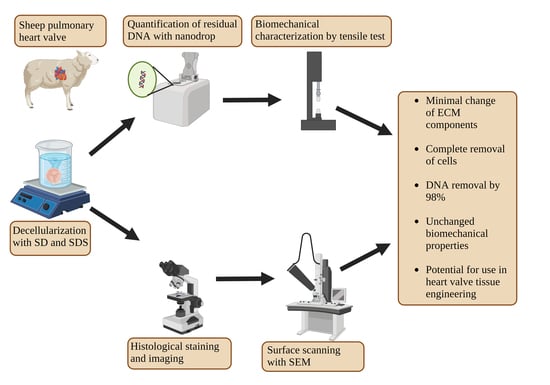
Characterization of a Decellularized Sheep Pulmonary Heart Valves and Analysis of Their Capability as a Xenograft Initial Matrix Material in Heart Valve Tissue Engineering
Abstract
1. Introduction
2. Experimental Design
2.1. Decellularization
2.2. Residual DNA Measurement
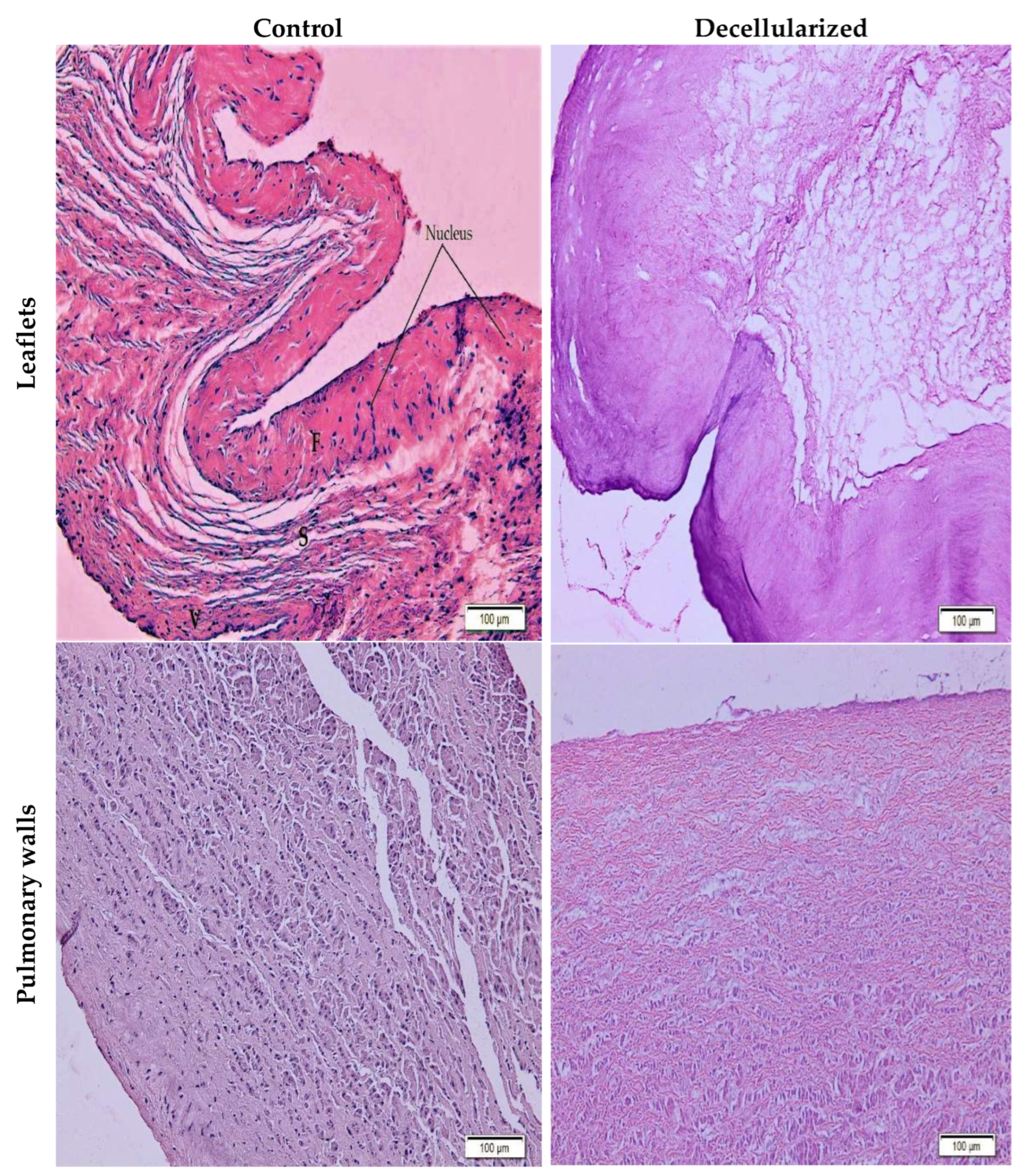
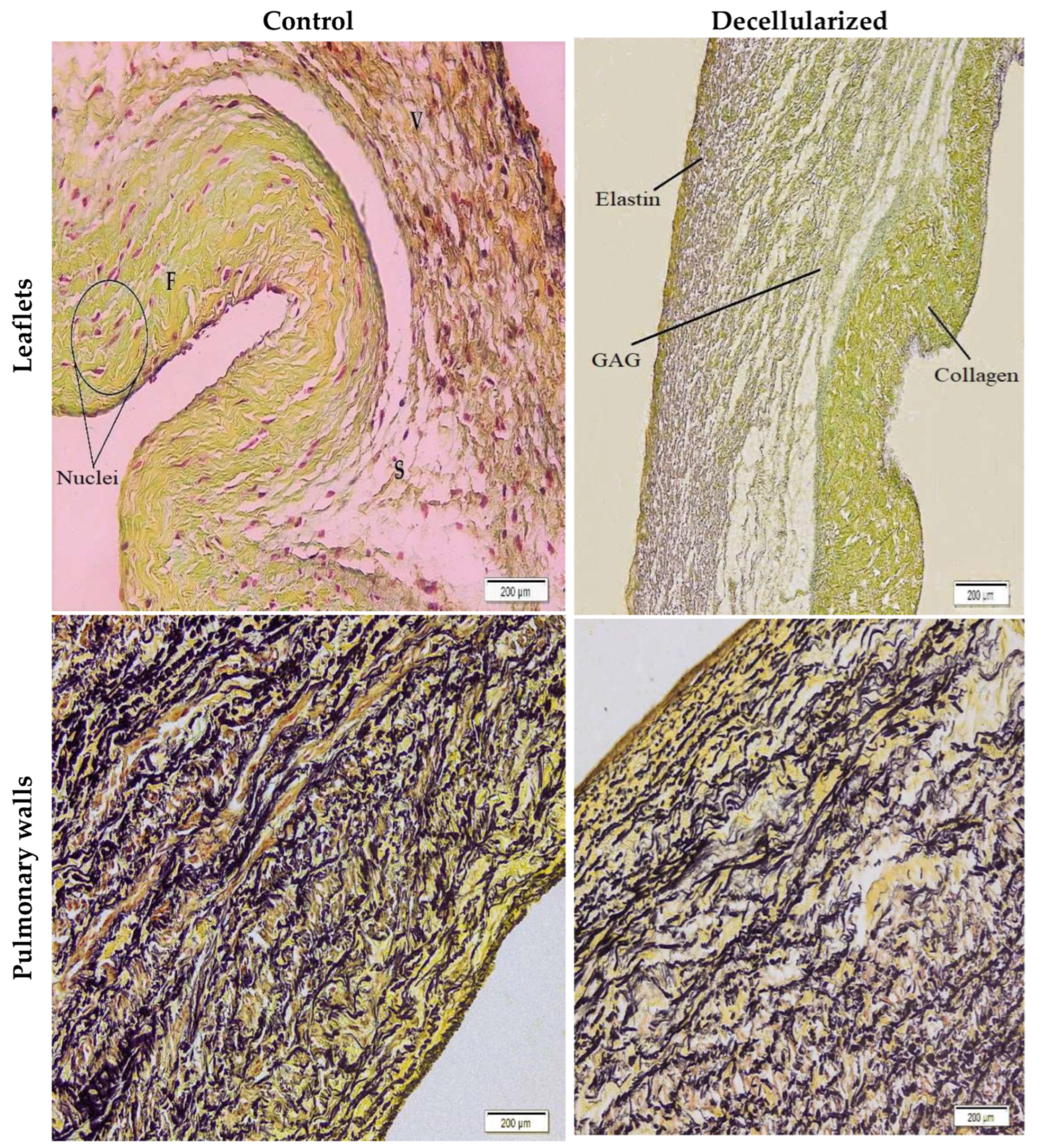
2.3. Histological Characterization
2.4. SEM Imaging
2.5. Tensile Test
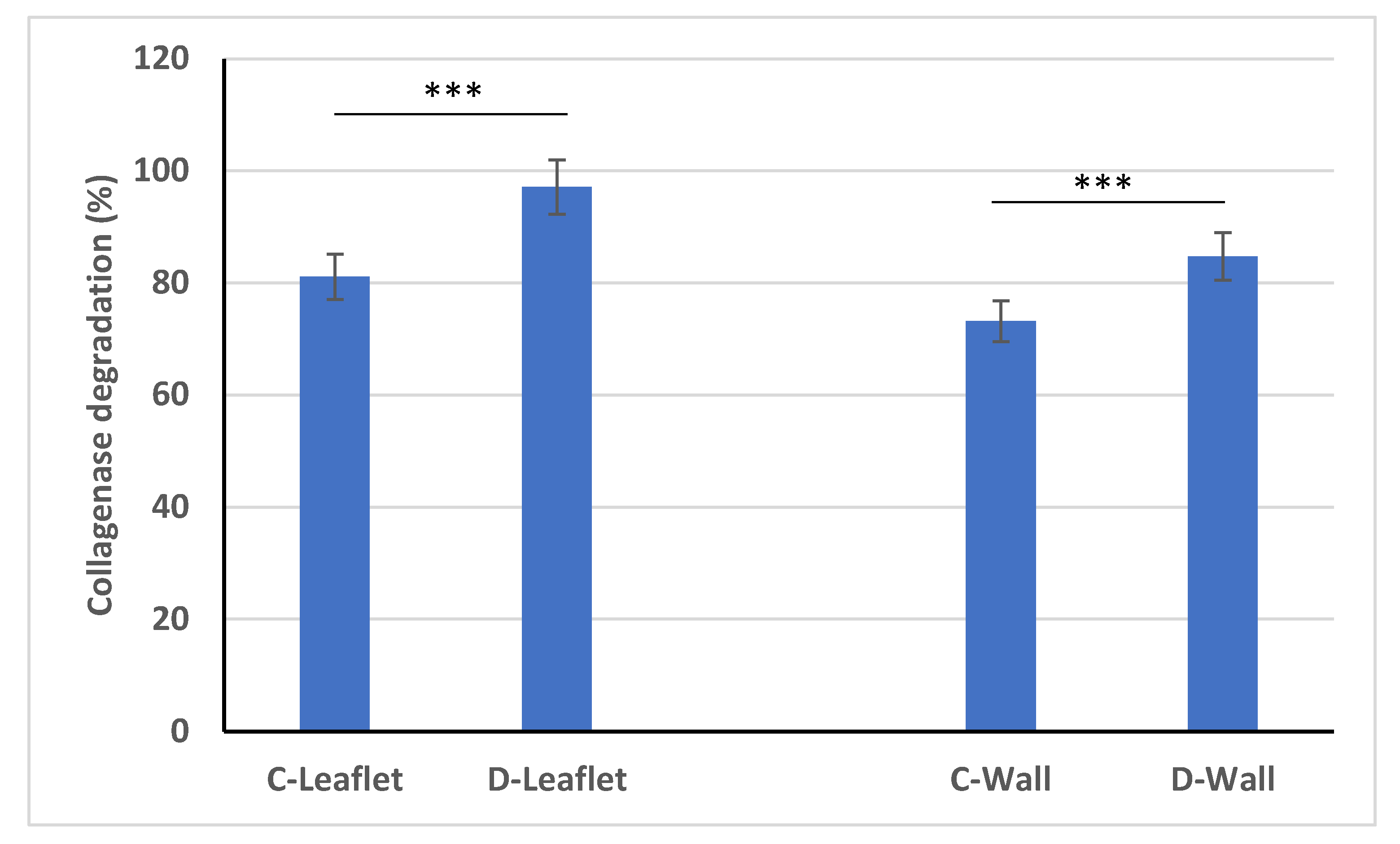
2.6. Collagenase Degradation Experiment
2.7. Swelling Ratio
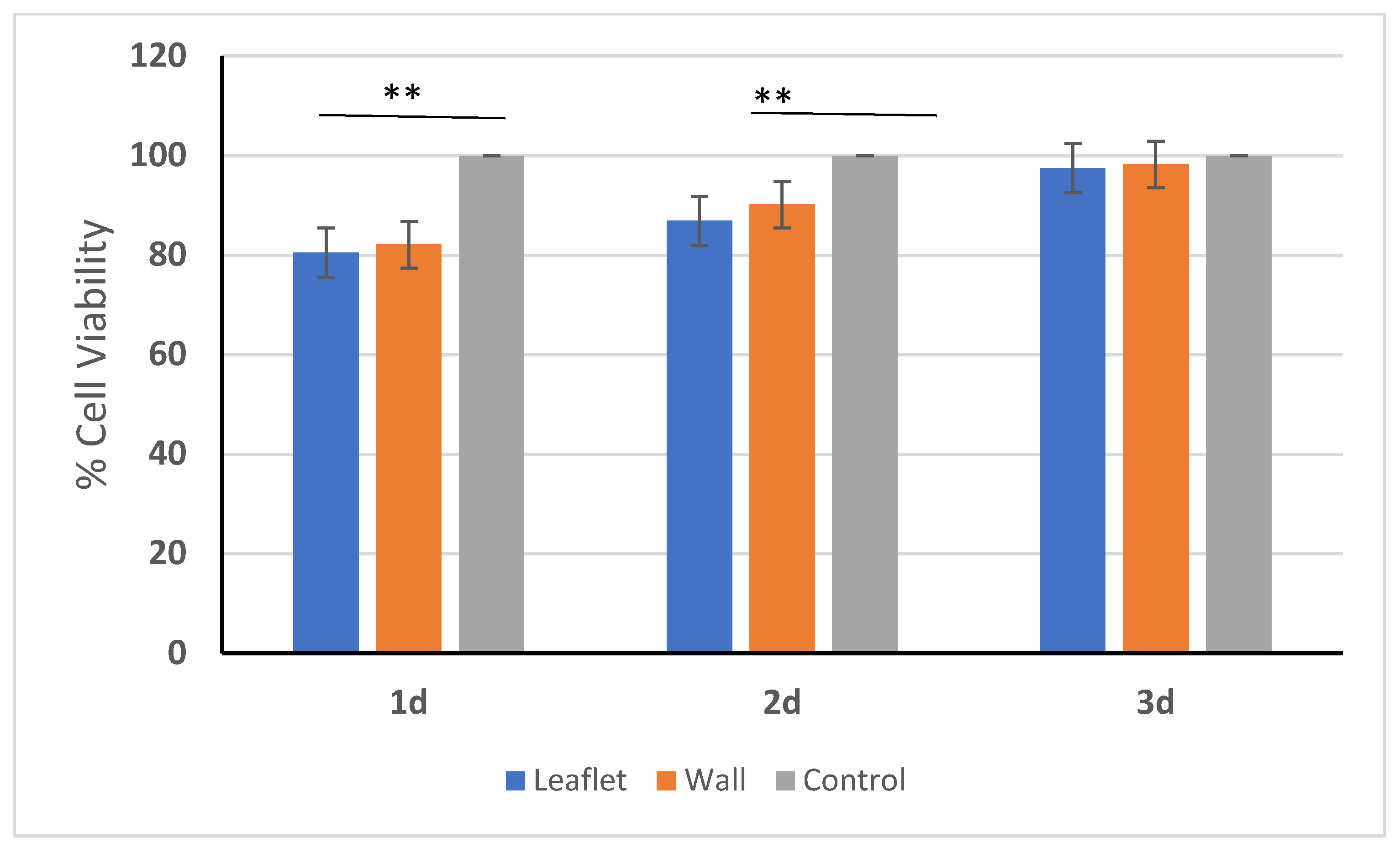
2.8. In Vitro Cytotoxicity Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Swelling Ratio
3.2. Residual DNA Content
3.3. SEM Imaging
3.4. Histological Assessment
3.5. Tensile Test
3.6. Collagenase Degradation
3.7. In Vitro Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucher, L.; Johnson, S. Coronary Artery Disease and Acute Coronary Syndrome. In Medical-Surgical Nursing: Assessment and Management of Clinical Problems; Lewis, S., Ed.; Elsevier: Toronto, ON, Canada, 2014; pp. 730–776. [Google Scholar]
- Korkmaz, F. Structural Infectious and Inflammatory Heart Diseases. In Care in Internal and Surgical Diseases; Karadakovan, A., Aslan, F., Eds.; Akademisyen Kitabevi: Adana, Turkey, 2022; pp. 465–488. [Google Scholar]
- Polat, C.; Enç, N. Heart Valve Diseases and Nursing Care. Turk. J. Cardiovasc. Nurs. 2015, 6, 42–57. [Google Scholar] [CrossRef][Green Version]
- Jahnavi, S.; Kumary, T.V.; Bhuvaneshwar, G.S.; Natarajan, T.S.; Verma, R.S. Engineering of a Polymer Layered Bio-Hybrid Heart Valve Scaffold. Mater. Sci. Eng. C 2015, 51, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Tillquist, M.N.; Maddox, T.M. Cardiac Crossroads: Deciding between Mechanical or Bioprosthetic Heart Valve Replacement. Patient Prefer. Adherence 2011, 5, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-Generation Tissue-Engineered Heart Valves with Repair, Remodelling and Regeneration Capacity. Nat. Rev. Cardiol. 2021, 18, 92–116. [Google Scholar] [CrossRef]
- Akpek, A. Analysis of Biocompatibility Characteristics of Stereolithography Applied Three Dimensional (3D) Bioprinted Artifical Heart Valves. J. Fac. Eng. Archit. Gazi Univ. 2018, 33, 929–938. [Google Scholar] [CrossRef]
- Akpek, A. Triküspit Kalp Kapakçıklarının Üç Boyutlu (3B) Biyobaskı Metotları Ile Fabrikasyonu. Süleyman Demirel Üniversitesi Fen Bilim. Enstitüsü Derg. 2017, 22, 740. [Google Scholar] [CrossRef]
- Wei, W.; Li, Y.; Yang, H.; Nassab, R.; Shahriyari, F.; Akpek, A.; Guan, X.; Liu, Y.; Taranejoo, S.; Tamayol, A.; et al. 3D Printed Anchoring Sutures for Permanent Shaping of Tissues. Macromol. Biosci. 2017, 17, 1700304. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines 2022, 10, 1095. [Google Scholar] [CrossRef]
- Boroumand, S.; Asadpour, S.; Akbarzadeh, A.; Faridi-Majidi, R.; Ghanbari, H. Heart Valve Tissue Engineering: An Overview of Heart Valve Decellularization Processes. Regen. Med. 2018, 13, 41–54. [Google Scholar] [CrossRef]
- Mela, P.; Hinderer, S.; Kandail, H.S.; Bouten, C.V.C.; Smits, A.I.P.M. Tissue-Engineered Heart Valves. In Principles of Heart Valve Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–176. [Google Scholar]
