Recent Advances in Metal–Organic Frameworks (MOFs) and Their Composites for Non-Enzymatic Electrochemical Glucose Sensors
Abstract
1. Introduction
2. Pristine MOFs as Modified Materials for Electrodes
2.1. Monometallic MOFs
2.1.1. Co-Based MOFs
2.1.2. Ni-Based MOFs
2.1.3. Cu-Based MOFs

2.2. Bimetallic MOFs
2.2.1. Co/Zn-MOFs
2.2.2. Co/Ni-MOFs
2.2.3. Ni/Cu-MOFs
2.2.4. Ni/Zn-MOFs
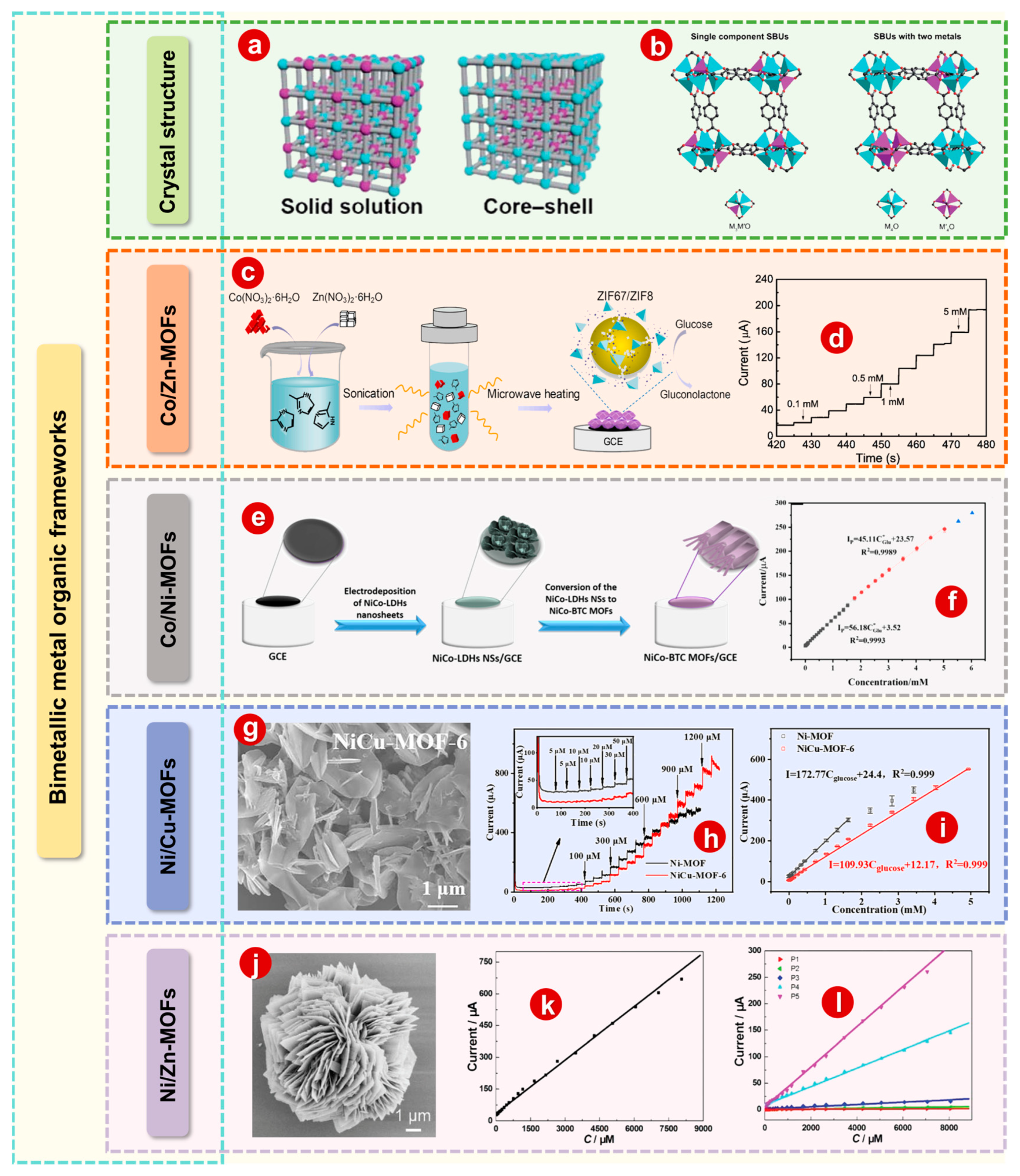
3. MOF Composites as Modified Materials for Electrodes
3.1. MOF/Carbon-Based Composites
3.1.1. MOF/Graphene
3.1.2. MOF/Reduced Graphene Oxide
3.1.3. MOF/Carbon Nanotubes
3.1.4. MOF/Carbon Nanohorns
3.1.5. MOF/Carbon Fibers
3.1.6. MOF/Carbon Cloth
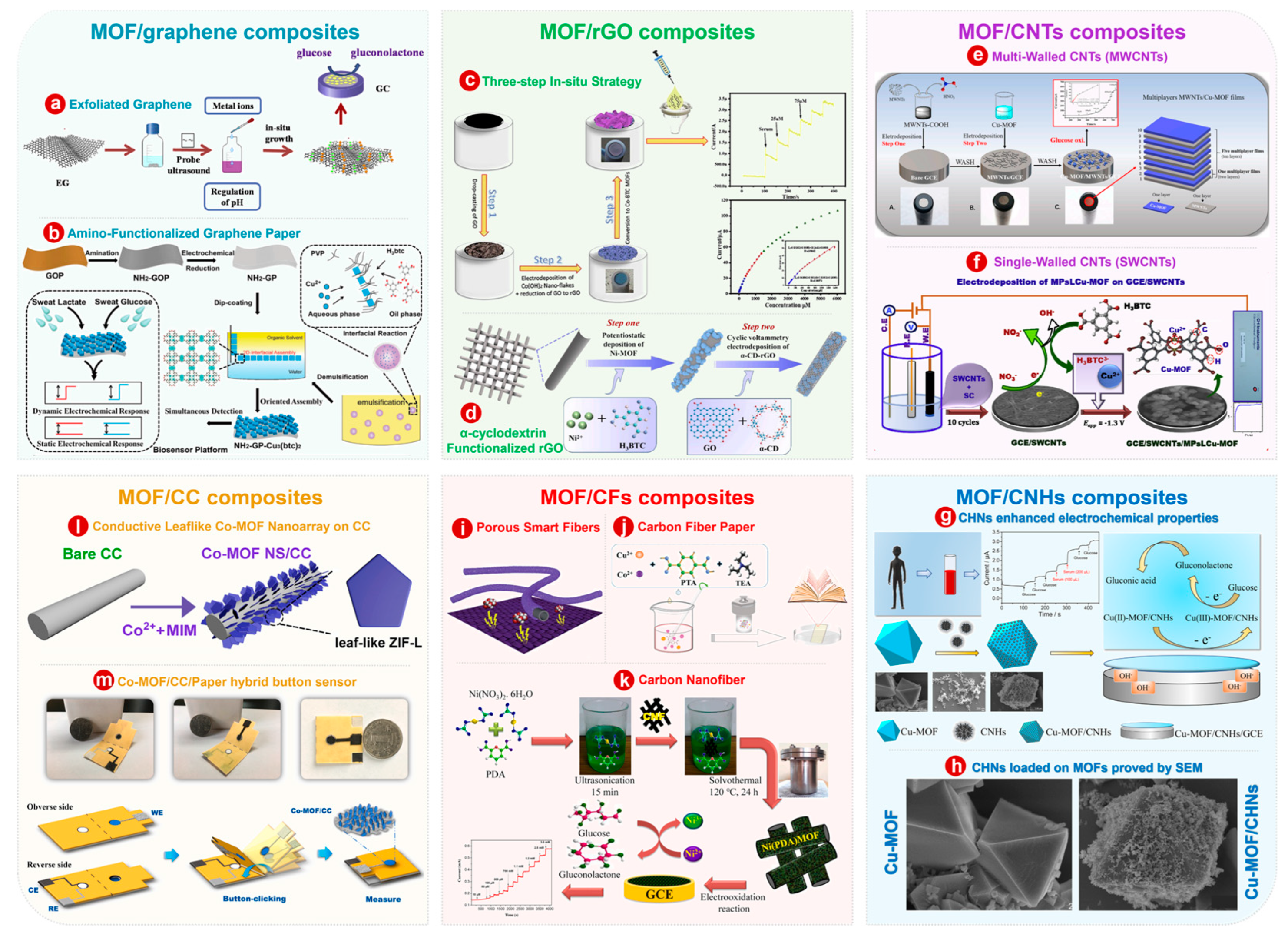
3.2. MOF/Metal-Based Composites
3.2.1. MOF/Au NPs
3.2.2. MOF/Ag NPs
3.2.3. MOF/Cu NPs
3.2.4. MOF/Nanoporous Au
3.2.5. MOF/Ni Foam
3.2.6. MOF/Cu Foam

3.3. MOF/Other Types of Functional Materials
3.3.1. MOF/Ionic Liquids
3.3.2. MOF/Conducting Polymer
3.3.3. MOF/MOF
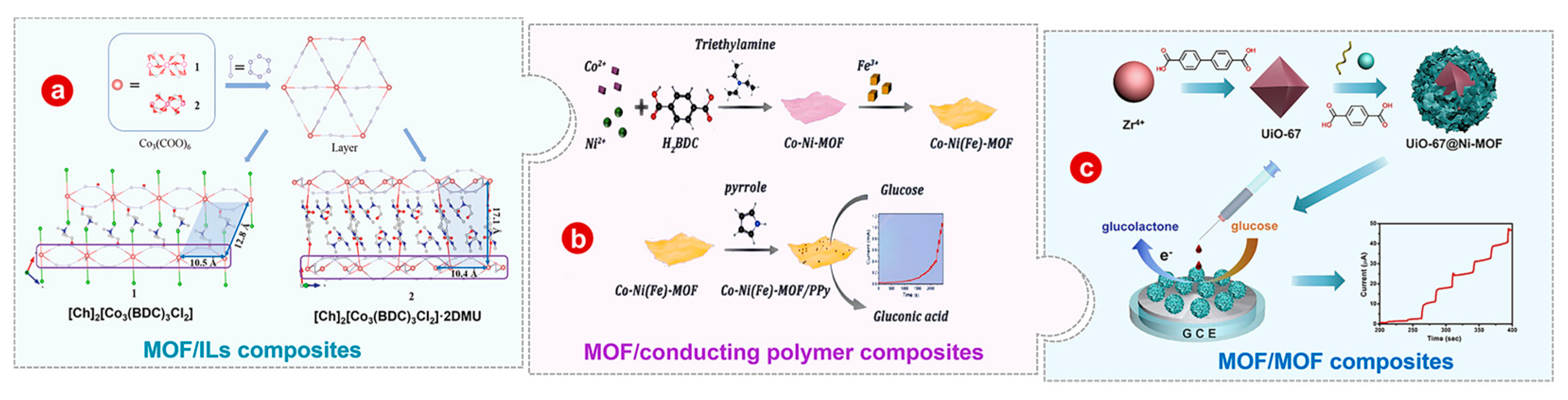
3.4. MOF/Two or More Kinds of Functional Material
4. Conclusions and Perspective
- (1)
- A variety of simple and effective synthesis methods (such as one-pot methods) have been gradually developed and perfected to make large-scale synthesis of the MOF and its composites possible. However, there is still a need to replace the precious metals used in existing MOF@metal NPs with low-cost but highly reactive metal NPs.
- (2)
- Aiming at improving the water stability of MOF s and MOF composites and reducing the content of other solvent molecules in the coordination center, it is necessary to develop a green way to synthesize MOFs and MOF composites in aqueous solution.
- (3)
- To gain further insight into the electrochemical mechanisms, in-depth studies through in situ test and theoretical simulations are necessary. In addition, advanced characterization instruments are necessary.
- (4)
- MOFs can be combined with nanofiber paper, portable fluorescence detectors and smartphones for development of convenient, quick and accurate electrochemical sensing techniques/equipment. Fluorescent detectors and smartphones are combined to develop simple, fast, accurate and portable methods of electrochemical sensing.
