Evaluation of Whole-Tumor Texture Analysis Based on MRI Diffusion Kurtosis and Biparametric VI-RADS Model for Staging and Grading Bladder Cancer
Abstract
1. Introduction
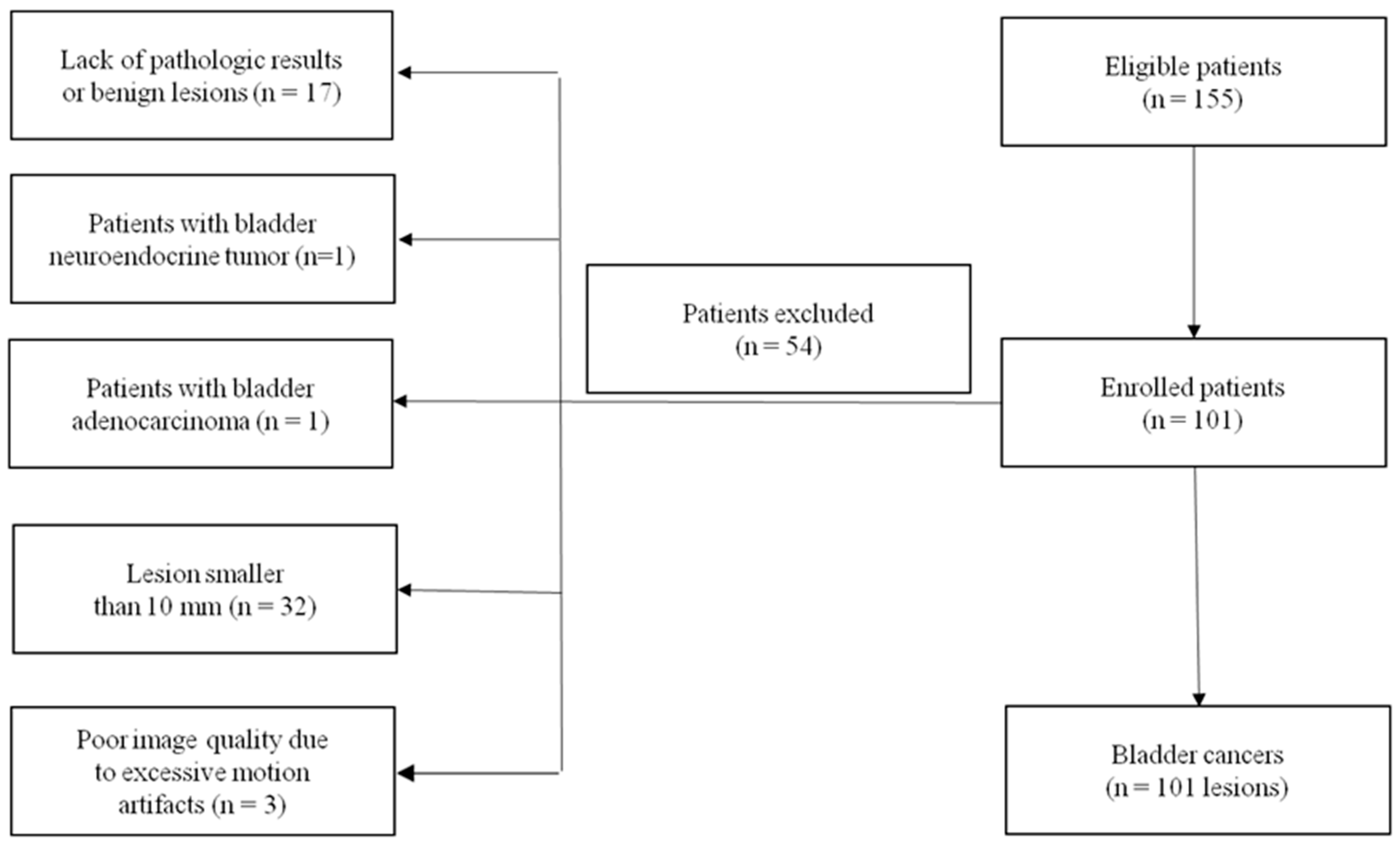
2. Materials and Methods
2.1. Patient Characteristics
2.2. Image Acquisition
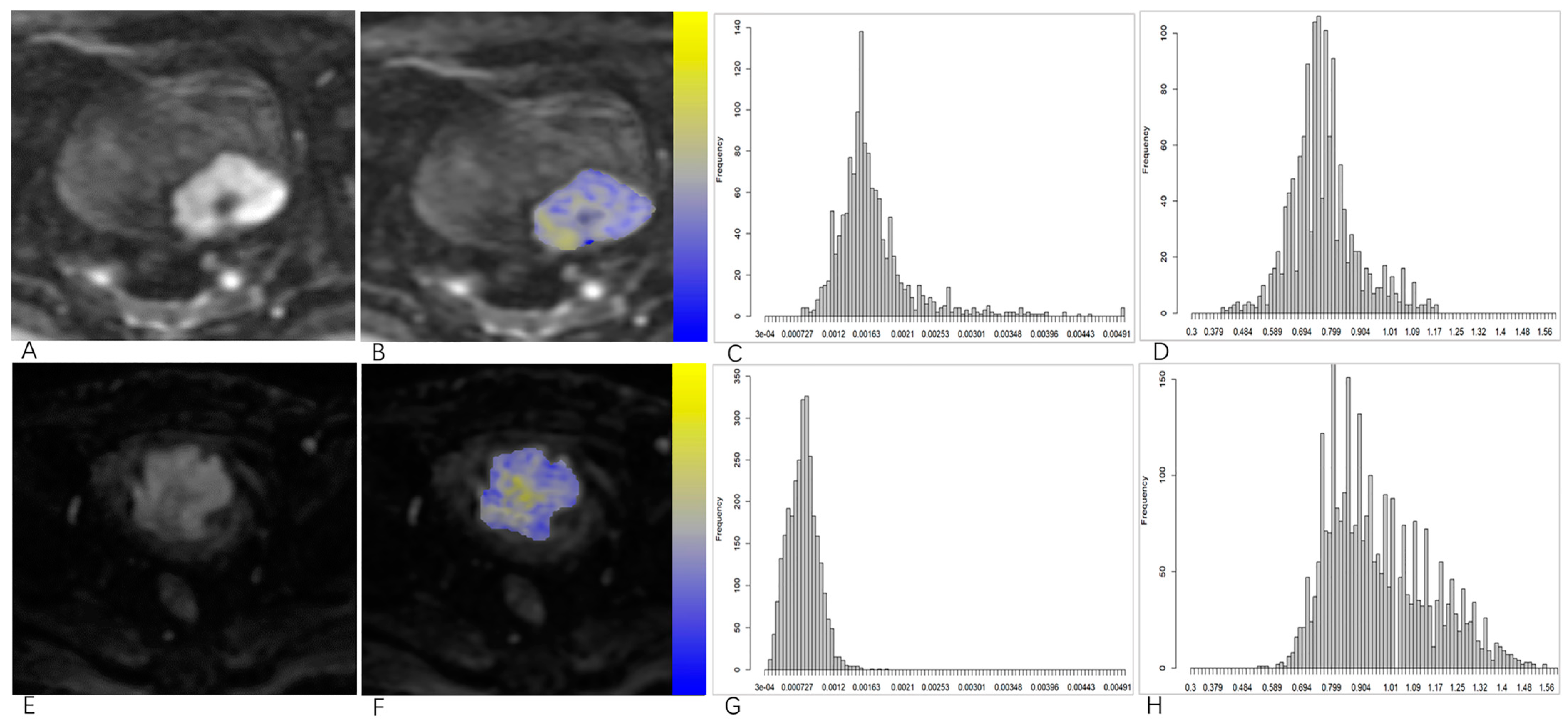
2.3. Image Processing and Analysis
2.4. VI-RADS Scoring
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Interobserver Agreement
3.3. Comparisons of Volumetric DKI Histogram Parameters and ADC Values between the NMIBC and MIBC Groups and Low- and High-Grade Groups
3.4. Diagnostic Performance of Multiple DKI Histogram Parameters
3.5. Comparisons of the Diagnostic Performance of DKI Histogram Parameters and ADC Values, as Well as Biparametric VI-RADS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)-2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Donat, S.M. Quality control in transurethral resection of bladder tumours. BJU Int. 2008, 102, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Ark, J.T.; Keegan, K.A.; Barocas, D.A.; Morgan, T.M.; Resnick, M.J.; You, C.; Cookson, M.S.; Penson, D.F.; Davis, R.; Clark, P.E.; et al. Incidence and predictors of understaging in patients with clinical T1 urothelial carcinoma undergoing radical cystectomy. BJU Int. 2014, 113, 894–899. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Zhang, X.; Liu, Y.; Ouyang, L.; Du, P.; Li, S.; Tian, Q.; Ling, J.; Guo, Y.; et al. Elaboration of a multisequence MRI-based radiomics signature for the preoperative prediction of the muscle-invasive status of bladder cancer: A double-center study. Eur. Radiol. 2020, 30, 4816–4827. [Google Scholar] [CrossRef]
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur. Urol. 2018, 74, 294–306. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, D.; Yu, H.; Shen, Y.; Tang, H.; Kamel, I.R.; Li, Z. Comparison of the Diagnostic Value of Monoexponential, Biexponential, and Stretched Exponential Diffusion-weighted MRI in Differentiating Tumor Stage and Histological Grade of Bladder Cancer. Acad. Radiol. 2019, 26, 239–246. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Hu, X.; Li, Z.; Feng, C.; Hu, D.; Kamel, I.R. Application of R2* and Apparent Diffusion Coefficient in Estimating Tumor Grade and T Category of Bladder Cancer. AJR Am. J. Roentgenol. 2020, 214, 383–389. [Google Scholar] [CrossRef]