- Simionescu, D.; Harpa, M.M.; Simionescu, A.; Oprita, C.; Movileanu, I. Tissue Engineering Heart Valves—A Review of More than Two Decades into Preclinical and Clinical Testing for Obtaining the Next Generation of Heart Valve Substitutes. Rom. J. Cardiol. 2021, 31, 501–510. [Google Scholar] [CrossRef]
- Ramm, R.; Niemann, H.; Petersen, B.; Haverich, A.; Hilfiker, A. Decellularized GGTA1-KO Pig Heart Valves Do Not Bind Preformed Human Xenoantibodies. Basic Res. Cardiol. 2016, 111, 39. [Google Scholar] [CrossRef]
- Kawecki, M.; Łabuś, W.; Klama-Baryla, A.; Kitala, D.; Kraut, M.; Glik, J.; Misiuga, M.; Nowak, M.; Bielecki, T.; Kasperczyk, A. A Review of Decellurization Methods Caused by an Urgent Need for Quality Control of Cell-Free Extracellular Matrix’ Scaffolds and Their Role in Regenerative Medicine. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 909–923. [Google Scholar] [CrossRef]
- VeDepo, M.C.; Detamore, M.S.; Hopkins, R.A.; Converse, G.L. Recellularization of Decellularized Heart Valves: Progress toward the Tissue-Engineered Heart Valve. J. Tissue Eng. 2017, 8, 204173141772632. [Google Scholar] [CrossRef]
- Copeland, K.M.; Wang, B.; Shi, X.; Simionescu, D.T.; Hong, Y.; Bajona, P.; Sacks, M.S.; Liao, J. Decellularization in Heart Valve Tissue Engineering. In Advances in Heart Valve Biomechanics; Springer International Publishing: Cham, Switzerland, 2018; pp. 289–317. [Google Scholar]
- Hussein, K.H.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Biocompatibility Evaluation of Tissue-Engineered Decellularized Scaffolds for Biomedical Application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of Decellularized Scaffolds for Stem Cell-Driven Tissue Engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef]
- Kasimir, M.-T.; Rieder, E.; Seebacher, G.; Silberhumer, G.; Wolner, E.; Weigel, G.; Simon, P. Comparison of Different Decellularization Procedures of Porcine Heart Valves. Int. J. Artif. Organs 2003, 26, 421–427. [Google Scholar] [CrossRef]
- Cebotari, S.; Mertsching, H.; Kallenbach, K.; Kostin, S.; Repin, O.; Batrinac, A.; Kleczka, C.; Ciubotaru, A.; Haverich, A. Construction of Autologous Human Heart Valves Based on an Acellular Allograft Matrix. Circulation 2002, 106, I-63–I-68. [Google Scholar] [CrossRef]
- Dainese, L.; Guarino, A.; Burba, I.; Esposito, G.; Pompilio, G.; Polvani, G.; Rossini, A. Heart Valve Engineering: Decellularized Aortic Homograft Seeded with Human Cardiac Stromal Cells. J. Heart Valve Dis. 2012, 21, 125–134. [Google Scholar]
- Cigliano, A.; Gandaglia, A.; Lepedda, A.J.; Zinellu, E.; Naso, F.; Gastaldello, A.; Aguiari, P.; De Muro, P.; Gerosa, G.; Spina, M.; et al. Fine Structure of Glycosaminoglycans from Fresh and Decellularized Porcine Cardiac Valves and Pericardium. Biochem. Res. Int. 2012, 2012, 979351. [Google Scholar] [CrossRef]
- Spina, M.; Naso, F.; Zancan, I.; Iop, L.; Dettin, M.; Gerosa, G. Biocompatibility Issues of Next Generation Decellularized Bioprosthetic Devices. Conf. Pap. Sci. 2014, 2014, 869240. [Google Scholar] [CrossRef]
