausea and vomiting in ambulatory surgery patients using physician-modifiable risk factors. J. Am. Med. Inform. Assoc. 2012, 19, 995–1002. [Google Scholar] [CrossRef][Green Version]
- Luo, J.; Huang, S.; Gong, M.; Dai, X.; Gao, M.; Yu, T.; Zhou, Z.; Zou, X. Comparison of artificial cervical arthroplasty versus anterior cervical discectomy and fusion for one-level cervical degenerative disc disease: A meta-analysis of randomized controlled trials. Eur. J. Orthop. Surg. Traumatol. 2015, 25 (Suppl. S1), S115–S125. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ding, F.; Li, P.; Ma, D. Cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) in symptomatic cervical degenerative disc diseases (CDDDs): An updated meta-analysis of prospective randomized controlled trials (RCTs). SpringerPlus 2016, 5, 1188. [Google Scholar] [CrossRef]
- Grasu, R.M.; Cata, J.P.; Dang, A.Q.; Tatsui, C.E.; Rhines, L.D.; Hagan, K.B.; Bhavsar, S.; Raty, S.R.; Arunkumar, R.; Potylchansky, Y.; et al. Implementation of an Enhanced Recovery After Spine Surgery program at a large cancer center: A preliminary analysis. J. Neurosurg. Spine 2018, 29, 588–598. [Google Scholar] [CrossRef]
| Variables (Unit) | N (%) or Median (IQR) | Cisatracurium and Neostigmine n = 201 | Rocuronium and Sugammadex n = 201 | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 178 (44.3%) | 94 (46.8%) | 84 (41.8%) | 0.315 |
| Male | 224 (55.7%) | 107 (53.2%) | 117 (58.2%) | |
| Age (years) | 63 (52–70.3) | 62 (51–70.5) | 64 (54.5–70.5) | 0.449 |
| Body weight (kg) | 65 (58–75) | 67 (57.5–76) | 65 (58–73) | 0.230 |
| ASA | ||||
| 1 | 2 (0.5%) | 1 (0.5%) | 1 (0.5%) | 1.000 |
| 2 | 184 (45.8%) | 92 (45.8%) | 92 (45.8%) | |
| 3 | 216 (53.7%) | 108 (53.7%) | 108 (53.7%) | |
| ASA | ||||
| 1 and 2 | 183 (46.3%) | 93 (46.3%) | 93 (46.3%) | 1.000 |
| 3 | 216 (53.7%) | 108 (53.7%) | 108 (53.7%) | |
| Apfel score | ||||
| 0 | 53 (20.1%) | 22 (19.5%) | 31 (20.5%) | 0.430 |
| 1 | 97 (36.7%) | 36 (31.9%) | 61 (40.4%) | |
| 2 | 88 (33.3%) | 43 (38.1%) | 45 (29.8%) | |
| ≧3 | 26 (9.8%) | 12 (10.6%) | 14 (9.3%) | |
| Hypertension | ||||
| No | 194 (48.3%) | 102 (50.7%) | 92 (45.8%) | 0.318 |
| Yes | 208 (51.7%) | 99 (49.3%) | 109 (54.2%) | |
| Diabetes mellitus | ||||
| No | 295 (73.4%) | 155 (77.1%) | 140 (69.7%) | 0.090 |
| Yes | 107 (26.6%) | 46 (22.9%) | 61 (30.3%) | |
| Cerebrovascular accident | ||||
| No | 389 (96.8%) | 196 (97.5%) | 193 (96.0%) | 0.398 |
| Yes | 13 (3.2%) | 5 (2.5%) | 8 (4.0%) | |