- Yoshida, S.; Takahara, T.; Kwee, T.C.; Waseda, Y.; Kobayashi, S.; Fujii, Y. DWI as an Imaging Biomarker for Bladder Cancer. AJR Am. J. Roentgenol. 2017, 208, 1218–1228. [Google Scholar] [CrossRef]
- El-Assmy, A.; Abou-El-Ghar, M.E.; Refaie, H.F.; Mosbah, A.; El-Diasty, T. Diffusion-weighted magnetic resonance imaging in follow-up of superficial urinary bladder carcinoma after transurethral resection: Initial experience. BJU Int. 2012, 110, E622–E627. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, H.G.; Zhang, R.Y.; Jin, D.; Xu, S.S.; Wu, G.Y.; Xu, J.R. Diffusion kurtosis imaging to assess correlations with clinicopathologic factors for bladder cancer: A comparison between the multi-b value method and the tensor method. Eur. Radiol. 2019, 29, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, B.; Tan, Q.; Liu, K.; Jiang, S.; Zhou, J. Prediction of muscle invasion of bladder cancer: A comparison between DKI and conventional DWI. Eur. J. Radiol. 2021, 136, 109522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.M.; Sun, H.; Shi, B.; Jin, Z.Y.; Xue, H.D. Quantitative CT texture analysis for evaluating histologic grade of urothelial carcinoma. Abdom. Radiol. 2017, 42, 561–568. [Google Scholar] [CrossRef]
- Razik, A.; Das, C.J.; Sharma, R.; Malla, S.; Sharma, S.; Seth, A.; Srivastava, D.N. Utility of first order MRI-Texture analysis parameters in the prediction of histologic grade and muscle invasion in urinary bladder cancer: A preliminary study. Br. J. Radiol. 2021, 94, 20201114. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, P.; Wang, Y.; Feng, C.; Shen, Y.; Hu, X.; Hu, D.; Meng, X.; Li, Z. Combining volumetric apparent diffusion coefficient histogram analysis with vesical imaging reporting and data system to predict the muscle invasion of bladder cancer. Abdom. Radiol. 2021, 46, 4301–4310. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sasaki, S.; Ito, M.; Okada, S.; Takahashi, S.; Kawai, T.; Suzuki, K.; Oshima, H.; Hara, M.; Shibamoto, Y. Urinary bladder cancer: Diffusion-weighted MR imaging—Accuracy for diagnosing T stage and estimating histologic grade. Radiology 2009, 251, 112–121. [Google Scholar] [CrossRef]