- Snyder, Y.; Jana, S. Strategies for Development of Decellularized Heart Valve Scaffolds for Tissue Engineering. Biomaterials 2022, 288, 121675. [Google Scholar] [CrossRef] [PubMed]
- Wollmann, L.C.; Suss, P.H.; Kraft, L.; Ribeiro, V.S.; Noronha, L.; da Costa, F.D.A.; Tuon, F.F. Histological and Biomechanical Characteristics of Human Decellularized Allograft Heart Valves After Eighteen Months of Storage in Saline Solution. Biopreserv. Biobank 2020, 18, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, K.H.; Sung, S.C.; Kim, Y.S. Effect of Ethanol Washing on Porcine Pulmonary Artery Wall Decellularization Using Sodium Dodecyl Sulfate. Artif. Organs 2022, 46, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Amadeo, F.; Boschetti, F.; Polvani, G.; Banfi, C.; Pesce, M.; Santoro, R. Aortic Valve Cell Seeding into Decellularized Animal Pericardium by Perfusion-Assisted Bioreactor. J. Tissue Eng. Regen. Med. 2018, 12, 1481–1493. [Google Scholar] [CrossRef]
- Seyrek, A.; Günal, G.; Aydin, H.M. Development of Antithrombogenic ECM-Based Nanocomposite Heart Valve Leaflets. ACS Appl. Bio. Mater. 2022, 5, 3883–3895. [Google Scholar] [CrossRef]
- Theodoridis, K.; Tudorache, I.; Calistru, A.; Cebotari, S.; Meyer, T.; Sarikouch, S.; Bara, C.; Brehm, R.; Haverich, A.; Hilfiker, A. Successful Matrix Guided Tissue Regeneration of Decellularized Pulmonary Heart Valve Allografts in Elderly Sheep. Biomaterials 2015, 52, 221–228. [Google Scholar] [CrossRef]
- van Steenberghe, M.; Schubert, T.; Gerelli, S.; Bouzin, C.; Guiot, Y.; Xhema, D.; Bollen, X.; Abdelhamid, K.; Gianello, P. Porcine Pulmonary Valve Decellularization with NaOH-Based vs Detergent Process: Preliminary In Vitro and In Vivo Assessments. J. Cardiothorac. Surg. 2018, 13, 34. [Google Scholar] [CrossRef]
- Hopkins, R.A.; Bert, A.A.; Hilbert, S.L.; Quinn, R.W.; Brasky, K.M.; Drake, W.B.; Lofland, G.K. Bioengineered Human and Allogeneic Pulmonary Valve Conduits Chronically Implanted Orthotopically in Baboons: Hemodynamic Performance and Immunologic Consequences. J. Thorac. Cardiovasc. Surg. 2013, 145, 1098–1107.e3. [Google Scholar] [CrossRef][Green Version]
- Godehardt, A.W.; Ramm, R.; Gulich, B.; Tönjes, R.R.; Hilfiker, A. Decellularized Pig Pulmonary Heart Valves—Depletion of Nucleic Acids Measured by Proviral PERV. Pol. Xenotransplant. 2020, 27, e12565. [Google Scholar] [CrossRef]
- Schoen, F.J.; Levy, R.J. Calcification of Tissue Heart Valve Substitutes: Progress Toward Understanding and Prevention. Ann. Thorac. Surg. 2005, 79, 1072–1080. [Google Scholar] [CrossRef]
- Christ, T.; Paun, A.C.; Grubitzsch, H.; Holinski, S.; Falk, V.; Dushe, S. Long-Term Results after the Ross Procedure with the Decellularized AutoTissue Matrix P® Bioprosthesis Used for Pulmonary Valve Replacement. Eur. J. Cardio-Thorac. Surg. 2019, 55, 885–892. [Google Scholar] [CrossRef]
- Cicha, I.; Rüffer, A.; Cesnjevar, R.; Glöckler, M.; Agaimy, A.; Daniel, W.G.; Garlichs, C.D.; Dittrich, S. Early Obstruction of Decellularized Xenogenic Valves in Pediatric Patients: Involvement of Inflammatory and Fibroproliferative Processes. Cardiovasc. Pathol. 2011, 20, 222–231. [Google Scholar] [CrossRef]
