Effect of MgCl2 Loading on the Yield and Performance of Cabbage-Based Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Carbon Materials
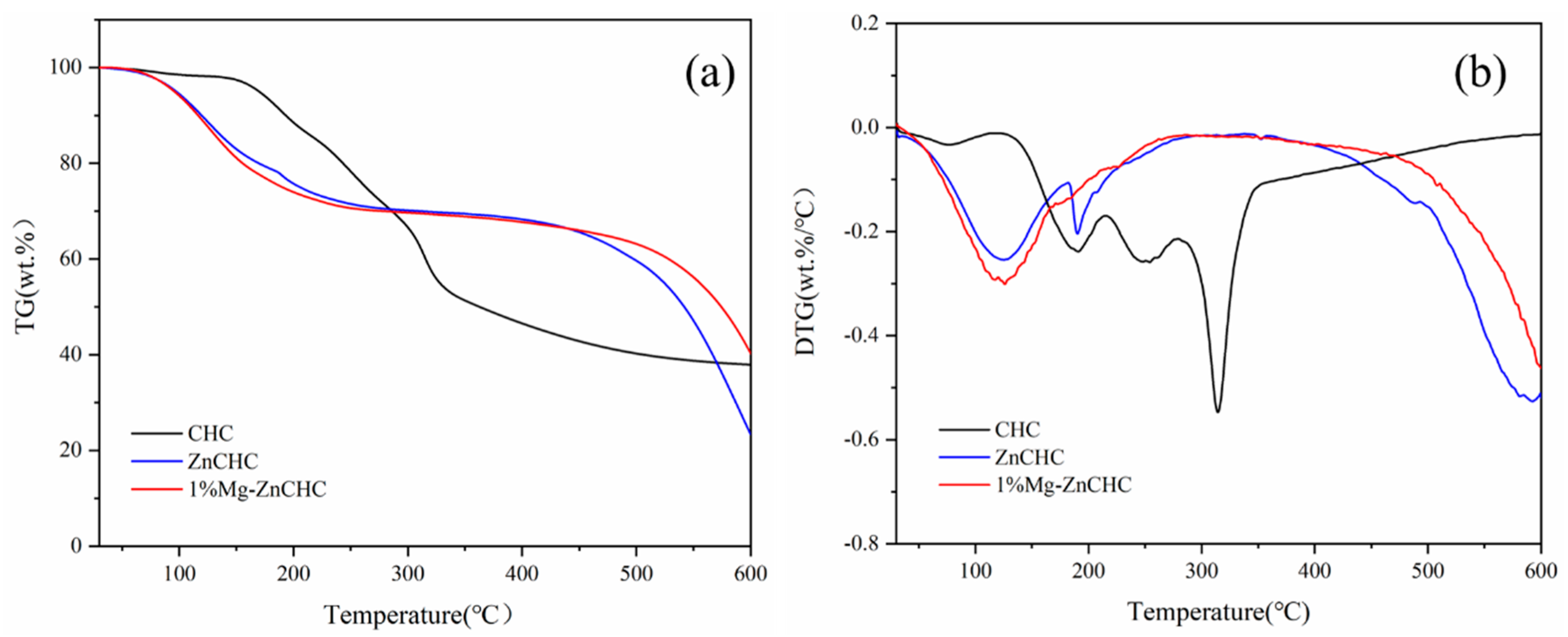
2.3. Thermogravimetric Test
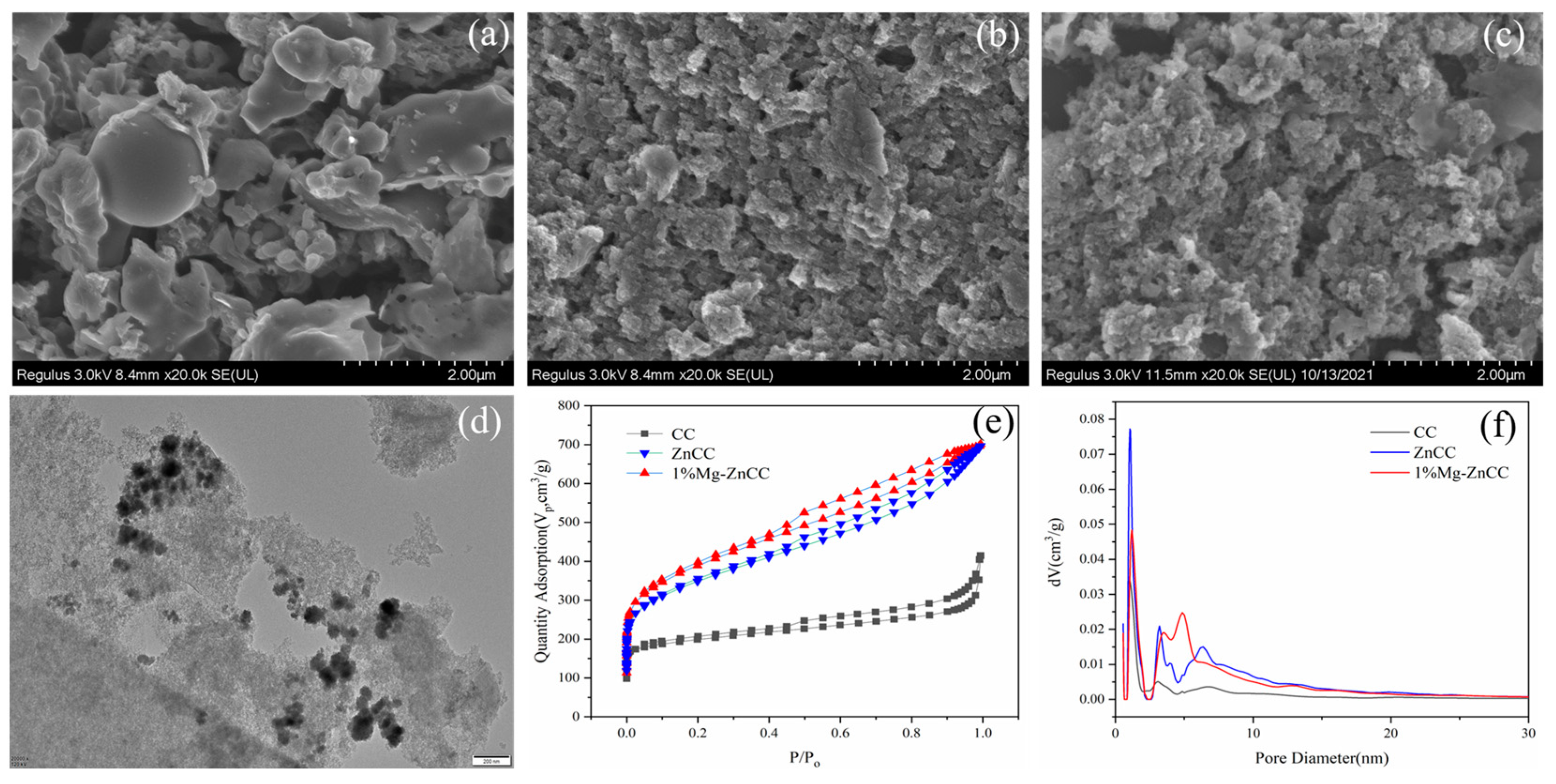
2.4. Characterization of Activated Carbon
2.5. Adsorption Experiment
3. Result and Discussion
3.1. Effect of MgCl2 Loading on the Thermal Degradation Process
3.2. Effect of MgCl2 Loading on Product Properties
3.2.1. Characterization of Carbon Materials
3.2.2. Effect of MgCl2 Doping on Carbon Yields
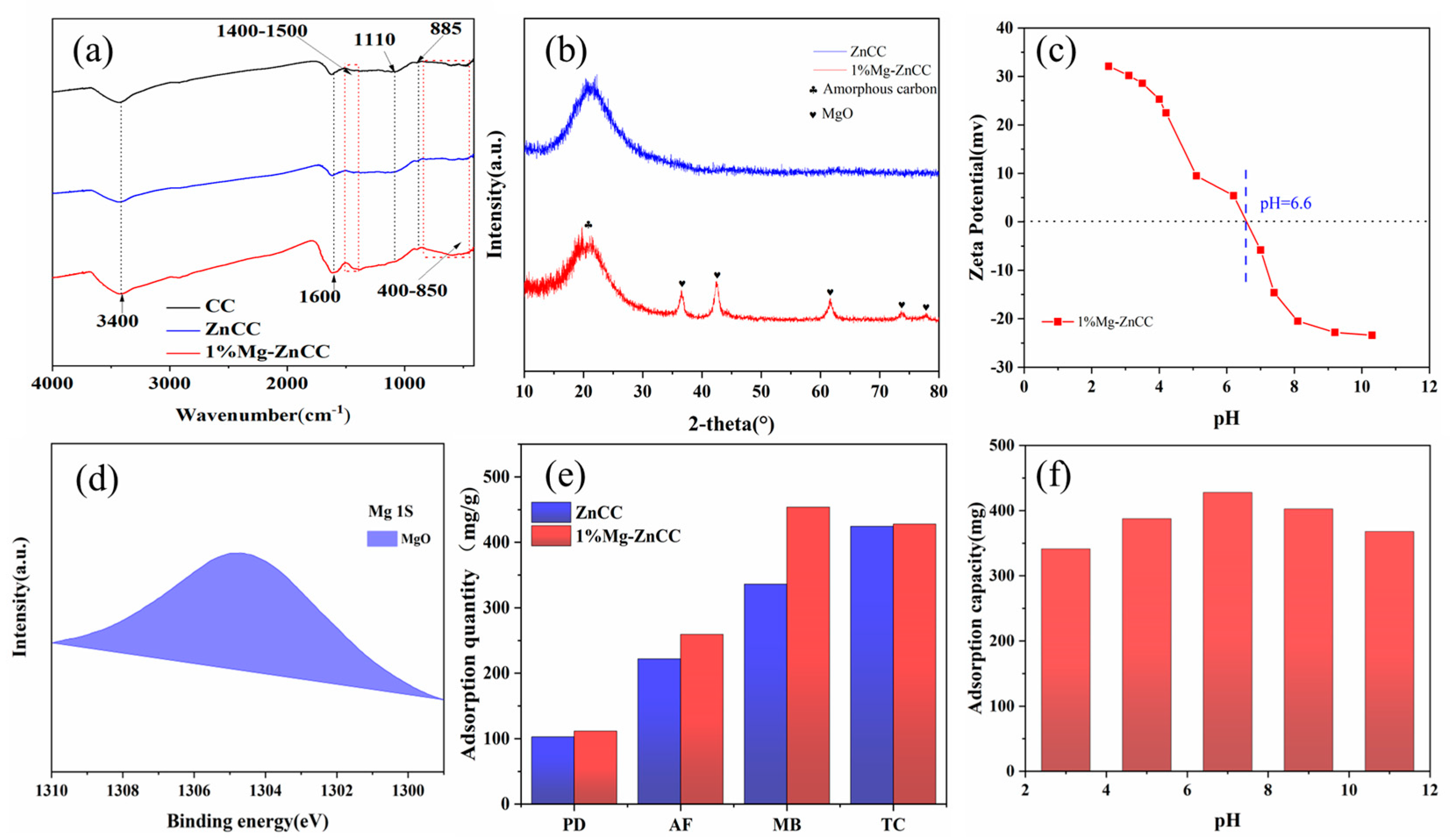
3.2.3. FTIR Analysis of Cabbage-Based Activated Carbon
3.2.4. XRD Analysis of Cabbage-Based Activated Carbon
3.2.5. Zeta Potential
3.2.6. XPS
3.3. Contaminant Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azevedo, S.G.; Sequeira, T.; Santos, M.; Mendes, L. Biomass-related sustainability: A review of the literature and interpretive structural modeling. Energy 2019, 171, 1107–1125. [Google Scholar] [CrossRef]
- Yang, X.; Kang, K.; Qiu, L.; Zhao, L.; Sun, R. Effects of carbonization conditions on the yield and fixed carbon content of biochar from pruned apple tree branches. Renew. Energy 2020, 146, 1691–1699. [Google Scholar] [CrossRef]
- Chen, T.; Liu, R.; Scott, N.R. Characterization of energy carriers obtained from the pyrolysis of white ash, switchgrass and corn stover—Biochar, syngas and bio-oil. Fuel Process. Technol. 2016, 142, 124–134. [Google Scholar] [CrossRef]
- Özçimen, D.; Karaosmanoğlu, F. Production and Characterization of bio-oil and biochar from rapeseed cake. Renew. Energy 2004, 29, 779–787. [Google Scholar] [CrossRef]
- Zhang, S.; Su, Y.; Xu, D.; Zhu, S.; Zhang, H.; Liu, X. Effects of torrefaction and organic-acid leaching pretreatment on the pyrolysis behavior of rice husk. Energy 2018, 149, 804–813. [Google Scholar] [CrossRef]