- Yajima, S.; Yoshida, S.; Takahara, T.; Arita, Y.; Tanaka, H.; Waseda, Y.; Yokoyama, M.; Ishioka, J.; Matsuoka, Y.; Saito, K.; et al. Usefulness of the inchworm sign on DWI for predicting pT1 bladder cancer progression. Eur. Radiol. 2019, 29, 3881–3888. [Google Scholar] [CrossRef]
- Arévalo, N.; Méndez, R.; Barrera, J. “Inchworm sign” in urinary bladder cancer. Abdom. Radiol. 2018, 43, 3509–3510. [Google Scholar] [CrossRef]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Y.; Dan, G.; Zhong, Z.; Karaman, M.M.; Li, Z.; Hu, D.; Zhou, X.J. Evaluation of a fractional-order calculus diffusion model and bi-parametric VI-RADS for staging and grading bladder urothelial carcinoma. Eur. Radiol. 2022, 32, 890–900. [Google Scholar] [CrossRef]
- Wang, F.; Jin, D.; Hua, X.L.; Zhao, Z.Z.; Wu, L.M.; Chen, W.B.; Wu, G.Y.; Chen, X.X.; Chen, H.G. Investigation of diffusion kurtosis imaging for discriminating tumors from inflammatory lesions after treatment for bladder cancer. J. Magn. Reson. Imaging 2018, 48, 259–265. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.; Cranston, D.; Fuggle, S.; Bicknell, R.; Harris, A.L. Different angiogenic pathways characterize superficial and invasive bladder cancer. Cancer Res. 1995, 55, 510–513. [Google Scholar] [PubMed]
- Qi, X.X.; Shi, D.F.; Ren, S.X.; Zhang, S.Y.; Li, L.; Li, Q.C.; Guan, L.M. Histogram analysis of diffusion kurtosis imaging derived maps may distinguish between low and high grade gliomas before surgery. Eur. Radiol. 2018, 28, 1748–1755. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, J.; Wang, Y.; Wu, G. Renal cell carcinoma: Preoperative evaluate the grade of histological malignancy using volumetric histogram analysis derived from magnetic resonance diffusion kurtosis imaging. Quant. Imaging Med. Surg. 2019, 9, 671–680. [Google Scholar] [CrossRef]