- Rüffer, A.; Purbojo, A.; Cicha, I.; Glöckler, M.; Potapov, S.; Dittrich, S.; Cesnjevar, R.A. Early Failure of Xenogenous De-Cellularised Pulmonary Valve Conduits—A Word of Caution! Eur. J. Cardio-Thorac. Surg. 2010, 38, 78–85. [Google Scholar] [CrossRef]
- Leyh, R.G.; Wilhelmi, M.; Rebe, P.; Fischer, S.; Kofidis, T.; Haverich, A.; Mertsching, H. In Vivo Repopulation of Xenogeneic and Allogeneic Acellular Valve Matrix Conduits in the Pulmonary Circulation. Ann. Thorac. Surg. 2003, 75, 1457–1463. [Google Scholar] [CrossRef]
- Tudorache, I.; Theodoridis, K.; Baraki, H.; Sarikouch, S.; Bara, C.; Meyer, T.; Höffler, K.; Hartung, D.; Hilfiker, A.; Haverich, A.; et al. Decellularized Aortic Allografts versus Pulmonary Autografts for Aortic Valve Replacement in the Growing Sheep Model: Haemodynamic and Morphological Results at 20 Months after Implantation. Eur. J. Cardio-Thorac. Surg. 2016, 49, 1228–1238. [Google Scholar] [CrossRef]
- Abdolghafoorian, H.; Farnia, P.; Sajadi Nia, R.S.; Bahrami, A.; Dorudinia, A.; Ghanavi, J. Effect of Heart Valve Decellularization on Xenograft Rejection. Exp. Clin. Transplant 2017, 15, 329–336. [Google Scholar] [CrossRef][Green Version]
- Ramm, R.; Goecke, T.; Köhler, P.; Tudorache, I.; Cebotari, S.; Ciubotaru, A.; Sarikouch, S.; Höffler, K.; Bothe, F.; Petersen, B.; et al. Immunological and Functional Features of Decellularized Xenogeneic Heart Valves after Transplantation into GGTA1-KO Pigs. Regen. Biomater. 2021, 8, rbab036. [Google Scholar] [CrossRef]
- Baraki, H.; Tudorache, I.; Braun, M.; Höffler, K.; Görler, A.; Lichtenberg, A.; Bara, C.; Calistru, A.; Brandes, G.; Hewicker-Trautwein, M.; et al. Orthotopic Replacement of the Aortic Valve with Decellularized Allograft in a Sheep Model. Biomaterials 2009, 30, 6240–6246. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.W.; Hilbert, S.L.; Converse, G.L.; Bert, A.A.; Buse, E.; Drake, W.B.; Armstrong, M.; Moriarty, S.J.; Lofland, G.K.; Hopkins, R.A. Enhanced Autologous Re-Endothelialization of Decellularized and Extracellular Matrix Conditioned Allografts Implanted Into the Right Ventricular Outflow Tracts of Juvenile Sheep. Cardiovasc. Eng. Technol. 2012, 3, 217–227. [Google Scholar] [CrossRef]
- Quinn, R.W.; Hilbert, S.L.; Bert, A.A.; Drake, B.W.; Bustamante, J.A.; Fenton, J.E.; Moriarty, S.J.; Neighbors, S.L.; Lofland, G.K.; Hopkins, R.A. Performance and Morphology of Decellularized Pulmonary Valves Implanted in Juvenile Sheep. Ann. Thorac. Surg. 2011, 92, 131–137. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, P.; Hu, D.; Al Shirbini, M.; Zhou, X.; Dong, N. Sequential Hydrophile and Lipophile Solubilization as an Efficient Method for Decellularization of Porcine Aortic Valve Leaflets: Structure, Mechanical Property and Biocompatibility Study. J. Tissue Eng. Regen. Med. 2018, 12, e828–e840. [Google Scholar] [CrossRef] [PubMed]
