Scatter Radiation Distribution to Radiographers, Nearby Patients and Caretakers during Portable and Pediatric Radiography Examinations
Abstract
1. Introduction
2. Materials and Methods
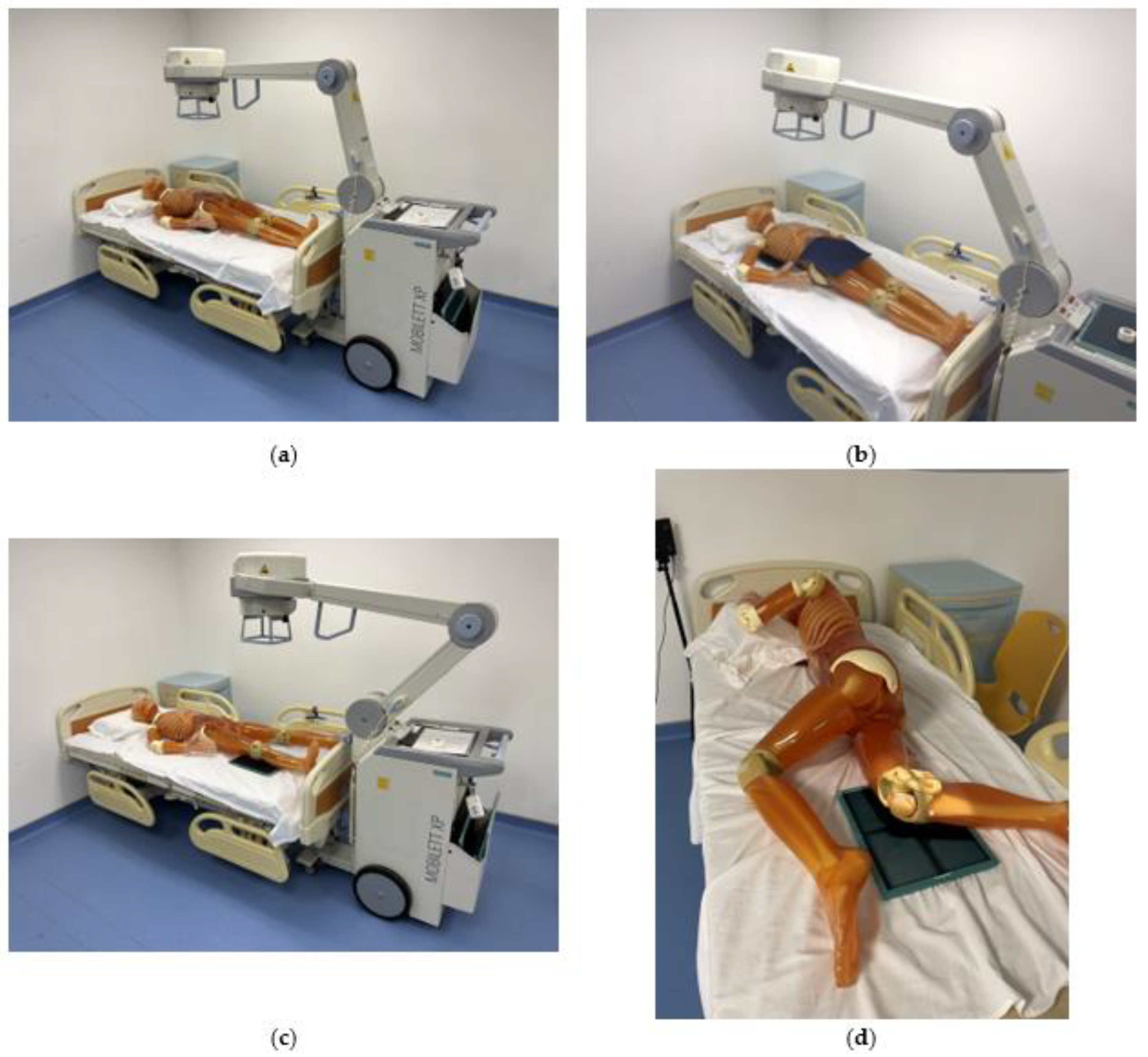
2.1. Experimental Materials and Machines
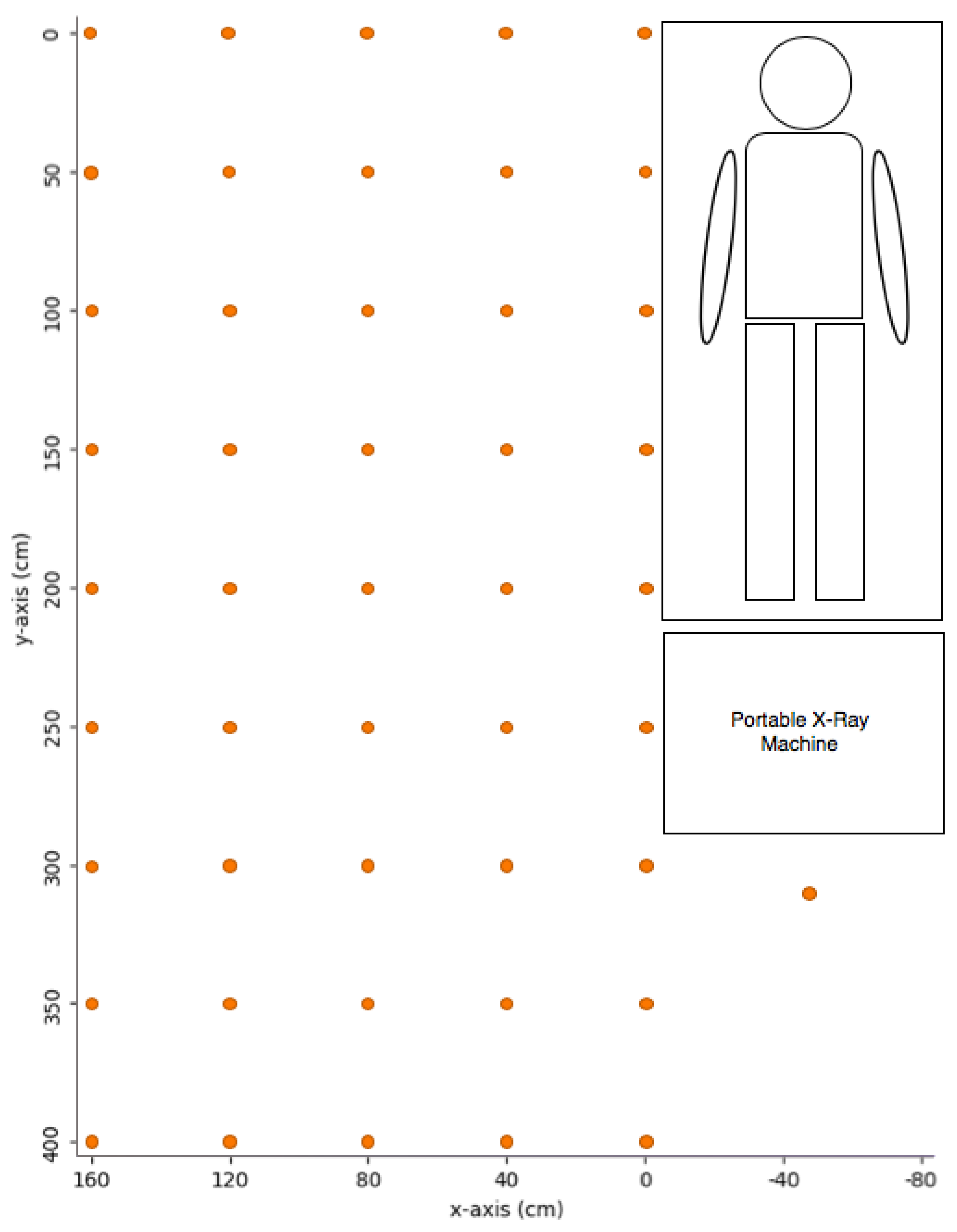
2.2. Exposure Settings and Measurement Points for Portable X-ray
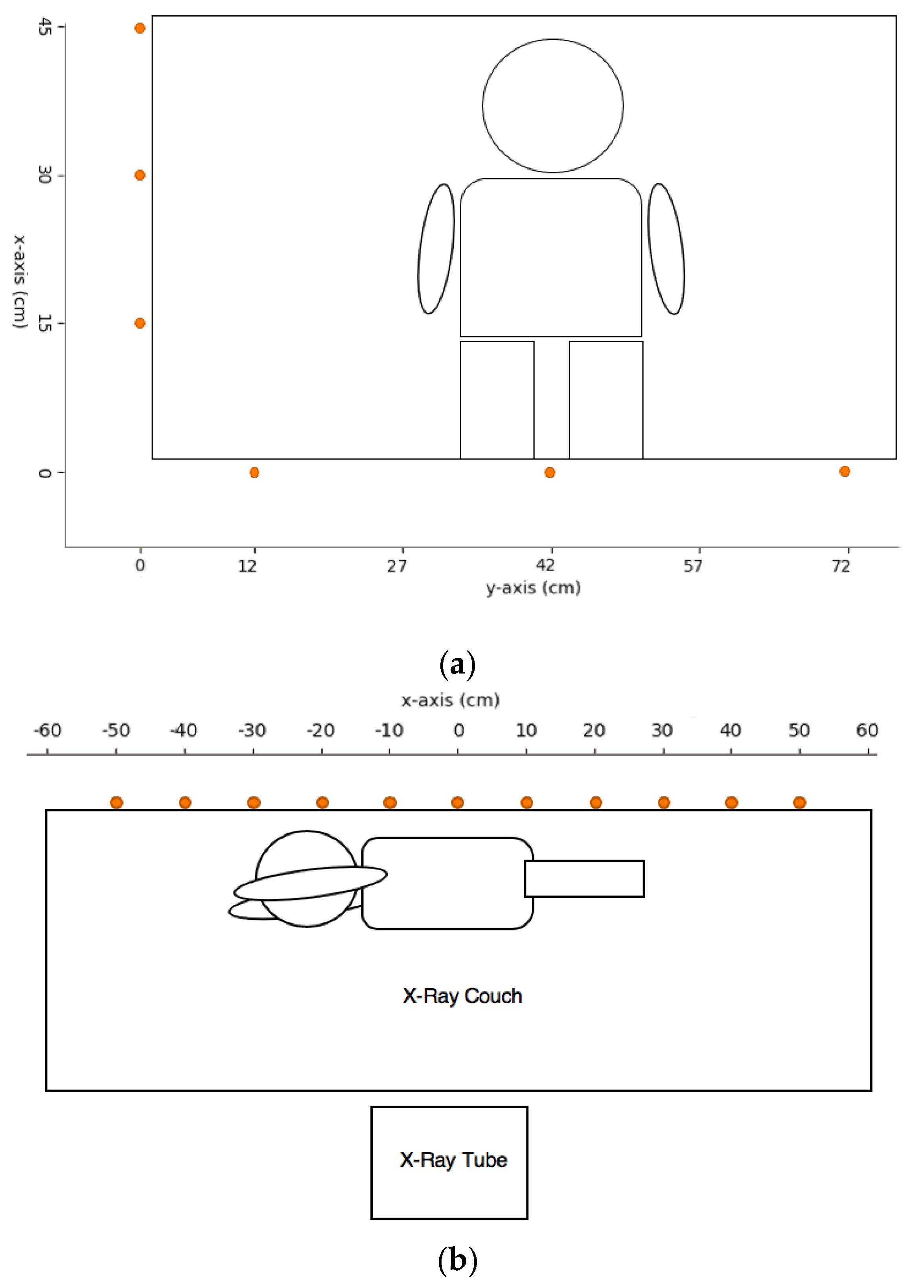
2.3. Exposure Settings and Measurement Points for Pediatric X-ray
2.4. Data Represenation and Statistical Analysis
3. Results
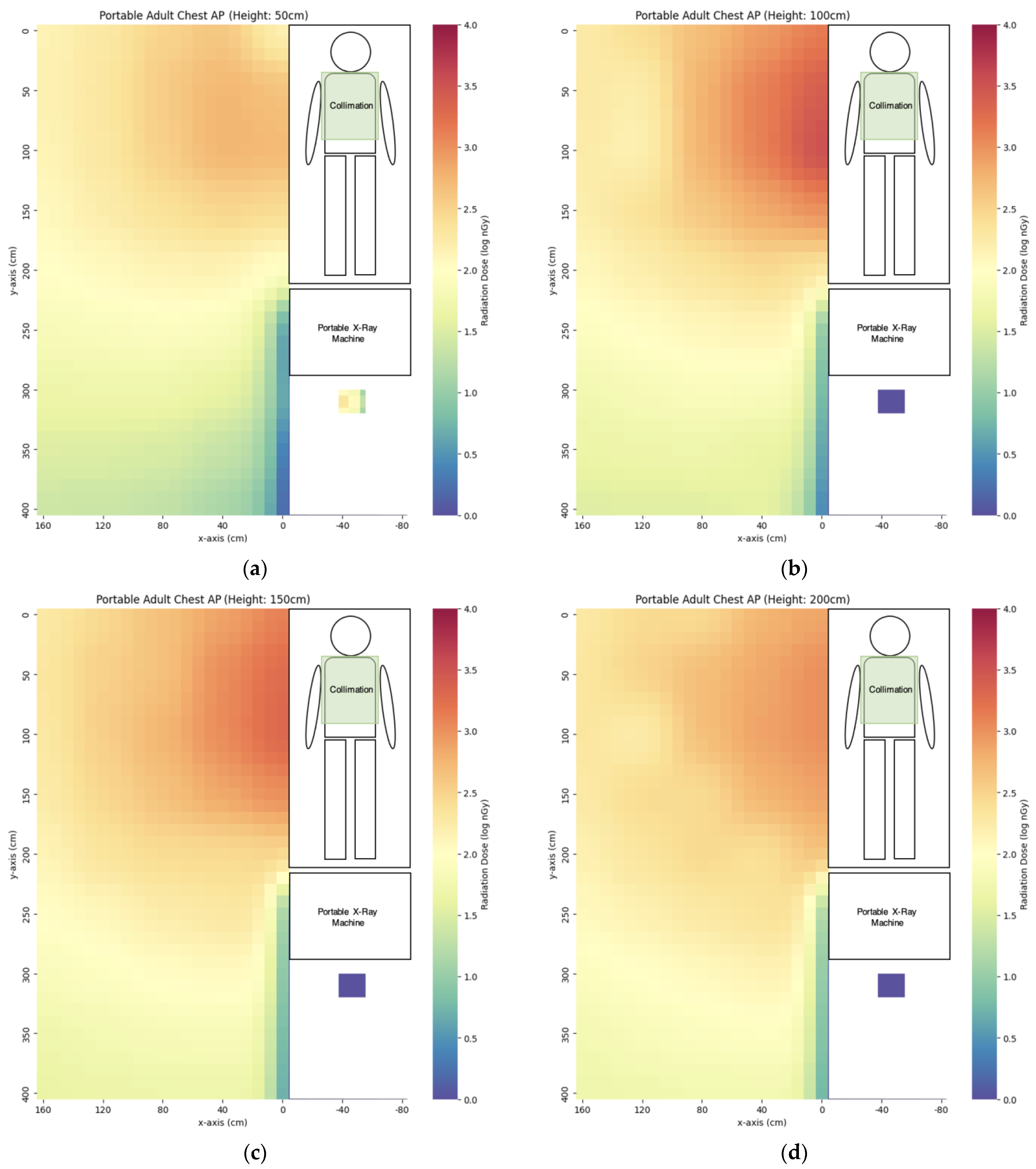
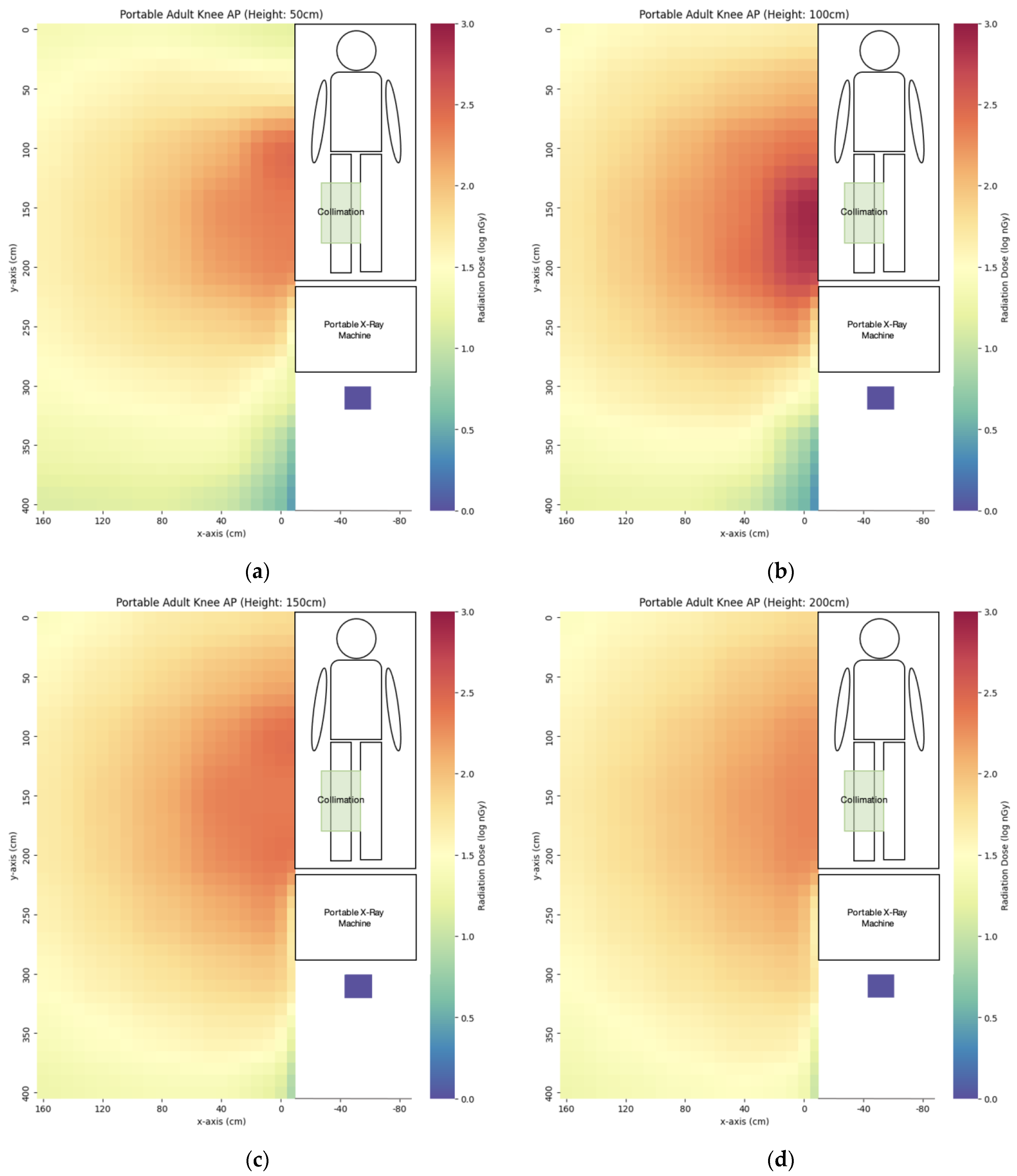