- Zhang, S.; Su, Y.; Xiong, Y.; Zhang, H. Physicochemical structure and reactivity of char from torrefied rice husk: Effects of inorganic species and torrefaction temperature. Fuel 2020, 262, 116667. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Özçimen, D.; Ersoy-Meriçboyu, A. Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renew. Energy 2010, 35, 1319–1324. [Google Scholar] [CrossRef]
- Thakkar, J.; Kumar, A.; Ghatora, S.; Canter, C. Energy balance and greenhouse gas emissions from the production and sequestration of charcoal from agricultural residues. Renew. Energy 2016, 94, 558–567. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Park, Y.K.; Yoo, M.L.; Lee, H.W.; Park, S.H.; Jung, S.C.; Park, S.S.; Kim, S.C. Effects of operation conditions on pyrolysis characteristics of agricultural residues. Renew. Energy 2012, 42, 125–130. [Google Scholar] [CrossRef]
- Lam, K.L.; Oyedun, A.O.; Hui, C.W. Experimental and Modelling Studies of Biomass Pyrolysis. Chin. J. Chem. Eng. 2012, 20, 543–550. [Google Scholar] [CrossRef]
- Singh, R.; Krishna, B.B.; Mishra, G.; Kumar, J.; Bhaskar, T. Strategies for selection of thermo-chemical processes for the valorization of biomass. Renew. Energy 2016, 98, 226–237. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, M.; Fan, X.; Song, J.; Peng, P.; Li, K.; Jia, W.; Song, H. IInfluence of pyrolysis temperature and feedstock on carbon fractions of biochar produced from pyrolysis of rice straw, pine wood, pig manure and sewage sludge. Chemosphere 2019, 218, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Cao, J.; Li, Y.; Howard, A.; Yu, K. Effect of pyrolysis temperature on characteristics of biochars derived from different feedstocks: A case study on ammonium adsorption capacity. Waste Manag. 2019, 87, 652–660. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Z.; Niu, W.; Yang, L.; Zhou, T.; Qin, D.; Niu, Z.; Yuan, Q. Effects of pyrolysis temperature on the physicochemical properties of gas and biochar obtained from pyrolysis of crop residues. Energy 2018, 143, 746–756. [Google Scholar] [CrossRef]