| Surgical indications for spine surgery | ||||
| Spinal fusion | 187 (46.5%) | 104 (51.7%) | 83 (41.3%) | 0.248 |
| Decompression | 45 (11.2%) | 18 (9.0%) | 27 (13.4%) | |
| Discectomy | 120 (29.9%) | 57 (28.4%) | 63 (31.3%) | |
| Excision of spinal cord tumor | 38 (9.5%) | 16 (8.0%) | 22 (10.9%) | |
| Others | 12 (3.0%) | 6 (3.0%) | 6 (3.0%) | |
| Variables (Unit) | N (%) or Median (IQR) | Cisatracurium and Neostigmine n = 201 | Rocuronium and Sugammadex n = 201 | p-Value |
|---|---|---|---|---|
| BIS-guided anesthesia | ||||
| No | 190 (47.3%) | 148 (73.6%) | 42 (20.9%) | <0.001 |
| Yes | 212 (52.7%) | 53 (26.4%) | 159 (79.1%) | |
| Duration of anesthesia (h) | 4.58 (3.48–5.98) | 4.67 (3.39–6.09) | 4.50 (3.49–5.90) | 0.647 |
| Sevoflurane consumption (mL/kg/h) | 0.17 (0.14–0.21) | 0.16 (0.13–0.21) | 0.18 (0.16–0.21) | 0.059 |
| Sevoflurane consumption (mL) | 50.01 (35.63–70.00) | 50.01 (35.00–70.14) | 50.01 (39.74–69.99) | 0.696 |
| Fluid administration (mL/kg/h) | 3.99 (3.01–4.92) | 4.05 (2.99–4.94) | 3.95 (3.03–4.94) | 0.985 |
| Urine output (mL/kg/h) | 1.28 (0.92–1.79) | 1.17 (0.91–1.66) | 1.41 (0.92–1.95) | 0.037 |
| Blood loss (mL/kg/h) | 0.67 (0.30–1.31) | 0.82 (0.32–1.34) | 0.66 (0.24–1.22) | 0.364 |
| Intraoperative MME (mg/h) | 3.48 (2.42–4.87) | 3.54 (2.46–5.01) | 3.47 (2.31–4.63) | 0.430 |
| LOS (day) | 7.0 (5.0–9.0) | 7.0 (5.0–10.0) | 6.0 (4.0–8.0) | <0.001 |
| Postoperative chest radiographs | ||||
| Normal | 370 (92.0%) | 179 (89.1%) | 191 (95.0%) | 0.027 |
| Abnormal † | 32 (8.0%) | 22 (10.9%) | 10 (5.0%) | |
| PONV at PACU | ||||
| No | 398 (99.0%) | 199 (99.0%) | 199 (99.0%) | 1.000 |
| Yes | 4 (1.0%) | 2 (1.0%) | 2 (1.0%) | |
| PONV in the ward | ||||
| No | 384 (95.5%) | 193 (96.0%) | 191 (95.0%) | 0.630 |
| Yes | 18 (4.5%) | 8 (4.0%) | 10 (5.0%) |
| Variables (Unit) | N (%) or Median (IQR) | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Female | 178 (44.3%) | 1 | 1 | ||
| Male | 224 (55.7%) | 0.70 (0.46–1.06) | 0.092 | 0.77 (0.41–1.45) | 0.422 |
| Age (y) | 63.0 (52.0–70.3) | 1.02 (1.00–1.03) | 0.031 | 1.00 (0.97–1.03) | 0.847 |
| Body weight (kg) | 65.0 (58.0–75.0) | 1.00 (0.98–1.01) | 0.536 | 1.01(0.98–1.04) | 0.456 |
| BIS | |||||
| None | 190 (47.3%) | 1 | 1 | ||
| Used | 212 (52.7%) | 0.39 (0.26–0.59) | <0.001 | 0.37 (0.18–0.73) | 0.005 |
| ASA 1 and 2 | 186 (46.3%) | 1 | 1 | ||
| ASA 3 | 216 (53.7%) | 3.24 (2.10–5.01) | <0.001 | 2.51 (1.32–4.78) | 0.005 |
| Cisatracurium and neostigmine | 201 (50.0%) | 1 | 1 | ||