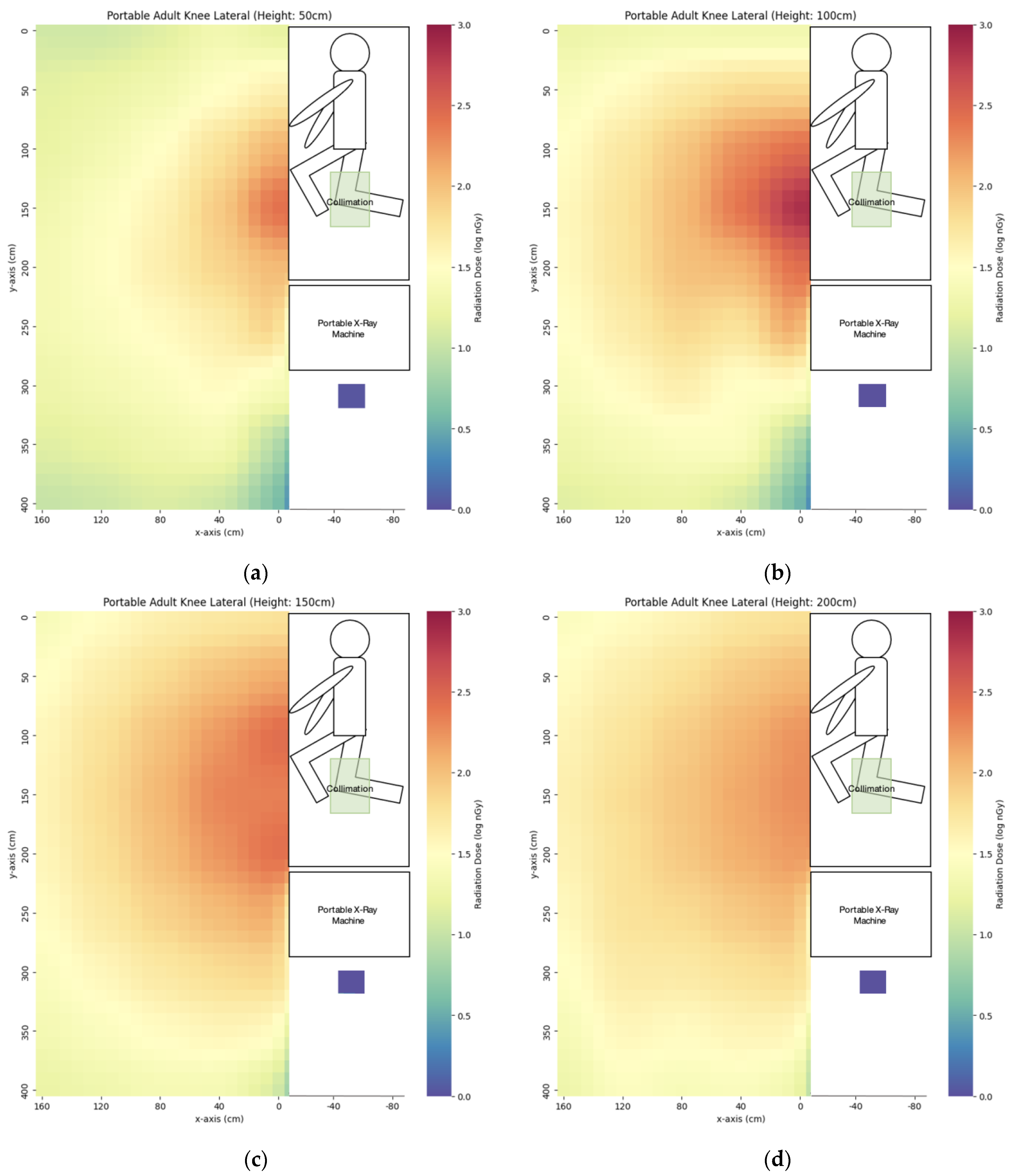
3.1. Portable X-ray
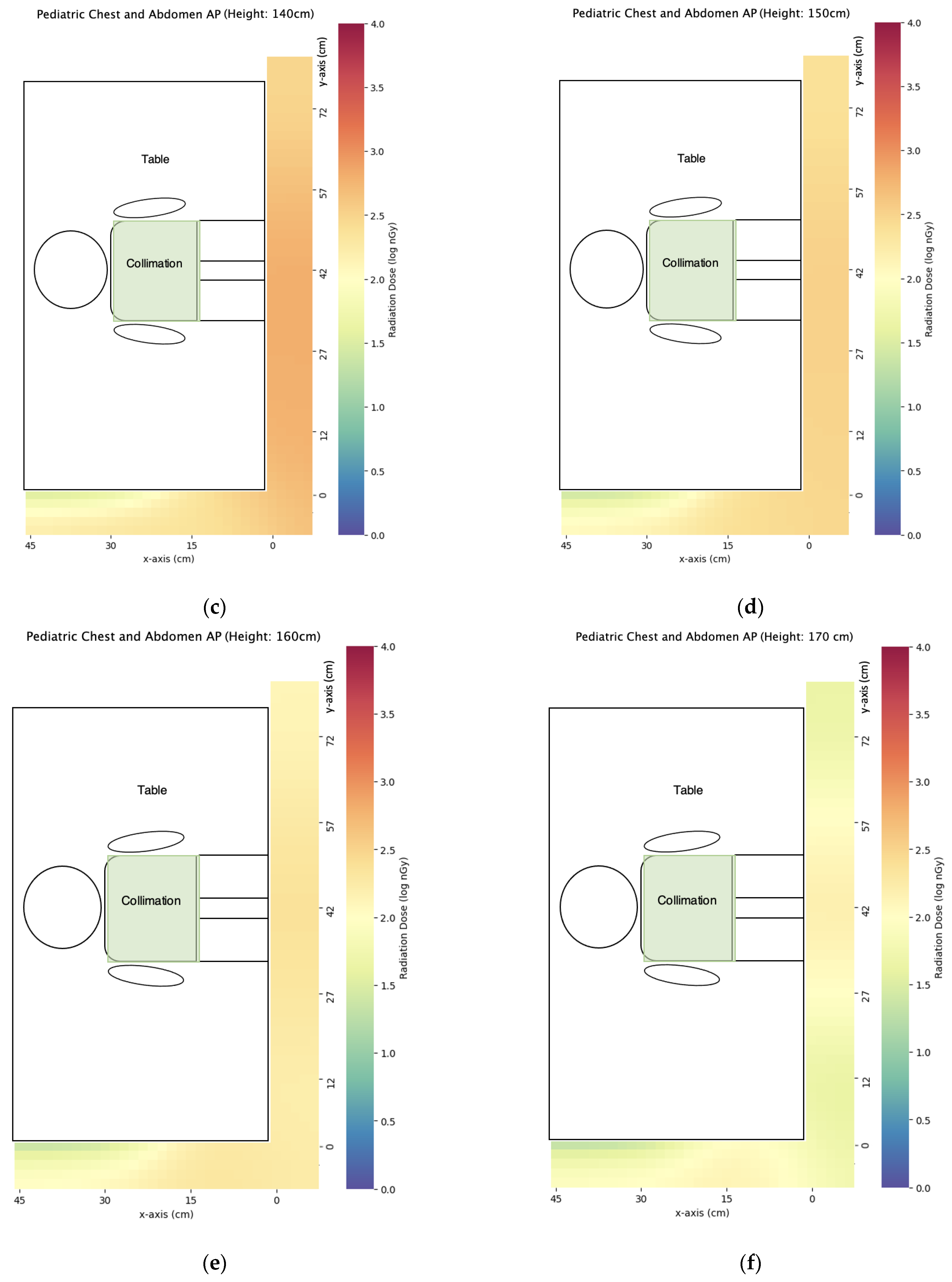
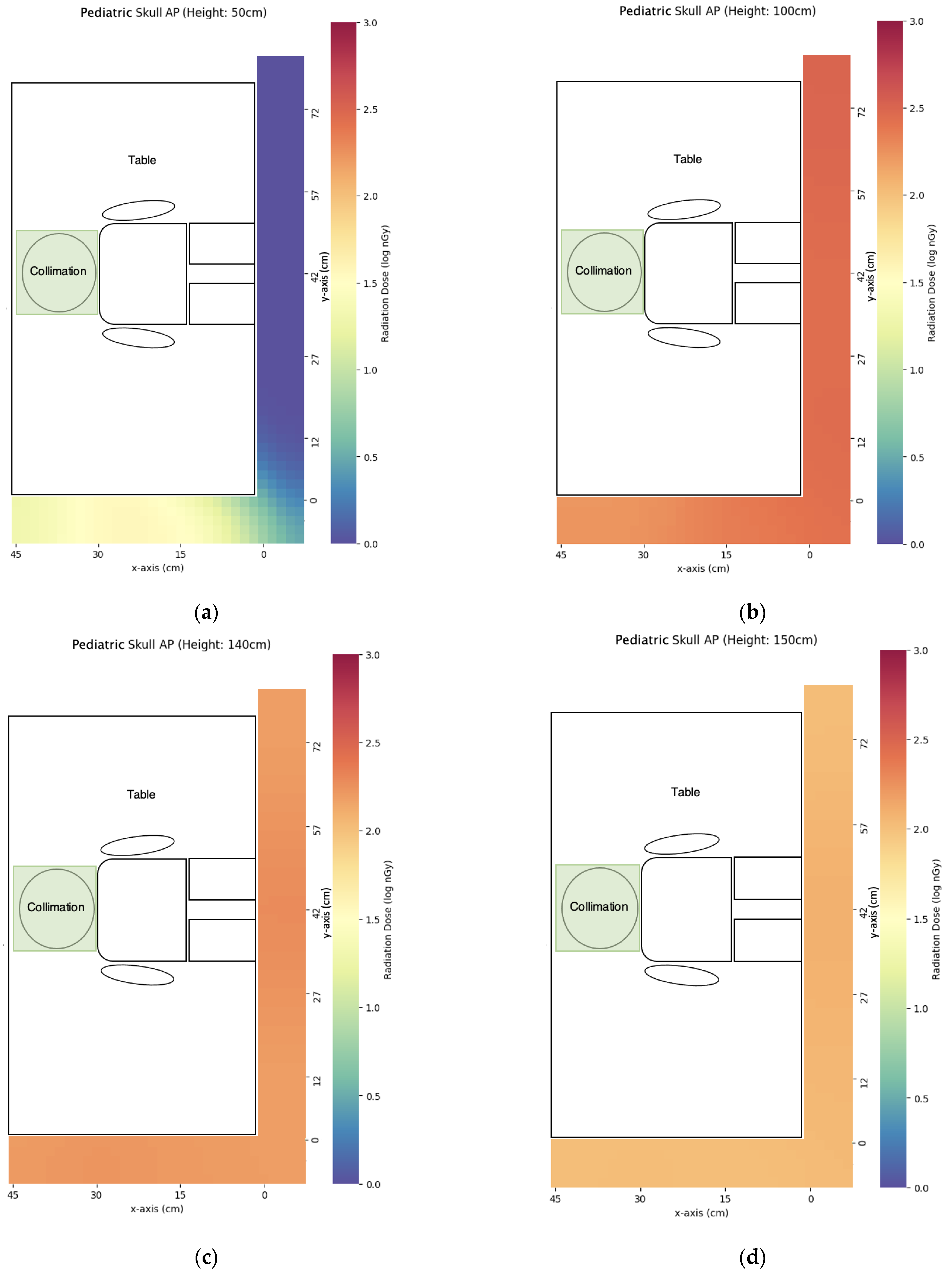
3.2. Pediatric X-ray
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazurov, A.I.; Potrakhov, N.N. Effect of Scattered X-ray Radiation on Imaging Quality and Techniques for Its Suppression. Biomed. Eng. 2015, 48, 241–245. [Google Scholar] [CrossRef]
- Moonkum, N.; Jitchom, S.; Sukaram, S.; Nimtrakool, N.; Boonrat, P.; Tochaikul, G. Determination of scattered radiation dose for radiological staff during portable chest examinations of COVID-19 patients. Radiol. Phys. Technol. 