- Chauvette, V.; Bouhout, I.; Tarabzoni, M.; Pham, M.; Wong, D.; Whitlock, R.; Chu, M.W.A.; El-Hamamsy, I.; Lefebvre, L.; Poirier, N.; et al. Pulmonary Homograft Dysfunction after the Ross Procedure Using Decellularized Homografts—A Multicenter Study. J. Thorac. Cardiovasc. Surg. 2022, 163, 1296–1305.e3. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, I.; Calistru, A.; Baraki, H.; Meyer, T.; Höffler, K.; Sarikouch, S.; Bara, C.; Görler, A.; Hartung, D.; Hilfiker, A.; et al. Orthotopic Replacement of Aortic Heart Valves with Tissue-Engineered Grafts. Tissue Eng. Part A 2013, 19, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.W.; Bert, A.A.; Converse, G.L.; Buse, E.E.; Hilbert, S.L.; Drake, W.B.; Hopkins, R.A. Performance of Allogeneic Bioengineered Replacement Pulmonary Valves in Rapidly Growing Young Lambs. J. Thorac. Cardiovasc. Surg. 2016, 152, 1156–1165.e4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Converse, G.L.; Buse, E.E.; Neill, K.R.; McFall, C.R.; Lewis, H.N.; VeDepo, M.C.; Quinn, R.W.; Hopkins, R.A. Design and Efficacy of a Single-Use Bioreactor for Heart Valve Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 249–259. [Google Scholar] [CrossRef]
- Mazine, A.; El-Hamamsy, I.; Verma, S.; Peterson, M.D.; Bonow, R.O.; Yacoub, M.H.; David, T.E.; Bhatt, D.L. Ross Procedure in Adults for Cardiologists and Cardiac Surgeons. J. Am. Coll. Cardiol. 2018, 72, 2761–2777. [Google Scholar] [CrossRef]
- Wollmann, L.C.F.; Laurindo, C.A.H.; da Costa, F.D.A.; Moreno, A.N. Efeito Da Criopreservação e/Ou Da Descelularização Na Matriz Extracelular de Condutos Valvados Porcinos. Rev. Bras. De Cir. Cardiovasc. 2011, 26, 490–496. [Google Scholar] [CrossRef]
- Ghatak, S.; Muthukumaran, R.B.; Nachimuthu, S.K. A Simple Method of Genomic DNA Extraction from Human Samples for PCR-RFLP Analysis. J. Biomol. Tech. 2013, 24, 224–231. [Google Scholar] [CrossRef]
- Hinderer, S.; Seifert, J.; Votteler, M.; Shen, N.; Rheinlaender, J.; Schäffer, T.E.; Schenke-Layland, K. Engineering of a Bio-Functionalized Hybrid off-the-Shelf Heart Valve. Biomaterials 2014, 35, 2130–2139. [Google Scholar] [CrossRef]
- Yu, T.; Chen, X.; Zhuang, W.; Tian, Y.; Liang, Z.; Kong, Q.; Hu, C.; Li, G.; Wang, Y. Nonglutaraldehyde Treated Porcine Pericardium with Good Biocompatibility, Reduced Calcification and Improved Anti-Coagulation for Bioprosthetic Heart Valve Applications. Chem. Eng. J. 2021, 414, 128900. [Google Scholar] [CrossRef]
- Luo, Y.; Lou, D.; Ma, L.; Gao, C. Optimizing Detergent Concentration and Processing Time to Balance the Decellularization Efficiency and Properties of Bioprosthetic Heart Valves. J. Biomed. Mater. Res. A 2019, 107, 2235–2243. [Google Scholar] [CrossRef]
- Lichtenberg, A.; Tudorache, I.; Cebotari, S.; Ringes-Lichtenberg, S.; Sturz, G.; Hoeffler, K.; Hurscheler, C.; Brandes, G.; Hilfiker, A.; Haverich, A. In Vitro Re-Endothelialization of Detergent Decellularized Heart Valves under Simulated Physiological Dynamic Conditions. Biomaterials 2006, 27, 4221–4229. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Findeisen, K.; Morticelli, L.; Goecke, T.; Kolbeck, L.; Ramm, R.; Höffler, H.; Brandes, G.; Korossis, S.; Haverich, A.; Hilfiker, A. Toward Acellular Xenogeneic Heart Valve Prostheses: Histological and Biomechanical Characterization of Decellularized and Enzymatically Deglycosylated Porcine Pulmonary Heart Valve Matrices. Xenotransplantation 2020, 27, e12617. [Google Scholar] [CrossRef]