| Affiliation | Materials | Linear Range (µM) | Sensitivity (µA mM−1 cm−2) | Detection Limit (µM, S/N = 3) | Reference |
|---|---|---|---|---|---|
| Monometallic MOFs | ZIF-67 HNPs | 50 to 3300 | 445.7 | 0.96 | [56] |
| Ni-based MOF | 10 to 800 | 635.9 | 6.68 | [61] | |
| Ni-MIL-77 NBs | 1 to 500 | 1.542 | 0.25 | [62] | |
| CPO-27-NiII | 40 to 6000 | 40.95 (µA mM−1) | 1.46 | [63] | |
| Ni-based MOF | 10 to 400 | 3297.1 | 8.97 | [64] | |
| Cu-based MOF | 2 to 1400 1400 to 4000 | 1044 682 | 0.6 | [68] | |
| Cu-based MOF | 5 to 3910 | / | 0.11 | [69] | |
| Bimetallic MOFs | CoZn-BTC | 1 to 255 255 to 2530 | 1218 510 | 4.7 | [78] |
| ZIF67/ZIF8 | 50 to 5000 | 833.61 | 6.5 | [79] | |
| E-NiCo-BTC | 1 to 1780 1780 to 5030 | 1789 1436 | 0.187 | [80] | |
| NiCoBP-Br | 0.5 to 6065.5 | 1755.51 | 0.0665 | [81] | |
| Ni@Cu-MOF | 5 to 2500 | 1703.33 | 1.67 | [82] | |
| NiCu-MOF-6 | 20 to 4930 | 1832 | 15 | [83] | |
| Ni3Zn-MOF | 0.5 to 5065 | 512.53 | 0.125 | [84] | |
| MOF/ carbon-based composites | Co-MOF/EG | 1 to 3330 | 23 (µA mM−1) | 0.58 | [99] |
| NH2-GP-Cu-MOF | 0.05 to 1775.5 | 5.36 | 0.03 | [33] | |
| Co3(BTC)2/rGO | 1 to 330 330 to 1380 | 1702 1002 | 0.33 | [103] | |
| α-CD-rGO/Ni-MOF | 0.65 to 4828 4828 to 9178 | 1385 760 | 0.3 | [104] | |
| Ni-MOF/CNTs | 1 to 1600 | 13.85 | 0.82 | [105] | |
| Cu-MOF/MWNTs | 0.5 to 11,840 | 3878 | 0.4 | [110] | |
| Ni(TPA)-SWCNT | 20 to 4400 | / | 4.6 | [111] | |
| SWCNTs-MPsLCu-MOF | 0.02 to 80 | 573 | 0.00172 | [112] | |
| Cu-MOF/CNHs | 0.25 to 1200 | / | 0.078 | [117] | |
| Co-PSF | 0.5 to 30 | 4835 | 0.3 | [120] | |
| CuCo-MOF/CFP | 1 to 1200 | 6861 | 0.12 | [121] | |
| Ni(PDA)MOF@CNF | 10 to 3000 | 9457.5 | 0.053 | [122] | |
| Co-MOF NS/CC | 4 to 4428 | 1113 | 1.2 | [131] | |
| Co-MOF/CC | 800 to 16,000 | / | 150 | [132] | |
| Ni/Co(HHTP)MOF/CC | 0.3 to 2312 | 3250 | 0.1 | [133] | |
| MOF/ metal-based composites | Au@Ni-BTC | 5 to 7400 | 1447.1 | 1.5 | [57] |
| Au NPs/Cu-TCPP | 160 to 8000 | / | 3.9 | [142] | |
| Ag@ZIF-67 | 2 to 1000 | 0.379 | 0.66 | [143] | |
| Ag@ Co(II)-based 3D porous MOF | 5 to 550 | 0.135 | 1.32 | [144] | |
| Cu-in-ZIF-8 | 0 to 700 | 412 | 2.76 | [146] | |
| NiCo-MOF/NPG | 1 to 8000 | 684.4 | 0.29 | [148] | |
| Co-MOF/NF | 1 to 3000 | 10,886 | 0.0013 | [150] | |
| Cu1Co2-MOF/NF | 50 to 500 | 8304.4 | 23 | [151] | |
| 1@Cu Fo | 2 to 2000 | 27,900 | 0.1 | [153] | |
| MOF/ ionic liquids | Co-MOF/IL | 5 to 900 | 169 | 1.6 | [156] |
| [Ch]2[Co3(BDC)3Cl2] | 10 to 1200 | 160.75 | 3.2 | [157] | |
| [Ch]2[Co3(BDC)4]·2DMU | 10 to 1200 | 71.71 | 3.2 | [157] | |
| MOF /conducting polymer | Co-Ni(Fe)-MOF/PPy | 2 to 3000 | 1805 | 1.13 | [160] |
| MOF@MOF | UiO-67@Ni-MOF | 5 to 3900 | / | 0.98 | [163] |
| MOF/ two or more kinds of functional material | ACF-rGO/Cu(INA)2 | 25 to 16,875 | 46.8 | 0.5 | [164] |
| Ag@TiO2@ZIF-67 | 48 to 1000 | 788 | 0.99 | [165] | |
| CoFe-PBA/Co-ZIF/NF | 1.4 to 1500 | 5270 | 0.02 | [70] | |
| Ag@ZIF-67/MWCNT | 33 to 400 | 13.014 | 0.49 | [166] | |
| Ni-MOF/rGO/CF | 6 to 2090 | 852 | 0.6 | [167] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens. Bioelectron. 2020, 165, 112331. [Google Scholar] [CrossRef]
- Adeel, M.; Asif, K.; Rahman, M.M.; Daniele, S.; Canzonieri, V.; Rizzolio, F. Glucose Detection Devices and Methods Based on Metal–Organic Frameworks and Related Materials. Adv. Funct. Mater. 2021, 31, 2106023. [Google Scholar] [CrossRef]
- Dong, Q.; Ryu, H.; Lei, Y. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef]
- Zimmet, P.Z.; Magliano, D.J.; Herman, W.H.; Shaw, J.E. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2014, 2, 56–64. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Strakosas, X.; Selberg, J.; Pansodtee, P.; Yonas, N.; Manapongpun, P.; Teodorescu, M.; Rolandi, M. A non-enzymatic glucose sensor enabled by bioelectronic pH control. Sci. Rep. 2019, 9, 10844. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- Nathan, D.M.; Cleary, P.A.; Backlund, J.-Y.C.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar]
- Zaidi, S.A.; Shin, J.H. Recent developments in nanostructure based electrochemical glucose sensors. Talanta 2016, 149, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Witkowska Nery, E.; Kundys, M.; Jeleń, P.S.; Jönsson-Niedziółka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Lee, A.-R. Recent developments in blood glucose sensors. J. Food Drug Anal. 2015, 23, 191–200. [Google Scholar] [CrossRef]
- Wei, M.; Qiao, Y.; Zhao, H.; Liang, J.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Lu, W.; Sun, X. Electrochemical non-enzymatic glucose sensors: Recent progress and perspectives. Chem. Commun. 2020, 56, 14553–14569. [Google Scholar] [CrossRef]
- Okuda-Shimazaki, J.; Yoshida, H.; Sode, K. FAD dependent glucose dehydrogenases—Discovery and engineering of representative glucose sensing enzymes. Bioelectrochemistry 2020, 132, 107414. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yan, Y. Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials: Opportunities and challenges. RSC Adv. 2016, 6, 84893–84905. [Google Scholar] [CrossRef]
- Niu, X.H.; Shi, L.B.; Zhao, H.L.; Lan, M.B. Advanced strategies for improving the analytical performance of Pt-based nonenzymatic electrochemical glucose sensors: A minireview. Anal. Methods 2016, 8, 1755–1764. [Google Scholar] [CrossRef]
- Wang, J.; Thomas, D.F.; Chen, A. Nonenzymatic Electrochemical Glucose Sensor Based on Nanoporous PtPb Networks. Anal. Chem. 2008, 80, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Jha, S.K. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens. Bioelectron. 2015, 67, 763–768. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Cincotto, F.H.; Moraes, F.C.; Fatibello-Filho, O. Square-wave adsorptive anodic stripping voltammetric determination of norfloxacin using a glassy carbon electrode modified with carbon black and CdTe quantum dots in a chitosan film. Microchim. Acta 2019, 186, 148. [Google Scholar] [CrossRef] [PubMed]