| Rocuronium and sugammadex | 201 (50.0%) | 0.43 (0.28–0.65) | <0.001 | 1.36 (0.68–2.74) | 0.388 |
| Duration of anesthesia (h) | 4.58 (3.48–5.98) | 1.36 (1.22–1.53) | <0.001 | 1.27 (1.00–1.63) | 0.053 |
| Sevoflurane consumption (mL) | 50.01 (35.63–70.0) | 1.02 (1.01–1.02) | <0.001 | 1.00 (0.99–1.02) | 0.834 |
| Fluid administration (mL/kg/h) | 3.99 (3.01–4.92) | 1.17 (1.03–1.32) | 0.012 | 1.30 (1.02–1.64) | 0.031 |
| Urine output (mL/kg/h) | 1.28 (0.92–1.79) | 1.12 (0.92–1.36) | 0.267 | 1.26 (0.84–1.90) | 0.267 |
| Blood loss (mL/kg/h) | 0.69 (0.30–1.31) | 1.70 (1.28–2.27) | <0.001 | 1.49 (1.05–2.12) | 0.028 |
| †† Intraoperative MME (mg) | 3.48 (2.42–4.87) | 0.85 (0.75–0.95) | 0.004 | 0.91 (0.74–1.11) | 0.362 |
| Diabetes mellitus | |||||
| None | 295 (73.4%) | 1 | 1 | ||
| Yes | 107 (26.6%) | 1.53 (0.97–2.40) | 0.066 | 0.89 (0.47–1.68) | 0.716 |
| Hypertension | |||||
| None | 194 (48.3%) | 1 | 1 | ||
| Yes | 208 (51.7%) | 1.41 (0.94–2.12) | 0.100 | 1.12 (0.56–2.24) | 0.754 |
| † Postoperative chest radiographs | |||||
| Normal | 370 (92.0%) | 1 | 1 | ||
| Abnormal † | 32 (8.0%) | 11.25 (4.23–29.94) | <0.001 | 7.66 (2.27–25.85) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, E.-B.; Li, Y.-Y.; Hung, K.-C.; Illias, A.M.; Tsai, Y.-F.; Yang, Y.-L.; Chin, J.-C.; Wu, S.-C. The Impact of Rocuronium and Sugammadex on Length of Stay in Patients Undergoing Open Spine Surgery: A Propensity Score-Matched Analysis. Bioengineering 2023, 10, 959. https://doi.org/10.3390/bioengineering10080959
Wu E-B, Li Y-Y, Hung K-C, Illias AM, Tsai Y-F, Yang Y-L, Chin J-C, Wu S-C. The Impact of Rocuronium and Sugammadex on Length of Stay in Patients Undergoing Open Spine Surgery: A Propensity Score-Matched Analysis. Bioengineering. 2023; 10(8):959. https://doi.org/10.3390/bioengineering10080959
Chicago/Turabian StyleWu, En-Bo, Yan-Yi Li, Kuo-Chuan Hung, Amina M. Illias, Yung-Fong Tsai, Ya-Ling Yang, Jo-Chi Chin, and Shao-Chun Wu. 2023. "The Impact of Rocuronium and Sugammadex on Length of Stay in Patients Undergoing Open Spine Surgery: A Propensity Score-Matched Analysis" Bioengineering 10, no. 8: 959. https://doi.org/10.3390/bioengineering10080959
APA StyleWu, E.-B., Li, Y.-Y., Hung, K.-C., Illias, A. M., Tsai, Y.-F., Yang, Y.-L., Chin, J.-C., & Wu, S.-C. (2023). The Impact of Rocuronium and Sugammadex on Length of Stay in Patients Undergoing Open Spine Surgery: A Propensity Score-Matched Analysis. Bioengineering, 10(8), 959. https://doi.org/10.3390/bioengineering10080959