2023, 16, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, K.; Hayashi, S.; Takenaka, M.; Yakushijin, T.; Nagaike, K.; Takada, R.; Yamai, T.; Matsumoto, K.; Yamamoto, M.; Omoto, S.; et al. Occupational radiation exposure to the lens of the eyes and its protection during endoscopic retrograde cholangiopancreatography. Sci. Rep. 2023, 13, 7824. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Moreno-Torres, M.; Contreras, A.M.; Núñez, M.I.; Guirado, D.; Peñas, L. Patient and staff dosimetry during radiographic procedures in an intensive care unit. J. Radiol. Prot. 2015, 35, 727–732. [Google Scholar] [CrossRef]
- Santos, W.S.; Maia, A.F. Evaluation of personal doses associated with the use of mobile X-rays in a Brazilian hospital. Radiat. Prot. Dosim. 2012, 150, 188–191. [Google Scholar] [CrossRef]
- Wilson, R.F.; Gainor, J.P.; Allen, B. The Effect of Stepping Back from the X-ray Table on Operator Radiation Exposure. Health Phys. 2021, 121, 522–530. [Google Scholar] [CrossRef]
- Schoeb, V. Healthcare service in Hong Kong and its Challenges: The role of health professionals within a social model of health. China Perspect. 2016, 2016, 51–58. [Google Scholar] [CrossRef]