- Alshik Edris, N.M.M.; Abdullah, J.; Kamaruzaman, S.; Sulaiman, Y. Voltammetric determination of hydroquinone, catechol, and resorcinol by using a glassy carbon electrode modified with electrochemically reduced graphene oxide-poly(Eriochrome black T) and gold nanoparticles. Microchim. Acta 2019, 186, 261. [Google Scholar] [CrossRef] [PubMed]
- Amani-Beni, Z.; Nezamzadeh-Ejhieh, A. A novel non-enzymatic glucose sensor based on the modification of carbon paste electrode with CuO nanoflower: Designing the experiments by response surface methodology (RSM). J. Colloid Interface Sci. 2017, 504, 186–196. [Google Scholar] [CrossRef]
- Parashuram, L.; Sreenivasa, S.; Akshatha, S.; Udayakumar, V.; Sandeep Kumar, S. A non-enzymatic electrochemical sensor based on ZrO2: Cu(I) nanosphere modified carbon paste electrode for electro-catalytic oxidative detection of glucose in raw Citrus aurantium var. sinensis. Food Chem. 2019, 300, 125178. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Sezgintürk, M.K. Indium tin oxide (ITO): A promising material in biosensing technology. Trends Anal. Chem. 2017, 97, 309–315. [Google Scholar] [CrossRef]
- Silah, H.; Erkmen, C.; Demir, E.; Uslu, B. Modified indium tin oxide electrodes: Electrochemical applications in pharmaceutical, biological, environmental and food analysis. Trends Anal. Chem. 2021, 141, 116289. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal–Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.-D.; Chen, J.-Y.; Li, Y.-W. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Zhang, Z.-H.; Zhang, X.; Zhou, Z.; Bu, X.-H. Metal–Organic Frameworks (MOFs) and MOF-Derived Materials for Energy Storage and Conversion. Electrochem. Energ. Rev. 2019, 2, 29–104. [Google Scholar] [CrossRef]
- Jiawei, G.; Yi, P.; Ting, Z.; Jiao, M.; Huan, P.; Yusuke, Y. Porphyrin-based framework materials for energy conversion. Nano Res. Energy 2022, 1, e9120009. [Google Scholar]
- Xu, Y.; Li, Q.; Xue, H.; Pang, H. Metal-organic frameworks for direct electrochemical applications. Coord. Chem. Rev. 2018, 376, 292–318. [Google Scholar] [CrossRef]
- Ling, W.; Liew, G.; Li, Y.; Hao, Y.; Pan, H.; Wang, H.; Ning, B.; Xu, H.; Huang, X. Materials and Techniques for Implantable Nutrient Sensing Using Flexible Sensors Integrated with Metal–Organic Frameworks. Adv. Mater. 2018, 30, 1800917. [Google Scholar] [CrossRef]
- Qin, Z.-S.; Dong, W.-W.; Zhao, J.; Wu, Y.-P.; Zhang, Q.-C.; Li, D.-S.; Wang, W.; Wang, F.; Xiao, F.; Liu, H. A water-stable Tb(iii)-based metal–organic gel (MOG) for detection of antibiotics and explosives. Inorg. Chem. Front. 2018, 5, 120–126. [Google Scholar] [CrossRef]
- Wang, K.-B.; Wang, S.-E.; Liu, J.-D.; Guo, Y.-X.; Mao, F.-F.; Wu, H.; Zhang, Q.-C. Fe-Based Coordination Polymers as Battery-Type Electrodes in Semi-Solid-State Battery–Supercapacitor Hybrid Devices. ACS Appl. Mater. Interfaces 2021, 13, 15315–15323. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, X.; Chai, H.; Huang, Y. Recent advances in the construction and analytical applications of metal-organicframeworks-based nanozymes. Trends Anal. Chem. 2018, 105, 391–403. [Google Scholar] [CrossRef]
- Ye, M.; Li, C.; Zhao, Y.; Qu, L. Graphene decorated with bimodal size of carbon polyhedrons for enhanced lithium storage. Carbon 2016, 106, 9–19. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Xu, J.; Ma, J.; Bai, Y.; Cao, S.; Zhang, S.; Pang, H. Metal-organic framework (MOF) composites as promising materials for energy storage applications. Adv. Colloid Interface Sci. 2022, 307, 102732. [Google Scholar] [CrossRef]
- Zheng, S.; Li, Q.; Xue, H.; Pang, H.; Xu, Q. A highly alkaline-stable metal oxide@metal–organic framework composite for high-performance electrochemical energy storage. Natl. Sci. Rev. 2020, 7, 305–314. [Google Scholar] [CrossRef]
- Xu, J.; Ma, J.; Peng, Y.; Cao, S.; Zhang, S.; Pang, H. Applications of metal nanoparticles/metal-organic frameworks composites in sensing field. Chin. Chem. Lett. 2023, 34, 107527. [Google Scholar] [CrossRef]
- Liu, S.; Teng, Z.; Liu, H.; Wang, T.; Wang, G.; Xu, Q.; Zhang, X.; Jiang, M.; Wang, C.; Huang, W.; et al. A Ce-UiO-66 Metal–Organic Framework-Based Graphene-Embedded Photocatalyst with Controllable Activation for Solar Ammonia Fertilizer Production. Angew. Chem. Int. Ed. 2022, 61, e202207026. [Google Scholar]
- Qiu, T.; Liang, Z.; Guo, W.; Tabassum, H.; Gao, S.; Zou, R. Metal–Organic Framework-Based Materials for Energy Conversion and Storage. ACS Energy Lett. 2020, 5, 520–532. [Google Scholar] [CrossRef]
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, B.; Guo, X.; Li, W.; Ma, W.; Wu, X.; Pang, H. MOF-derived metal sulfides for electrochemical energy applications. Energy Storage Mater. 2022, 51, 840–872. [Google Scholar] [CrossRef]
- Peng, Y.; Bai, Y.; Liu, C.; Cao, S.; Kong, Q.; Pang, H. Applications of metal–organic framework-derived N, P, S doped materials in electrochemical energy conversion and storage. Coord. Chem. Rev. 2022, 466, 214602. [Google Scholar] [CrossRef]
- Daud, A.D.; Lim, H.N.; Ibrahim, I.; Endot, N.A.; Gowthaman, N.S.K.; Jiang, Z.T.; Cordova, K.E. An effective metal-organic framework-based electrochemical non-enzymatic glucose sensor. J. Electroanal. Chem. 2022, 921, 116676. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Liang, H.; Lin, H.; Zhao, G. A non-enzymatic glucose sensor with enhanced anti-interference ability based on a MIL-53(NiFe) metal–organic framework. J. Mater. Chem. B 2019, 7, 7006–7013. [Google Scholar] [CrossRef]
- Wang, L.; Deng, N.; Wang, G.; Ju, J.; Cheng, B.; Kang, W. Constructing Amino-Functionalized Flower-like Metal–Organic Framework Nanofibers in Sulfonated Poly(ether sulfone) Proton Exchange Membrane for Simultaneously Enhancing Interface Compatibility and Proton Conduction. ACS Appl. Mater. Interfaces 2019, 11, 39979–39990. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, Q.; Lu, S.; Chen, G.; Gao, S.; Lu, W.; Sun, X. High-performance non-enzymatic glucose detection: Using a conductive Ni-MOF as an electrocatalyst. J. Mater. Chem. B 2020, 8, 5411–5415. [Google Scholar] [CrossRef]