- Syedain, Z.H.; Bradee, A.R.; Kren, S.; Taylor, D.A.; Tranquillo, R.T. Decellularized Tissue-Engineered Heart Valve Leaflets with Recellularization Potential. Tissue Eng. Part A 2013, 19, 759–769. [Google Scholar] [CrossRef]
- Flameng, W.; De Visscher, G.; Mesure, L.; Hermans, H.; Jashari, R.; Meuris, B. Coating with Fibronectin and Stromal Cell–Derived Factor-1α of Decellularized Homografts Used for Right Ventricular Outflow Tract Reconstruction Eliminates Immune Response–Related Degeneration. J. Thorac. Cardiovasc. Surg. 2014, 147, 1398–1404.e2. [Google Scholar] [CrossRef]
- Haupt, J.; Lutter, G.; Gorb, S.N.; Simionescu, D.T.; Frank, D.; Seiler, J.; Paur, A.; Haben, I. Detergent-Based Decellularization Strategy Preserves Macro- and Microstructure of Heart Valves. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 230–236. [Google Scholar] [CrossRef]
- VeDepo, M.C.; Buse, E.E.; Quinn, R.W.; Williams, T.D.; Detamore, M.S.; Hopkins, R.A.; Converse, G.L. Species-Specific Effects of Aortic Valve Decellularization. Acta Biomater. 2017, 50, 249–258. [Google Scholar] [CrossRef]
- Granados, M.; Morticelli, L.; Andriopoulou, S.; Kalozoumis, P.; Pflaum, M.; Iablonskii, P.; Glasmacher, B.; Harder, M.; Hegermann, J.; Wrede, C.; et al. Development and Characterization of a Porcine Mitral Valve Scaffold for Tissue Engineering. J. Cardiovasc. Transl. Res. 2017, 10, 374–390. [Google Scholar] [CrossRef]
- Vásquez-Rivera, A.; Oldenhof, H.; Hilfiker, A.; Wolkers, W.F. Spectral Fingerprinting of Decellularized Heart Valve Scaffolds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 214, 95–102. [Google Scholar] [CrossRef]
- Rieder, E.; Kasimir, M.-T.; Silberhumer, G.; Seebacher, G.; Wolner, E.; Simon, P.; Weigel, G. Decellularization Protocols of Porcine Heart Valves Differ Importantly in Efficiency of Cell Removal and Susceptibility of the Matrix to Recellularization with Human Vascular Cells. J. Thorac. Cardiovasc. Surg. 2004, 127, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.G.; Park, J.K.; Lee, W.Y. Tissue-Engineered Heart Valve Leaflets: An Effective Method of Obtaining Acellularized Valve Xenografts. Int. J. Artif. Organs 2002, 25, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Lü, X.; Chang, J.; Zhou, Y.; Zhang, H. Quercetin-Crosslinked Porcine Heart Valve Matrix: Mechanical Properties, Stability, Anticalcification and Cytocompatibility. Acta Biomater. 2010, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Huang, S.; Ma, L. A Novel Detergent-Based Decellularization Combined with Carbodiimide Crosslinking for Improving Anti-Calcification of Bioprosthetic Heart Valve. Biomed. Mater. 2021, 16, 045022. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, K.; Müller, J.; Ramm, R.; Findeisen, K.; Andrée, B.; Korossis, S.; Haverich, A.; Hilfiker, A. Effects of Combined Cryopreservation and Decellularization on the Biomechanical, Structural and Biochemical Properties of Porcine Pulmonary Heart Valves. Acta Biomater. 2016, 43, 71–77. [Google Scholar] [CrossRef]