- Chida, K. What are useful methods to reduce occupational radiation exposure among radiological medical workers, especially for interventional radiology personnel? Radiol. Phys. Technol. 2022, 15, 101–115. [Google Scholar] [CrossRef]
- Yeung, P.; Pinson, J.A.; Lawson, M.; Leong, C.; Badawy, M.K. COVID-19 pandemic and the effect of increased utilisation of mobile X-ray examinations on radiation dose to radiographers. J. Med. Radiat. Sci. 2022, 69, 147–155. [Google Scholar] [CrossRef]
- Stewart, F.A.; Akleyev, A.V.; Hauer-Jensen, M.; Hendry, J.H.; Kleiman, N.J.; Macvittie, T.J.; Aleman, B.M.; Edgar, A.B.; Mabuchi, K.; Muirhead, C.R.; et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs—Threshold doses for tissue reactions in a radiation protection context. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [CrossRef]
- Matsubara, K.; Takei, Y.; Mori, H.; Kobayashi, I.; Noto, K.; Igarashi, T.; Suzuki, S.; Akahane, K. A multicenter study of radiation doses to the eye lenses of medical staff performing non-vascular imaging and interventional radiology procedures in Japan. Phys. Med. 2020, 74, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cornacchia, S.; Errico, R.; La Tegola, L.; Maldera, A.; Simeone, G.; Fusco, V.; Niccoli-Asabella, A.; Rubini, G.; Guglielmi, G. The new lens dose limit: Implication for occupational radiation protection. Radiol. Med. 2019, 124, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Haga, Y.; Sota, M.; Inaba, Y.; Chida, K. Performance of the DOSIRIS™ eye lens dosimeter. J. Radiol. Prot. 2019, 39, N19–N26. [Google Scholar] [CrossRef] [PubMed]
- United Nations Scientific Committee on the Effects of Atomic Radiation Sources and Effects of Ionizing Radiation. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 1993 Report; United Nations Scientific Committee: Vienna, Austria, 1993.