- Yu, S.; Park, J.; Kim, M.; Ryu, C.; Park, J. Characterization of biochar and byproducts from slow pyrolysis of hinoki cypress. Bioresour. Technol. Rep. 2019, 6, 217–222. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Hamzah, Z.; Daud, M.Z.M.; Zakaria, Z. The Effects of Holding Time and the Sweeping Nitrogen Gas Flowrates on the Pyrolysis of EFB using a Fixed–Bed Reactor. Procedia Eng. 2013, 53, 185–191. [Google Scholar] [CrossRef]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Huang, H.; Chen, Y. Influence of pyrolysis temperature and holding time on properties of biochar derived from medicinal herb (radix isatidis) residue and its effect on soil CO2 emission. J. Anal. Appl. Pyrolysis 2014, 110, 277–284. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.-M.; Dimin, M.; Juoi, J.M.; Husin, M.H.M.; Mitan, N.M.M. Influence of heating temperature and holding time on biochars derived from rubber wood sawdust via slow pyrolysis. J. Anal. Appl. Pyrolysis 2014, 107, 31–39. [Google Scholar] [CrossRef]
- Safdari, M.-S.; Amini, E.; Weise, D.R.; Fletcher, T.H. Heating rate and temperature effects on pyrolysis products from live wildland fuels. Fuel 2019, 242, 295–304. [Google Scholar] [CrossRef]
- Zanzi, R.; Sjöström, K.; Björnbom, E. Rapid pyrolysis of agricultural residues at high temperature. Biomass Bioenergy 2002, 23, 357–366. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Zhu, S.; Liu, X.; Xiong, Y.; Zhang, H. Effects of MgCl2 and Mg(NO3)2 loading on catalytic pyrolysis of sawdust for bio-oil and MgO-impregnated biochar production. J. Anal. Appl. Pyrolysis 2020, 152, 104962. [Google Scholar] [CrossRef]
- Hill, S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley: Chichester, UK, 2006. [Google Scholar]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.K.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Carvalho, W.S.; Cunha, I.F.; Pereira, M.S.; Ataíde, C.H. Thermal decomposition profile and product selectivity of analytical pyrolysis of sweet sorghum bagasse: Effect of addition of inorganic salts. Ind. Crop. Prod. 2015, 74, 372–380. [Google Scholar] [CrossRef]
- Shimada, N.; Kawamoto, H.; Saka, S. Different action of alkali/alkaline earth metal chlorides on cellulose pyrolysis. J. Anal. Appl. Pyrolysis 2008, 81, 80–87. [Google Scholar] [CrossRef]