- Golub, K.W.; Sulmonetti, T.P.; Darunte, L.A.; Shealy, M.S.; Jones, C.W. Metal–Organic-Framework-Derived Co/Cu–Carbon Nanoparticle Catalysts for Furfural Hydrogenation. ACS Appl. Nano Mater. 2019, 2, 6040–6056. [Google Scholar] [CrossRef]
- Tian, F.; Qiao, C.; Zheng, R.; Ru, Q.; Sun, X.; Zhang, Y.; Meng, C. Synthesis of bimetallic–organic framework Cu/Co-BTC and the improved performance of thiophene adsorption. RSC Adv. 2019, 9, 15642–15647. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.-F.; Li, C.; Xu, Q. Bimetallic metal–organic frameworks and their derivatives. Chem. Sci. 2020, 11, 5369–5403. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Hua, Y.; Li, X.; Chen, C.; Pang, H. Cobalt based metal-organic frameworks and their derivatives for electrochemical energy conversion and storage. Chem. Eng. J. 2019, 370, 37–59. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Q.; Xie, Q.; Ou, H.; Lin, X.; Zeb, A.; Hu, L.; Wu, Y.; Ma, G. Recent progress in Co–based metal–organic framework derivatives for advanced batteries. J. Mater. Sci. Technol. 2022, 96, 262–284. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Q.; Guan, J. A binuclear Co-based metal–organic framework towards efficient oxygen evolution reaction. Chem. Commun. 2021, 57, 5016–5019. [Google Scholar] [CrossRef]
- Chen, Q.; Chu, D.; Yan, L.; Lai, H.; Chu, X.-Q.; Ge, D.; Chen, X. Enhanced non-enzymatic glucose sensing based on porous ZIF-67 hollow nanoprisms. New J. Chem. 2021, 45, 10031–10039. [Google Scholar] [CrossRef]
- Chen, J.; Yin, H.; Zhou, J.; Wang, L.; Gong, J.; Ji, Z.; Nie, Q. Efficient Nonenzymatic Sensors Based on Ni-MOF Microspheres Decorated with Au Nanoparticles for Glucose Detection. J. Electron. Mater. 2020, 49, 4754–4763. [Google Scholar] [CrossRef]
- Wang, F.; Hu, J.; Peng, Y.; Wu, X.; Xue, H.; Pang, H. Application and Modification of Nickel-based Metal-organic frameworks in Electrochemical Sensing. Adv. Sens. Energy Mater. 2023, 2, 100053. [Google Scholar] [CrossRef]
- Du, P.; Dong, Y.; Liu, C.; Wei, W.; Liu, D.; Liu, P. Fabrication of hierarchical porous nickel based metal-organic framework (Ni-MOF) constructed with nanosheets as novel pseudo-capacitive material for asymmetric supercapacitor. J. Colloid Interface Sci. 2018, 518, 57–68. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Diao, Y.; He, Q.; Lu, C.; Singh, A.; Kumar, A.; Liu, J.; Lan, Q. Recent advances in the electrochemical applications of Ni-based metal organic frameworks (Ni-MOFs) and their derivatives. Chemosphere 2022, 307, 135729. [Google Scholar] [CrossRef]
- Gumilar, G.; Kaneti, Y.V.; Henzie, J.; Chatterjee, S.; Na, J.; Yuliarto, B.; Nugraha, N.; Patah, A.; Bhaumik, A.; Yamauchi, Y. General synthesis of hierarchical sheet/plate-like M-BDC (M = Cu, Mn, Ni, and Zr) metal–organic frameworks for electrochemical non-enzymatic glucose sensing. Chem. Sci. 2020, 11, 3644–3655. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zheng, S.; Li, X.; Zhang, G.; Guo, X.; Xue, H.; Pang, H. Facile synthesis of ultrathin Ni-MOF nanobelts for high-efficiency determination of glucose in human serum. J. Mater. Chem. B 2017, 5, 5234–5239. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.M.; Ahmed, F.; Sutradhar, S.C.; Ryu, T.; Kim, W. A Ni-based redox-active metal-organic framework for sensitive and non-enzymatic detection of glucose. J. Electroanal. Chem. 2018, 822, 43–49. [Google Scholar] [CrossRef]
- Xuan, X.; Qian, M.; Pan, L.; Lu, T.; Han, L.; Yu, H.; Wan, L.; Niu, Y.; Gong, S. A longitudinally expanded Ni-based metal–organic framework with enhanced double nickel cation catalysis reaction channels for a non-enzymatic sweat glucose biosensor. J. Mater. Chem. B 2020, 8, 9094–9109. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiong, P.; Zheng, C.; Qiu, H.; Wei, M. Metal–organic frameworks: A new promising class of materials for a high performance supercapacitor electrode. J. Mater. Chem. A 2014, 2, 16640–16644. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Li, T.; Bai, Y.; Wang, Y.; Xu, H.; Jin, H. Advances in transition-metal (Zn, Mn, Cu)-based MOFs and their derivatives for anode of lithium-ion batteries. Coord. Chem. Rev. 2020, 410, 213221. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Khaki Sanati, E.; Hosseini, H. Direct growth of metal-organic frameworks thin film arrays on glassy carbon electrode based on rapid conversion step mediated by copper clusters and hydroxide nanotubes for fabrication of a high performance non-enzymatic glucose sensing platform. Biosens. Bioelectron. 2018, 112, 100–107. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.-W.; Ma, Y.; Zhang, J.-J.; Mo, G.-Q.; Du, H.-J.; Ye, J.-S. Cu(II) Metal-Organic Framework Encapsulated in Carbon Paste Electrode for High-Performance Non-Enzymatic Glucose Sensing. Chin. J. Anal. Chem. 2020, 48, e20038–e20046. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, D.; Gu, M.; Lu, C.; Yi, F.-Y.; Ma, X. MOF-Derived Bimetallic CoFe-PBA Composites as Highly Selective and Sensitive Electrochemical Sensors for Hydrogen Peroxide and Nonenzymatic Glucose in Human Serum. ACS Appl. Mater. Interfaces 2020, 12, 35365–35374. [Google Scholar] [CrossRef]
- Guo, Q.; Zeng, W.; Liu, S.; Li, Y. In situ formation of Co3O4 hollow nanocubes on carbon cloth-supported NiCo2O4 nanowires and their enhanced performance in non-enzymatic glucose sensing. Nanotechnology 2020, 31, 265501. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-Metal MOFs: Unique Opportunities in Metal–Organic Framework (MOF) Functionality and Design. Angew. Chem. Int. Ed. 2019, 58, 15188–15205. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, K.-Y.; Day, G.S.; Zhou, H.-C. The chemistry of multi-component and hierarchical framework compounds. Chem. Soc. Rev. 2019, 48, 4823–4853. [Google Scholar] [CrossRef]