- Hennessy, R.S.; Jana, S.; Tefft, B.J.; Helder, M.R.; Young, M.D.; Hennessy, R.R.; Stoyles, N.J.; Lerman, A. Supercritical Carbon Dioxide–Based Sterilization of Decellularized Heart Valves. JACC Basic Transl. Sci. 2017, 2, 71–84. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, J.; Ding, J.; Xu, J.; Zhong, H.; Lei, S. A Novel Approach to Prepare a Tissue Engineering Decellularized Valve Scaffold with Poly(Ethylene Glycol)–Poly(ε-Caprolactone). RSC Adv. 2016, 6, 14427–14438. [Google Scholar] [CrossRef]
- Zhai, W.; Chang, J.; Lin, K.; Wang, J.; Zhao, Q.; Sun, X. Crosslinking of Decellularized Porcine Heart Valve Matrix by Procyanidins. Biomaterials 2006, 27, 3684–3690. [Google Scholar] [CrossRef]
- Roosens, A.; Somers, P.; De Somer, F.; Carriel, V.; Van Nooten, G.; Cornelissen, R. Impact of Detergent-Based Decellularization Methods on Porcine Tissues for Heart Valve Engineering. Ann. Biomed. Eng. 2016, 44, 2827–2839. [Google Scholar] [CrossRef]
- Sierad, L.N.; Shaw, E.L.; Bina, A.; Brazile, B.; Rierson, N.; Patnaik, S.S.; Kennamer, A.; Odum, R.; Cotoi, O.; Terezia, P.; et al. Functional Heart Valve Scaffolds Obtained by Complete Decellularization of Porcine Aortic Roots in a Novel Differential Pressure Gradient Perfusion System. Tissue Eng. Part C Methods 2015, 21, 1284–1296. [Google Scholar] [CrossRef]



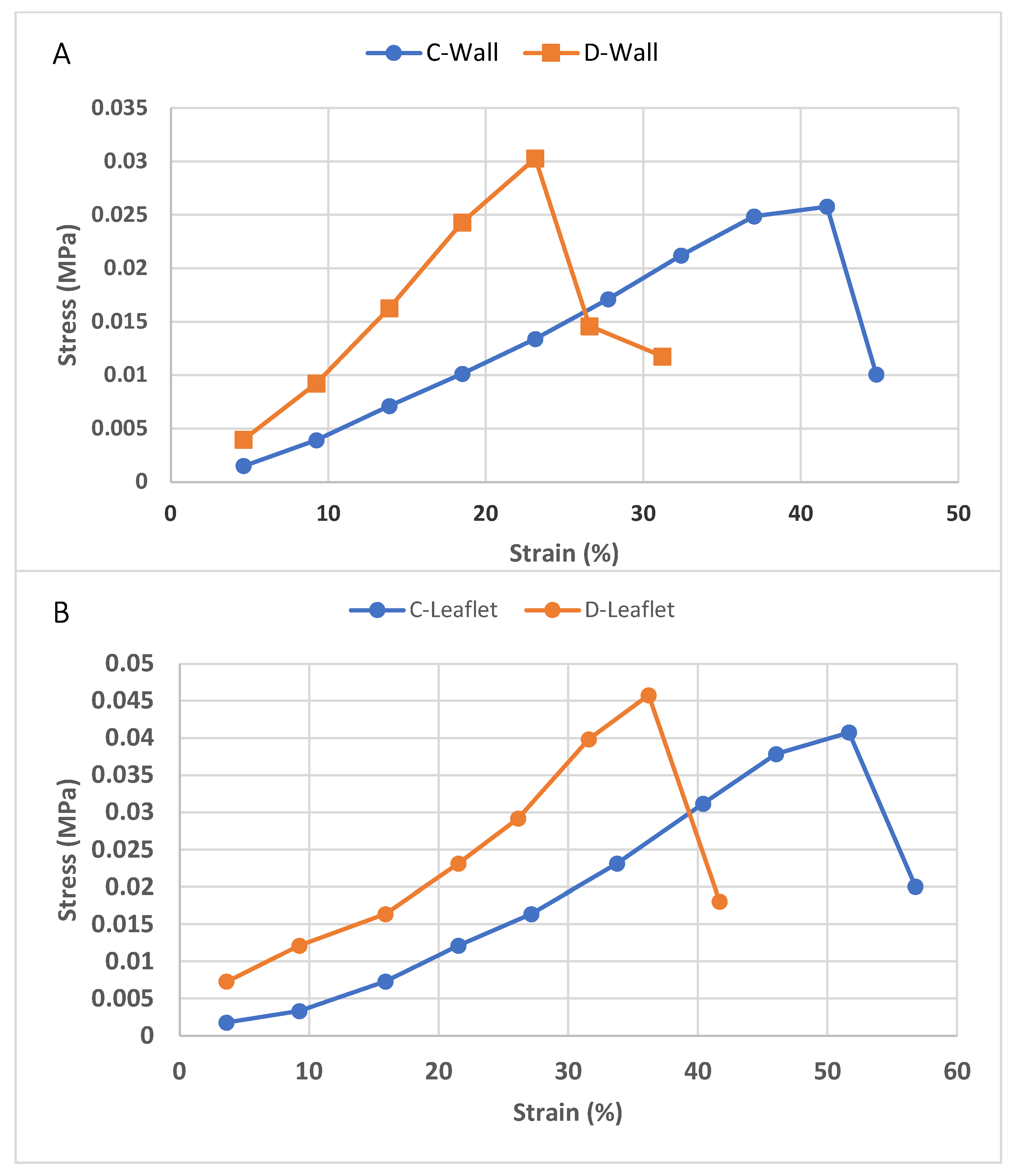
| UTS (MPa) | Modulus of Elasticity (MPa) | |
|---|---|---|
| C-Leaflet | 0.041 ± 0.02 | 0.0042 ± 0.0017 |
| D-Leaflet | 0.049 ± 0.01 | 0.0023 ± 0.001 |
| C-Wall | 0.033 ± 0.02 | 0.0037 ± 0.002 |
| D-Wall | 0.047 ± 0.015 | 0.011 ± 0.003 |
| Heart Valve Type | Decell. Agents | General Finding | Reference |
|---|---|---|---|
| Porcine aortic valve | Trypsin-EDTA + Triton X100 + SD | DNA removal after decellularization was 88.3%, exposed fibers seen in SEM images, the modulus of elasticity in the circumferential sections of the native and decellularized samples were determined as 13.8 kPa and 10.5 kPa, respectively, and no statistically significant difference was found. | [55] |
| Porcine aortic valve | N-Lauroylsarcosine sodium salt | DNA removal after decellularization was 92%, weight losses of native and decellularized samples after collagenase treatment were 80% and 90%, respectively, the decellularization process did not change the modulus of elasticity, while the values of the native and decellularized samples (circumferential direction) were measured as 12.8 MPa and 13 MPa, respectively. | [68] |
| Porcine pulmonary valve | Triton X-100 + SDS | DNA was reduced by 66% after decellularization of pulmonary wall samples, the UTS value of the native samples sectioned in the circumferential direction was determined as 0.5 MPa, and a statistically significant increase to 1.2 MPa was observed after decellularization; similarly, a significant increase from 1.2 MPa to 3.5 MPa was observed in the modulus of elasticity. | [69] |
| Porcine pulmonary valve | Trypsin-EDTA | The UTS values of the circumferential sections of native and decellularized leaflet samples were determined as 8.2 MPa and 7.8 MPa, respectively, while the elastic modulus values were measured as 24 MPa and 25 MPa, respectively, and there was no statistically significant difference between these values. | [67] |