- Lim, C.S.; Tirumani, S.; van der Pol, C.B.; Alessandrino, F.; Sonpavde, G.P.; Silverman, S.G.; Shinagare, A.B. Use of Quantitative T2-Weighted and Apparent Diffusion Coefficient Texture Features of Bladder Cancer and Extravesical Fat for Local Tumor Staging After Transurethral Resection. AJR Am. J. Roentgenol. 2019, 212, 1060–1069. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Tian, Q.; Li, B.; Wu, Y.; Yang, Z.; Liang, Z.; Liu, Y.; Cui, G.; Lu, H. Radiomics assessment of bladder cancer grade using texture features from diffusion-weighted imaging. J. Magn. Reson. Imaging 2017, 46, 1281–1288. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, X.; Ouyang, H.; Zhang, J.; Chen, S.; Xie, L.; Zhao, X. Whole-tumor texture model based on diffusion kurtosis imaging for assessing cervical cancer: A preliminary study. Eur. Radiol. 2021, 31, 5576–5585. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010, 23, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Jiang, J.; Zhao, L.; Zhang, J.; Zhang, S.; Yao, Y.; Yang, S.; Shi, J.; Shen, N.; Su, C.; et al. Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget 2015, 6, 42380–42393. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Delli Pizzi, A.; Mastrodicasa, D.; Marchioni, M.; Primiceri, G.; Di Fabio, F.; Cianci, R.; Seccia, B.; Sessa, B.; Mincuzzi, E.; Romanelli, M.; et al. Bladder cancer: Do we need contrast injection for MRI assessment of muscle invasion? A prospective multi-reader VI-RADS approach. Eur. Radiol. 2021, 31, 3874–3883. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | No. (%) | |
|---|---|---|
| age (year) | 63.5 ± 9.8 (36–84) | |
| gender | male | 87 |
| female | 14 | |
| diameter | ≤3 cm | 75 |
| >3 cm | 26 | |
| stage | ≤T1 | 67 |
| ≥T2 | 34 | |
| grade | high grade | 70 |
| low grade | 31 | |
| surgical method | TURBT | 69 |
| radical or partial bladder resection | 32 |
| MD | MK | |||||
|---|---|---|---|---|---|---|
| NMIBC | MIBC | p | NMIBC | MIBC | p | |
| mean | 1.92 ± 0.38 | 1.42 ± 0.34 | 0.00 | 0.69 ± 0.16 | 0.81 ± 0.20 | 0.00 |
| 5th | 1.19 ± 0.39 | 0.91 ± 0.25 | 0.00 | 0.38 ± 0.50 | 0.38 ± 0.27 | 0.99 |
| 25th | 1.53 ± 0.38 | 1.11 ± 0.28 | 0.00 | 0.60 ± 0.15 | 0.69 ± 0.22 | 0.04 |
| 50th | 1.86 ± 0.42 | 1.31 ± 0.33 | 0.00 | 0.72 ± 0.15 | 0.84 ± 0.20 | 0.00 |
| 75th | 2.75 ± 0.33 | 1.63 ± 0.46 | 0.00 | 0.81 ± 0.18 | 0.96 ± 0.22 | 0.00 |
| 90th | 2.75 ± 0.33 | 2.06 ± 0.56 | 0.00 | 0.92 ± 0.33 | 1.06 ± 0.25 | 0.04 |
| inhomogeneity | 0.29 ± 0.08 | 0.32 ± 0.09 | 0.12 | 0.38 ± 0.76 | 0.29± 0.16 | 0.52 |