- Li, A.; Ge, W.; Liu, L.; Qiu, G. Preparation, adsorption performance and mechanism of MgO-loaded biochar in wastewater treatment: A review. Environ. Res. 2022, 212, 113341. [Google Scholar] [CrossRef]
- Tang, X.; Ran, G.; Li, J.; Zhang, Z.; Xiang, C. Extremely efficient and rapidly adsorb methylene blue using porous adsorbent prepared from waste paper: Kinetics and equilibrium studies. J. Hazard. Mater. 2021, 402, 123579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, L.; Yu, H.; Yan, T.; Li, X. Adsorption of phosphate from aqueous solution by vegetable biochar/layered double oxides: Fast removal and mechanistic studies. Bioresour. Technol. 2019, 284, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Islam, M.S.; Arifin, M.T.; Firoz, S.H. Synthesis, Characterization and Sorption Properties of Biochar, Chitosan and ZnO-Based Binary Composites towards a Cationic Dye. Sustainability 2022, 14, 14571. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, J.; Zhang, Y.; Zhou, J.; Shi, B. Conversion of tannery solid waste to an adsorbent for high-efficiency dye removal from tannery wastewater: A road to circular utilization. Chemosphere 2021, 263, 127987. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, J.; Hou, D.; Tsang, D.C.; Ok, Y.S.; Alessi, D.S. Synthesis of MgO-coated corncob biochar and its application in lead stabilization in a soil washing residue. Environ. Int. 2019, 122, 357–362. [Google Scholar] [CrossRef]
- Myglovets, M.; Poddubnaya, O.I.; Sevastyanova, O.; Lindström, M.E.; Gawdzik, B.; Sobiesiak, M.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Preparation of carbon adsorbents from lignosulfonate by phosphoric acid activation for the adsorption of metal ions. Carbon 2014, 80, 771–783. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B.; Zimmerman, A.; Harris, W. Biomass-facilitated production of activated magnesium oxide nanoparticles with extraordinary CO2 capture capacity. Chem. Eng. J. 2018, 334, 81–88. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Tian, K.; Ding, Y.-W.; Yu, H.-Q. Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ. Sci. Technol. 2013, 47, 9397–9403. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Khan, K.Y.; Li, T.; Yang, X.; He, Z. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Nan, H.; Pei, L.; Huang, Y.; Cao, X.; Xu, X.; Zhao, L. Metal chloride-loaded biochar for phosphorus recovery: Noteworthy roles of inherent minerals in precursor. Chemosphere 2021, 266, 128991. [Google Scholar] [CrossRef]
- Dai, Y.; Li, J.; Shan, D. Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs. Chemosphere 2020, 238, 124432. [Google Scholar] [CrossRef]
- Rouhani, M.; Ashrafi, S.D.; Taghavi, K.; Joubani, M.N.; Jaafari, J. Evaluation of tetracycline removal by adsorption method using magnetic iron oxide nanoparticles (Fe3O4) and clinoptilolite from aqueous solutions. J. Mol. Liq. 2022, 356, 119040. [Google Scholar] [CrossRef]
- Huang, K.; Yang, S.; Liu, X.; Zhu, C.; Qi, F.; Wang, K.; Wang, J.; Wang, Q.; Wang, T.; Ma, P. Adsorption of antibiotics from wastewater by cabbage-based N, P co-doped mesoporous carbon materials. J. Clean. Prod. 2023, 391, 136174. [Google Scholar] [CrossRef]
- Zhai, S.; Li, M.; Peng, H.; Wang, D.; Fu, S. Cost-effective resource utilization for waste biomass: A simple preparation method of photo-thermal biochar cakes (BCs) toward dye wastewater treatment with solar energy. Environ. Res. 2021, 194, 110720. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: Review. Environ. Toxicol. Pharmacol. 2017, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Q.; Zheng, C. Sorption of sulfadiazine, norfloxacin, metronidazole, and tetracycline by granular activated carbon: Kinetics, mechanisms, and isotherms. Water Air. Soil Pollut 2017, 228, 129–141. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Ngo, H.H.; Wen, H.; Li, N.; Wu, W. Performance evaluation of powdered activated carbon for removing 28 types of antibiotics CWom water. J. Environ. Manag 2016, 172, 193–200. [Google Scholar] [CrossRef]