- Nishi, K.; Fujibuchi, T.; Yoshinaga, T. Development and evaluation of the effectiveness of educational material for radiological protection that uses augmented reality and virtual reality to visualise the behaviour of scattered radiation. J. Radiol. Prot. 2022, 42, 011506. [Google Scholar] [CrossRef]
- Nishi, K.; Fujibuchi, T.; Yoshinaga, T. Development of an application to visualise the spread of scattered radiation in radiography using augmented reality. J. Radiol. Prot. 2020, 40, 1299. [Google Scholar] [CrossRef]
- Takata, T.; Nakabayashi, S.; Kondo, H.; Yamamoto, M.; Furui, S.; Shiraishi, K.; Kobayashi, T.; Oba, H.; Okamoto, T.; Kotoku, J. Mixed Reality Visualization of Radiation Dose for Health Professionals and Patients in Interventional Radiology. J. Med. Syst. 2021, 45, 38. [Google Scholar] [CrossRef]
- Otomo, K.; Inaba, Y.; Abe, K.; Onodera, M.; Suzuki, T.; Sota, M.; Haga, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Spatial Scattering Radiation to the Radiological Technologist during Medical Mobile Radiography. Bioengineering 2023, 10, 259. [Google Scholar] [CrossRef]
- Alzen, G.; Benz-Bohm, G. Radiation protection in pediatric radiology. Dtsch. Ärzteblatt Int. 2011, 108, 407–414. [Google Scholar] [CrossRef]
- Alemayehu, T.G.; Bogale, G.G.; Bazie, G.W. Occupational radiation exposure dose and associated factors among radiology personnel in Eastern Amhara, Ethiopia. PLoS ONE 2023, 18, e0286400. [Google Scholar] [CrossRef]
- Koenig, A.M.; Maas, J.; Viniol, S.; Etzel, R.; Fiebich, M.; Thomas, R.P.; Mahnken, A.H. Scatter radiation reduction with a radiation-absorbing pad in interventional radiology examinations. Eur. J. Radiol. 2020, 132, 109245. [Google Scholar] [CrossRef]
- Haga, Y.; Chida, K.; Kaga, Y.; Sota, M.; Meguro, T.; Zuguchi, M. Occupational eye dose in interventional cardiology procedures. Sci. Rep. 2017, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Chida, K.; Munehisa, M.; Sato, T.; Inaba, Y.; Suzuki, M.; Zuguchi, M. Non-Lead Protective Aprons for the Protection of Interventional Radiology Physicians from Radiation Exposure in Clinical Settings: An Initial Study. Diagnostics 2021, 11, 1613. [Google Scholar] [CrossRef] [PubMed]






| Projection | Field Size | kVp | mAs | SID |
|---|---|---|---|---|
| AP abdomen | 35 cm × 43 cm | 81 | 11 | 100 cm |
| AP chest | 35 cm × 43 cm | 81 | 11 | 100 cm |
| AP right knee | 24 cm × 30 cm | 60 | 5 | 100 cm |
| Lateral right knee | 24 cm × 30 cm | 60 | 5 | 100 cm |
| Projection | Field Size | kVp | mAs | SID |
|---|---|---|---|---|
| AP chest and abdomen | 14 cm × 25 cm | 65 | 3.2 | 110 cm |
| AP skull | 14 cm × 14 cm | 65 | 3.2 | 110 cm |
| Abdomen in lateral decubitus view | 14 cm × 20 cm | 65 | 3.2 | 110 cm |
| Projection | Longest Cord Extension | Hiding behind the Portable X-ray Machine |
|---|---|---|
| AP abdomen | 177 ± 8 | 14 ± 0 |
| AP chest | 43 ± 1 | 3 ± 0 |
| AP right knee | 19 ± 1 | 5 ± 0 |
| Lateral right knee | 17 ± 1 | 24 ± 0 |
| Projection | x = 0 cm | x = 40 cm | x = 80 cm | x = 120 cm | x = 160 cm |
|---|---|---|---|---|---|
| AP abdomen | 6760 ± 251 | 3323 ± 28 | 1788 ± 50 | 1008 ± 36 | 580 ± 42 |
| AP chest | 2588 ± 422 | 1302 ± 4 | 604 ± 14 | 160 ± 10 | 187 ± 6 |
| AP right knee | 117 ± 2 | 89 ± 1 | 66 ± 3 | 52 ± 1 | 37 ± 0 |
| Lateral right knee | 69 ± 1 | 67 ± 1 | 50 ± 1 | 34 ± 0 | 25 ± 1 |
| Projection | z = 50 cm | x = 100 cm | x = 140 cm | x = 150 cm | x = 160 cm | x = 170 cm |
|---|---|---|---|---|---|---|
| AP chest and abdomen | 33 ± 1 | 1490 ± 109 | 659 ± 7 | 338 ± 7 | 248 ± 1 | 170 ± 7 |
| AP skull | 1 ± 0 | 532 ± 14 | 194 ± 2 | 127 ± 2 | 90 ± 1 | 73 ± 1 |
| Abdomen in right lateral decubitus view | 86 ± 1 | 172 ± 11 | 167 ± 4 | 117 ± 3 | 79 ± 2 | 87 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tam, S.-Y.; Fung, Y.-Y.; Lau, S.-Y.; Lam, W.-N.; Wong, E.T.-H. Scatter Radiation Distribution to Radiographers, Nearby Patients and Caretakers during Portable and Pediatric Radiography Examinations. Bioengineering 2023, 10, 779. https://doi.org/10.3390/bioengineering10070779
Tam S-Y, Fung Y-Y, Lau S-Y, Lam W-N, Wong ET-H. Scatter Radiation Distribution to Radiographers, Nearby Patients and Caretakers during Portable and Pediatric Radiography Examinations. Bioengineering. 2023; 10(7):779. https://doi.org/10.3390/bioengineering10070779
Chicago/Turabian StyleTam, Shing-Yau, Yuen-Ying Fung, Sum-Yi Lau, Wang-Ngai Lam, and Edward Ting-Hei Wong. 2023. "Scatter Radiation Distribution to Radiographers, Nearby Patients and Caretakers during Portable and Pediatric Radiography Examinations" Bioengineering 10, no. 7: 779. https://doi.org/10.3390/bioengineering10070779
APA StyleTam, S.-Y., Fung, Y.-Y., Lau, S.-Y., Lam, W.-N., & Wong, E. T.-H. (2023). Scatter Radiation Distribution to Radiographers, Nearby Patients and Caretakers during Portable and Pediatric Radiography Examinations. Bioengineering, 10(7), 779. https://doi.org/10.3390/bioengineering10070779