- Foo, M.L.; Matsuda, R.; Kitagawa, S. Functional Hybrid Porous Coordination Polymers. Chem. Mater. 2014, 26, 310–322. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Gohari Derakhshandeh, P.; Depauw, H.; Coudert, F.-X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kaushal, S.; Singh, P.P. Bimetallic metal organic frameworks heterogeneous catalysts: Design, construction, and applications. Mater. Today Energy 2021, 20, 100667. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, Y.; Liu, Y.; Zhang, W.; Dai, Z.; Srivastava, D.; Kumar, A.; Pan, Y.; Liu, J. Recent advances in bimetallic metal–organic frameworks (BMOFs): Synthesis, applications and challenges. New J. Chem. 2022, 46, 13818–13837. [Google Scholar] [CrossRef]
- Ataei Kachouei, M.; Shahrokhian, S.; Ezzati, M. Bimetallic CoZn-MOFs easily derived from CoZn-LDHs, as a suitable platform in fabrication of a non-enzymatic electrochemical sensor for detecting glucose in human fluids. Sens. Actuators B Chem. 2021, 344, 130254. [Google Scholar] [CrossRef]
- Li, X.; Dong, H.; Fan, Q.; Chen, K.; Sun, D.; Hu, T.; Ni, Z. One-pot, rapid microwave-assisted synthesis of bimetallic metal–organic framework for efficient enzyme-free glucose detection. Microchem. J. 2022, 179, 107468. [Google Scholar] [CrossRef]
- Ezzati, M.; Shahrokhian, S.; Hosseini, H. In Situ Two-Step Preparation of 3D NiCo-BTC MOFs on a Glassy Carbon Electrode and a Graphitic Screen Printed Electrode as Nonenzymatic Glucose-Sensing Platforms. ACS Sustain. Chem. Eng. 2020, 8, 14340–14352. [Google Scholar] [CrossRef]
- Li, P.; Bai, Y.; Zhang, G.; Guo, X.; Meng, X.; Pang, H. Surface-halogen-introduced 2D NiCo bimetallic MOFs via a modulation method for elevated electrochemical glucose sensing. Inorg. Chem. Front. 2022, 9, 5853–5861. [Google Scholar] [CrossRef]
- Xue, Z.; Jia, L.; Zhu, R.-R.; Du, L.; Zhao, Q.-H. High-performance non-enzymatic glucose electrochemical sensor constructed by transition nickel modified Ni@Cu-MOF. J. Electroanal. Chem. 2020, 858, 113783. [Google Scholar] [CrossRef]
- Pan, W.; Zheng, Z.; Wu, X.; Gao, J.; Liu, Y.; Yuan, Q.; Gan, W. Facile synthesis of 2D/3D hierarchical NiCu bimetallic MOF for non-enzymatic glucose sensor. Microchem. J. 2021, 170, 106652. [Google Scholar] [CrossRef]
- Zheng, S.; Li, B.; Tang, Y.; Li, Q.; Xue, H.; Pang, H. Ultrathin nanosheet-assembled [Ni3(OH)2(PTA)2(H2O)4]·2H2O hierarchical flowers for high-performance electrocatalysis of glucose oxidation reactions. Nanoscale 2018, 10, 13270–13276. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Metal-organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Qian, R.; Ling, P.; Cui, L.; Ju, H. Design and sensing applications of metal–organic framework composites. Trends Anal. Chem. 2014, 58, 71–78. [Google Scholar] [CrossRef]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X.; Gascon, J. Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2015, 14, 48–55. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Energy Applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef]
- Du, M.; Li, Q.; Zhao, Y.; Liu, C.-S.; Pang, H. A review of electrochemical energy storage behaviors based on pristine metal–organic frameworks and their composites. Coord. Chem. Rev. 2020, 416, 213341. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Composites of metal–organic frameworks: Preparation and application in adsorption. Mater. Today 2014, 17, 136–146. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Vellingiri, K.; Deep, A.; Kwon, E.E.; Bolan, N.; Kim, K.-H. Metal–organic framework composites as electrocatalysts for electrochemical sensing applications. Coord. Chem. Rev. 2018, 357, 105–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Luhana, C.; Wang, H.; Li, M.; Guo, L. Facile synthesis of a Cu-based MOF confined in macroporous carbon hybrid material with enhanced electrocatalytic ability. Chem. Commun. 2013, 49, 6885–6887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nsabimana, A.; Zhu, L.; Bo, X.; Han, C.; Li, M.; Guo, L. Metal organic frameworks/macroporous carbon composites with enhanced stability properties and good electrocatalytic ability for ascorbic acid and hemoglobin. Talanta 2014, 129, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-W.; Sun, T.-J.; Hu, J.-L.; Wang, S.-D. Composites of metal–organic frameworks and carbon-based materials: Preparations, functionalities and applications. J. Mater. Chem. A 2016, 4, 3584–3616. [Google Scholar] [CrossRef]
- Travlou, N.A.; Singh, K.; Rodríguez-Castellón, E.; Bandosz, T.J. Cu–BTC MOF–graphene-based hybrid materials as low concentration ammonia sensors. J. Mater. Chem. A 2015, 3, 11417–11429. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, K.; Liu, G.; Chen, Y.; Wang, M.; Li, S.; Li, R. Recent advances on graphene: Synthesis, properties and applications. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107051. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Liu, H.; Zhai, Y.; Li, L.; Wen, H. 2D MOF with electrochemical exfoliated graphene for nonenzymatic glucose sensing: Central metal sites and oxidation potentials. Anal. Chim. Acta 2020, 1122, 9–19. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Ezzati, M.; Hosseini, H. Fabrication of a sensitive and fast response electrochemical glucose sensing platform based on co-based metal-organic frameworks obtained from rapid in situ conversion of electrodeposited cobalt hydroxide intermediates. Talanta 2020, 210, 120696. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, Y.; Liu, M.; Wang, H.; Ren, J.; Tian, Y.; Liu, X.; Zhou, Y.; Wang, J.; Zhu, W.; et al. In-situ two-step electrodeposition of α-CD-rGO/Ni-MOF composite film for superior glucose sensing. J. Alloys Compd. 2022, 923, 166418. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Ye, B. An efficient electrochemical glucose sensor based on porous nickel-based metal organic framework/carbon nanotubes composite (Ni-MOF/CNTs). J. Alloys Compd. 2018, 767, 651–656. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Nayak, P.; Nair, S.P.; Ramaprabhu, S. Enzyme-less and low-potential sensing of glucose using a glassy carbon electrode modified with palladium nanoparticles deposited on graphene-wrapped carbon nanotubes. Microchim. Acta 2016, 183, 1055–1062. [Google Scholar] [CrossRef]