- Wang, S.-L.; Tzou, Y.-M.; Lu, Y.-H.; Sheng, G. Removal of 3-chlorophenol from water using rice-straw-based carbon. J. Hazard. Mater. 2007, 147, 313–318. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Coconut husk derived activated carbon via microwave induced activation: Effects of activation agents, preparation parameters and adsorption performance. Chem. Eng. J. 2012, 184, 57–65. [Google Scholar] [CrossRef]
- Huang, C.; Puziy, A.M.; Sun, T.; Poddubnaya, O.I.; Suarez-García, F.; Tascon, J.M.D.; Hulicova-Jurcakova, D. Capacitive behaviours of phosphorus-rich carbons derived CWom lignocelluloses. Electrochim. Acta 2014, 137, 219–227. [Google Scholar] [CrossRef]
- Angin, D. Utilization of activated carbon produced CWom CWuit juice industry solid waste for the adsorption of yellow 18 CWom aqueous solutions. Bioresour. Technol. 2014, 259–266. [Google Scholar] [CrossRef]
- Arjmand, C.; Kaghazchi, T.; Latifi, S.M.; Soleimani, M. Chemical production of activated carbon CWom nutshells and date stones. Chem. Eng. Technol. 2010, 29, 986–991. [Google Scholar] [CrossRef]
- Roy, H.; Sarkar, D.; Pervez, N.; Paul, S.; Cai, Y.; Naddeo, V.; Firoz, S.H.; Islam, S. Synthesis, Characterization and Performance Evaluation of Burmese Grape (Baccaurea ramiflora) Seed Biochar for Sustainable Wastewater Treatment. Water 2023, 15, 394. [Google Scholar] [CrossRef]
- Bagheri, N.; Abedi, J. Adsorption of methane on corn cobs based activated carbon. Chem. Eng. Res. Des. 2011, 89, 2038–2043. [Google Scholar] [CrossRef]
- Li, S.; You, T.; Guo, Y.; Yao, S.; Xiao, M.; Zhang, Z.; Shen, Y. High ispersions of nano zero valent iron supported on biochar by one-step carbothermal synthesis and its application in chromate removal. RSC Adv. 2019, 9, 12435–12848. [Google Scholar]
- Sze, M.F.F.; McKay, G. An adsorption diffusion model for removal of parachlorophenol by activated carbon derived CWom bituminous coal. Environ. Pollut 2010, 158, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Basaleh, A.A.; Al-Malack, M.H.; Saleh, T.A. Methylene Blue removal using polyamide-vermiculite nanocomposites:Kinetics, equilibrium and thermodynamic study. Environ. Chem. Eng. 2019, 7, 103–107. [Google Scholar]