| Porcine aortic valve | SDS | The UTS values of the circumferential sections of native and decellularized leaflet samples were determined as 4.21 MPa and 1.93 MPa, respectively, while the stiffness values were measured as 7.50 MPa and 3.24 MPa, respectively, and a critical decrease of biomechanical properties was reported after decellularization. | [70] |
| Porcine aortic valve | SD | DNA removal after decellularization was 93%, the modulus of elasticity of the radial sections of the native and decellularized samples was 3.8 MPa and 3.6 MPa, the UTS values were measured as 1 MPa and 0.7 MPa, respectively, and no statistically significant difference was found. | [71] |
| Porcine aortic valve | Triton X100 + SD + EDTA | Weight loss in the decellularized group after collagenase II treatment was 100%, while the UTS value of the native samples taken in the circumferential direction was 8.9 MPa; this value almost did not change after decellularization, while the elastic modulus values were measured as 21.8 MPa and 25 MPa, respectively. | [72] |
| Porcine aortic valve | SD | DNA removal after decellularization was 72%, there was a significant increase in stiffness after decellularization (9.17 to 11.49 N/mm), and there was no significant difference in work to maximum load values. | [73] |
| Porcine aortic valve | SDS + Triton X100 + SD + EDTA | DNA removal after decellularization was 90%; with Movat’s pentachrome staining, it was observed that collagens and elastins were preserved and the amount of GAG decreased. There was no statistically significant difference in biaxial mechanical properties in both radial and circumferential sections of native and decellularized samples. | [74] |
| Porcine aortic valve | Triton X-100 + SD + IGEPAL CA-630 | Both modulus of elasticity and UTS values in the radial direction of native and decellularized leaflets were found to be approximately 2 MPa, and no significant difference was observed between the two groups. No cell nuclei were found in histological staining. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İnal, M.S.; Darcan, C.; Akpek, A. Characterization of a Decellularized Sheep Pulmonary Heart Valves and Analysis of Their Capability as a Xenograft Initial Matrix Material in Heart Valve Tissue Engineering. Bioengineering 2023, 10, 949. https://doi.org/10.3390/bioengineering10080949
İnal MS, Darcan C, Akpek A. Characterization of a Decellularized Sheep Pulmonary Heart Valves and Analysis of Their Capability as a Xenograft Initial Matrix Material in Heart Valve Tissue Engineering. Bioengineering. 2023; 10(8):949. https://doi.org/10.3390/bioengineering10080949
Chicago/Turabian Styleİnal, Müslüm Süleyman, Cihan Darcan, and Ali Akpek. 2023. "Characterization of a Decellularized Sheep Pulmonary Heart Valves and Analysis of Their Capability as a Xenograft Initial Matrix Material in Heart Valve Tissue Engineering" Bioengineering 10, no. 8: 949. https://doi.org/10.3390/bioengineering10080949
APA Styleİnal, M. S., Darcan, C., & Akpek, A. (2023). Characterization of a Decellularized Sheep Pulmonary Heart Valves and Analysis of Their Capability as a Xenograft Initial Matrix Material in Heart Valve Tissue Engineering. Bioengineering, 10(8), 949. https://doi.org/10.3390/bioengineering10080949