- Das, D.; Das, A.; Reghunath, M.; Nanda, K.K. Phosphine-free avenue to Co2P nanoparticle encapsulated N,P co-doped CNTs: A novel non-enzymatic glucose sensor and an efficient electrocatalyst for oxygen evolution reaction. Green Chem. 2017, 19, 1327–1335. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Ye, J. Enzyme-free glucose sensor based on layer-by-layer electrodeposition of multilayer films of multi-walled carbon nanotubes and Cu-based metal framework modified glassy carbon electrode. Biosens. Bioelectron. 2019, 135, 45–49. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Chen, L.; Yang, J.; Wang, Q. High-performance non-enzymatic glucose sensor by hierarchical flower-like nickel(II)-based MOF/carbon nanotubes composite. Mater. Sci. Eng. C 2019, 96, 41–50. [Google Scholar] [CrossRef]
- Arul, P.; Gowthaman, N.S.K.; John, S.A.; Tominaga, M. Tunable electrochemical synthesis of 3D nucleated microparticles like Cu-BTC MOF-carbon nanotubes composite: Enzyme free ultrasensitive determination of glucose in a complex biological fluid. Electrochim. Acta 2020, 354, 136673. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.-K.; Tománek, D. Electronic and structural properties of carbon nanohorns. Phys. Rev. B 2000, 62, R2291–R2294. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, Properties, Functionalization, and Applications of Carbon Nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, G. Single-walled carbon nanohorns and their applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Fiston, M.N.; Qian, J.; Kingsford, O.J. Highly sensitive electrochemical sensing of para-chloronitrobenzene using a carbon nanohorn–nanotube hybrid modified electrode. Anal. Methods 2019, 11, 1125–1130. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.; Yang, P.; Chen, Y.; Tao, J.; Hu, J.; Zhao, P. Carbon nanohorns enhanced electrochemical properties of Cu-based metal organic framework for ultrasensitive serum glucose sensing. J. Electroanal. Chem. 2020, 862, 114018. [Google Scholar] [CrossRef]
- Newcomb, B.A. Processing, structure, and properties of carbon fibers. Compos. Part A Appl. Sci. Manuf. 2016, 91, 262–282. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.; Huang, Y.; Guo, Z.; Shao, L. Building Nanoporous Metal–Organic Frameworks “Armor” on Fibers for High-Performance Composite Materials. ACS Appl. Mater. Interfaces 2017, 9, 5590–5599. [Google Scholar] [CrossRef]
- Zhao, Z.; Kong, Y.; Lin, X.; Liu, C.; Liu, J.; He, Y.; Yang, L.; Huang, G.; Mei, Y. Oxide nanomembrane induced assembly of a functional smart fiber composite with nanoporosity for an ultra-sensitive flexible glucose sensor. J. Mater. Chem. A 2020, 8, 26119–26129. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Yu, F.; Wu, J.; Liu, Z.; Peng, B. Multifunctional book-like CuCo-MOF for highly sensitive glucose detection and electrocatalytic oxygen evolution. New J. Chem. 2021, 45, 16714–16721. [Google Scholar] [CrossRef]
- Dey, B.; Ahmad, M.W.; Sarkhel, G.; Yang, D.-J.; Choudhury, A. Fabrication of porous nickel (II)-based MOF@carbon nanofiber hybrid mat for high-performance non-enzymatic glucose sensing. Mater. Sci. Semicond. Process. 2022, 142, 106500. [Google Scholar] [CrossRef]
- Mishra, A.; Shetti, N.P.; Basu, S.; Raghava Reddy, K.; Aminabhavi, T.M. Carbon Cloth-based Hybrid Materials as Flexible Electrochemical Supercapacitors. ChemElectroChem 2019, 6, 5771–5786. [Google Scholar]
- Ma, J.; Li, J.; Guo, R.; Xu, H.; Shi, F.; Dang, L.; Liu, Z.; Sun, J.; Lei, Z. Direct growth of flake-like metal-organic framework on textile carbon cloth as high-performance supercapacitor electrode. J. Power Sources 2019, 428, 124–130. [Google Scholar]
- Shi, H.; Wen, G.; Nie, Y.; Zhang, G.; Duan, H. Flexible 3D carbon cloth as a high-performing electrode for energy storage and conversion. Nanoscale 2020, 12, 5261–5285. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.-W.; Wu, Y.-X.; Wei, T.-C.; Geng, D.; Mei, J.; Liu, H.; Lau, W.-M.; Liu, L.-M. Three-dimensional hierarchical interwoven nitrogen-doped carbon nanotubes/CoxNi1-x-layered double hydroxides ultrathin nanosheets for high-performance supercapacitors. Electrochim. Acta 2016, 203, 21–29. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, M.; Hu, N.; Zhang, W.; Luo, Z.; Wang, R.; Wang, J.; Huang, L.; Suo, Y.; Wang, J. Traditional NiCo2S4 Phase with Porous Nanosheets Array Topology on Carbon Cloth: A Flexible, Versatile and Fabulous Electrocatalyst for Overall Water and Urea Electrolysis. ACS Sustain. Chem. Eng. 2018, 6, 5011–5020. [Google Scholar] [CrossRef]
- Torrinha, Á.; Morais, S. Electrochemical (bio)sensors based on carbon cloth and carbon paper: An overview. Trends Anal. Chem. 2021, 142, 116324. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, R.; He, F.; Hu, W.; Cao, X.; Jia, J.; Lu, W.; Sun, X. A comparative study of electrocatalytic oxidation of glucose on conductive Ni-MOF nanosheet arrays with different ligands. New J. Chem. 2020, 44, 17849–17853. [Google Scholar] [CrossRef]
- Chen, T.; Wang, F.; Cao, S.; Bai, Y.; Zheng, S.; Li, W.; Zhang, S.; Hu, S.-X.; Pang, H. In Situ Synthesis of MOF-74 Family for High Areal Energy Density of Aqueous Nickel–Zinc Batteries. Adv. Mater. 2022, 34, 2201779. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, W.; Li, Y.; Ma, Y.; Wang, J.; Hu, N.; Suo, Y.; Wang, J. Conductive Leaflike Cobalt Metal–Organic Framework Nanoarray on Carbon Cloth as a Flexible and Versatile Anode toward Both Electrocatalytic Glucose and Water Oxidation. Inorg. Chem. 2018, 57, 8422–8428. [Google Scholar] [CrossRef]