- Kawamoto, H.; Yamamoto, D.; Saka, S. Influence of neutral inorganic chlorides on primary and secondary char formation from cellulose. Wood Sci. 2008, 54, 242–246. [Google Scholar] [CrossRef]
| Name | Specific Surfaceeik (m2/g) | Aperture (nm) | Hole Capacity (cc/g) | ||||
|---|---|---|---|---|---|---|---|
| Microporous | External Surface | Total | Microporous | Mesopore | Total | ||
| CC | 262.165 | 200.08 | 462.245 | 5.67 | 0.114 | 0.418 | 0.5326 |
| ZnCC | 289.967 | 738.149 | 1028.12 | 10.61 | 0.133 | 0.7972 | 0.9302 |
| 1%Mg-ZnCC | 229.193 | 855.444 | 1084.64 | 11.44 | 0.106 | 0.9174 | 1.0234 |
| Name | C [%] | H [%] | O [%] | N [%] | Charcoal Yield | Carbon Yield |
|---|---|---|---|---|---|---|
| Cabbage | 31.58 | 5.203 | 38.274 | 4.54 | ||
| CC | 66.12 | 1.211 | 14.7033 | 5.16 | 39.08 | 81.81 |
| ZnCC | 76.14 | 2.037 | 7.9948 | 3.54 | 19.26 | 46.44 |
| 1%Mg-ZnCC | 74.58 | 3.247 | 9.0961 | 2.77 | 24.81 | 58.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Huang, K.; Xue, M.; Zhang, Y.; Wang, J.; Liu, L. Effect of MgCl2 Loading on the Yield and Performance of Cabbage-Based Biochar. Bioengineering 2023, 10, 836. https://doi.org/10.3390/bioengineering10070836
Zhu C, Huang K, Xue M, Zhang Y, Wang J, Liu L. Effect of MgCl2 Loading on the Yield and Performance of Cabbage-Based Biochar. Bioengineering. 2023; 10(7):836. https://doi.org/10.3390/bioengineering10070836
Chicago/Turabian StyleZhu, Cui, Kuncheng Huang, Mengyuan Xue, Yiming Zhang, Jiaquan Wang, and Lu Liu. 2023. "Effect of MgCl2 Loading on the Yield and Performance of Cabbage-Based Biochar" Bioengineering 10, no. 7: 836. https://doi.org/10.3390/bioengineering10070836
APA StyleZhu, C., Huang, K., Xue, M., Zhang, Y., Wang, J., & Liu, L. (2023). Effect of MgCl2 Loading on the Yield and Performance of Cabbage-Based Biochar. Bioengineering, 10(7), 836. https://doi.org/10.3390/bioengineering10070836