| skewness | 0.43 ± 0.63 | 1.31 ± 0.69 | 0.00 | −1.05 ± 0.91 | −0.78 ± 0.72 | 0.13 |
| kurtosis | −0.37 ± 1.04 | 2.45 ± 3.41 | 0.00 | 2.25 ± 2.71 | 1.76 ± 2.21 | 0.33 |
| entropy | 3.88 ± 0.40 | 3.89 ± 0.26 | 0.86 | 3.78 ± 0.25 | 3.84 ± 0.25 | 0.25 |
| MD | MK | |||||
|---|---|---|---|---|---|---|
| High Grade | Low Grade | p | High Grade | Low Grade | p | |
| mean | 1.66 ± 0.41 | 1.97 ± 0.44 | 0.00 | 0.78 ± 0.17 | 0.61 ± 0.15 | 0.00 |
| 5th | 1.03 ± 0.32 | 1.27 ± 0.42 | 0.00 | 0.43 ± 0.49 | 0.29 ± 0.24 | 0.14 |
| 25th | 1.30 ± 0.34 | 1.59 ± 0.47 | 0.00 | 0.67 ± 0.17 | 0.53 ± 0.16 | 0.00 |
| 50th | 1.56 ± 0.41 | 1.92 ± 0.51 | 0.00 | 0.81 ± 0.17 | 0.64 ± 0.13 | 0.00 |
| 75th | 1.99 ± 0.54 | 2.34 ± 0.54 | 0.00 | 0.92 ± 0.19 | 0.73 ± 0.15 | 0.00 |
| 90th | 2.45 ± 0.56 | 2.68 ± 0.42 | 0.04 | 1.04 ± 0.33 | 0.80 ± 0.18 | 0.00 |
| inhomogeneity | 0.31 ± 0.09 | 0.27 ± 0.08 | 0.01 | 0.37 ± 0.75 | 0.31 ± 0.15 | 0.64 |
| skewness | 0.88 ± 0.74 | 0.37 ± 0.74 | 0.00 | −0.84 ± 0.77 | −1.22 ± 1.00 | 0.06 |
| kurtosis | 0.91 ± 2.82 | −1.47 ± 1.44 | 0.05 | 1.81 ± 2.02 | 2.73 ± 3.42 | 0.17 |
| entropy | 3.93 ± 0.26 | 3.77 ± 0.50 | 0.10 | 3.83 ± 0.25 | 3.72 ± 0.23 | 0.04 |
| MD | MK | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Cut-Off Value | 95% CI | Sensitivity | Specificity | AUC | Cut-Off Value | 95% CI | Sensitivity | Specificity | |
| mean | 0.85 | 1.63 | 0.76 to 0.91 | 73.5 | 85.1 | 0.67 | 0.87 | 0.57 to 0.76 | 44.1 | 89.6 |
| 5th | 0.76 | 1.05 | 0.66 to 0.84 | 79.4 | 67.2 | 0.59 | 0.52 | 0.49 to 0.69 | 44.1 | 77.6 |
| 25th | 0.83 | 1.33 | 0.74 to 0.89 | 82.4 | 73.1 | 0.67 | 0.76 | 0.57 to 0.76 | 47.1 | 91.0 |
| 50th | 0.86 | 1.51 | 0.78 to 0.92 | 76.5 | 85.1 | 0.69 | 0.90 | 0.59 to 0.78 | 44.1 | 92.5 |
| 75th | 0.87 | 2.14 | 0.79 to 0.93 | 94.1 | 67.2 | 0.69 | 0.84 | 0.59 to 0.78 | 76.5 | 58.2 |
| 90th | 0.87 | 2.53 | 0.79 to 0.93 | 88.2 | 80.1 | 0.70 | 0.93 | 0.60 to 0.78 | 70.1 | 64.2 |
| skewness | 0.84 | 1.11 | 0.75 to 0.90 | 64.7 | 88.1 | 0.61 | −1.26 | 0.51 to 0.70 | 82.4 | 44.8 |
| kurtosis | 0.84 | −0.08 | 0.76 to 0.91 | 79.4 | 73.1 | 0.53 | 2.30 | 0.43 to 0.63 | 79.4 | 40.3 |
| MD | MK | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Cut-Off Value | 95% CI | Sensitivity | Specificity | AUC | Cut-Off Value | 95% CI | Sensitivity | Specificity | |
| mean | 0.68 | 2.08 | 0.58 to 0.77 | 88.6 | 41.9 | 0.77 | 0.73 | 0.67 to 0.84 | 58.6 | 80.1 |
| 5th | 0.66 | 1.02 | 0.56 to 0.75 | 51.4 | 77.4 | 0.62 | 0.54 | 0.52 to 0.71 | 31.4 | 90.3 |
| 25th | 0.67 | 1.67 | 0.57 to 0.76 | 87.1 | 38.7 | 0.72 | 0.69 | 0.62 to 0.81 | 47.1 | 87.1 |
| 50th | 0.69 | 2.07 | 0.59 to 0.78 | 91.4 | 41.9 | 0.78 | 0.75 | 0.69 to 0.86 | 60.0 | 83.9 |
| 75th | 0.66 | 2.26 | 0.56 to 0.75 | 68.6 | 58.1 | 0.79 | 0.85 | 0.70 to 0.87 | 65.7 | 87.1 |
| 90th | 0.62 | 2.76 | 0.52 to 0.72 | 65.7 | 58.1 | 0.79 | 0.79 | 0.69 to 0.86 | 65.7 | 83.9 |
| inhomogeneity | 0.64 | 0.31 | 0.54 to 0.73 | 50.0 | 77.4 | 0.53 | 0.43 | 0.43 to 0.63 | 91.4 | 29.0 |
| skewness | 0.68 | 0.16 | 0.58 to 0.77 | 91.4 | 41.9 | 0.59 | −1.80 | 0.49 to 0.69 | 95.7 | 35.5 |
| entropy | 0.55 | 3.60 | 0.45 to 0.65 | 88.6 | 32.3 | 0.67 | 3.78 | 0.57 to 0.76 | 72.9 | 71.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Li, S.; He, K.; Hu, H.; Feng, C.; Li, Z.; Wang, Y. Evaluation of Whole-Tumor Texture Analysis Based on MRI Diffusion Kurtosis and Biparametric VI-RADS Model for Staging and Grading Bladder Cancer. Bioengineering 2023, 10, 745. https://doi.org/10.3390/bioengineering10070745
Meng X, Li S, He K, Hu H, Feng C, Li Z, Wang Y. Evaluation of Whole-Tumor Texture Analysis Based on MRI Diffusion Kurtosis and Biparametric VI-RADS Model for Staging and Grading Bladder Cancer. Bioengineering. 2023; 10(7):745. https://doi.org/10.3390/bioengineering10070745
Chicago/Turabian StyleMeng, Xiaoyan, Shichao Li, Kangwen He, Henglong Hu, Cui Feng, Zhen Li, and Yanchun Wang. 2023. "Evaluation of Whole-Tumor Texture Analysis Based on MRI Diffusion Kurtosis and Biparametric VI-RADS Model for Staging and Grading Bladder Cancer" Bioengineering 10, no. 7: 745. https://doi.org/10.3390/bioengineering10070745
APA StyleMeng, X., Li, S., He, K., Hu, H., Feng, C., Li, Z., & Wang, Y. (2023). Evaluation of Whole-Tumor Texture Analysis Based on MRI Diffusion Kurtosis and Biparametric VI-RADS Model for Staging and Grading Bladder Cancer. Bioengineering, 10(7), 745. https://doi.org/10.3390/bioengineering10070745