- Wei, X.; Guo, J.; Lian, H.; Sun, X.; Liu, B. Cobalt metal-organic framework modified carbon cloth/paper hybrid electrochemical button-sensor for nonenzymatic glucose diagnostics. Sens. Actuators B Chem. 2021, 329, 129205. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Zhangsun, H.; Zhao, S.; Zhao, Y.; Wang, L. Carbon cloth-supported nanorod-like conductive Ni/Co bimetal MOF: A stable and high-performance enzyme-free electrochemical sensor for determination of glucose in serum and beverage. Food Chem. 2021, 349, 129202. [Google Scholar] [CrossRef]
- Morozan, A.; Jaouen, F. Metal organic frameworks for electrochemical applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, F.; Li, C.; Zhang, T.; Zhang, H.; Cai, W.; Li, Y. Black Gold: Plasmonic Colloidosomes with Broadband Absorption Self-Assembled from Monodispersed Gold Nanospheres by Using a Reverse Emulsion System. Angew. Chem. Int. Ed. 2015, 54, 9596–9600. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Mookherjee, R. Convective assembly of linear gold nanoparticle arrays at the micron scale for surface enhanced Raman scattering. Nano Res. 2011, 4, 1117–1128. [Google Scholar] [CrossRef]
- Huang, K.; Guo, S.; Wang, R.; Lin, S.; Hussain, N.; Wei, H.; Deng, B.; Long, Y.; Lei, M.; Tang, H.; et al. Two-dimensional MOF/MOF derivative arrays on nickel foam as efficient bifunctional coupled oxygen electrodes. Chin. J. Catal. 2020, 41, 1754–1760. [Google Scholar] [CrossRef]
- Ruditskiy, A.; Xia, Y. Toward the Synthesis of Sub-15 nm Ag Nanocubes with Sharp Corners and Edges: The Roles of Heterogeneous Nucleation and Surface Capping. J. Am. Chem. Soc. 2016, 138, 3161–3167. [Google Scholar] [CrossRef]
- Men, D.; Zhou, F.; Hang, L.; Li, X.; Duan, G.; Cai, W.; Li, Y. A functional hydrogel film attached with a 2D Au nanosphere array and its ultrahigh optical diffraction intensity as a visualized sensor. J. Mater. Chem. C 2016, 4, 2117–2122. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Hang, L.; Zhou, F.; Men, D.; Li, H.; Li, X.; Zhang, H.; Liu, G.; Cai, W.; Li, C.; Li, Y. Functionalized periodic Au@MOFs nanoparticle arrays as biosensors for dual-channel detection through the complementary effect of SPR and diffraction peaks. Nano Res. 2017, 10, 2257–2270. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, Y.; Wu, Y.; Guo, X.; Ying, Y.; Wen, Y.; Yang, H. Enzyme-Free Tandem Reaction Strategy for Surface-Enhanced Raman Scattering Detection of Glucose by Using the Composite of Au Nanoparticles and Porphyrin-Based Metal–Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 55324–55330. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.; Dai, L.; He, Z.; Wang, L. A novel electrochemical sensor for glucose detection based on Ag@ZIF-67 nanocomposite. Sens. Actuators B Chem. 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, W.-J.; Lu, Y.-K.; Liu, G.; Hou, L.; Wang, Y.-Y. Nonenzymatic Glucose Sensing and Magnetic Property Based On the Composite Formed by Encapsulating Ag Nanoparticles in Cluster-Based Co-MOF. Inorg. Chem. 2019, 58, 16743–16751. [Google Scholar] [CrossRef] [PubMed]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, X.; Liu, T.; Zhao, H.; Lan, M. Encapsulating Cu nanoparticles into metal-organic frameworks for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2016, 227, 583–590. [Google Scholar] [CrossRef]
- Li, W.; Ma, L.; Peng, P.; Jia, Q.; Wan, Z.; Zhu, Y.; Guo, W. Microstructural evolution and deformation behavior of fiber laser welded QP980 steel joint. Mater. Sci. Eng. A 2018, 717, 124–133. [Google Scholar] [CrossRef]
- Li, W.; Lv, S.; Wang, Y.; Zhang, L.; Cui, X. Nanoporous gold induced vertically standing 2D NiCo bimetal-organic framework nanosheets for non-enzymatic glucose biosensing. Sens. Actuators B Chem. 2019, 281, 652–658. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Li, R.; Ye, C.; Zhao, G.; Wang, Y. Ni-Based metal–organic framework derived Ni@C nanosheets on a Ni foam substrate as a supersensitive non-enzymatic glucose sensor. J. Mater. Chem. B 2017, 5, 5549–5555. [Google Scholar] [CrossRef]
- Li, Y.; Xie, M.; Zhang, X.; Liu, Q.; Lin, D.; Xu, C.; Xie, F.; Sun, X. Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection. Sens. Actuators B Chem. 2019, 278, 126–132. [Google Scholar] [CrossRef]
- Du, Q.; Liao, Y.; Shi, N.; Sun, S.; Liao, X.; Yin, G.; Huang, Z.; Pu, X.; Wang, J. Facile synthesis of bimetallic metal–organic frameworks on nickel foam for a high performance non-enzymatic glucose sensor. J. Electroanal. Chem. 2022, 904, 115887. [Google Scholar] [CrossRef]
- Xiaohong, Z.; Zhidong, Z.; Xiongwei, L.; Jian, L.; Guohua, H. A maltose, L-rhamnose sensor based on porous Cu foam and electrochemical amperometric i-t scanning method. J. Food Meas. Charact. 2017, 11, 548–555. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Q.; Yu, F.; Ran, G.Y.; Wang, H.Y.; Shepherd, N.D.; D’Alessandro, D.M.; Kurmoo, M.; Zuo, J.L. A Metal-Organic Framework Based on a Nickel Bis(dithiolene) Connector: Synthesis, Crystal Structure, and Application as an Electrochemical Glucose Sensor. J. Am. Chem. Soc. 2020, 142, 20313–20317. [Google Scholar] [CrossRef] [PubMed]
- Shomal, R.; Ogubadejo, B.; Shittu, T.; Mahmoud, E.; Du, W.; Al-Zuhair, S. Advances in Enzyme and Ionic Liquid Immobilization for Enhanced in MOFs for Biodiesel Production. Molecules 2021, 26, 3512. [Google Scholar] [CrossRef]
- Xia, X.; Hu, G.; Li, W.; Li, S. Understanding Reduced CO2 Uptake of Ionic Liquid/Metal–Organic Framework (IL/MOF) Composites. ACS Appl. Nano Mater. 2019, 2, 6022–6029. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, N.; Cao, P.; Lin, M.; Xu, L.; Ma, H. Electrochemical non-enzymatic glucose sensor using ionic liquid incorporated cobalt-based metal-organic framework. Microchem. J. 2020, 159, 105343. [Google Scholar] [CrossRef]
- Ma, Z.-Z.; Ma, Y.; Liu, B.; Xu, L.; Jiao, H. A high-performance Co-MOF non-enzymatic electrochemical sensor for glucose detection. New J. Chem. 2021, 45, 21350–21358. [Google Scholar] [CrossRef]
- Ayranci, R. The Rapid and Practical Route to Cu@PCR Sensor: Modification of Copper Nanoparticles Upon Conducting Polymer for a Sensitive Non-Enzymatic Glucose Sensor. Electroanalysis 2021, 33, 268–275. [Google Scholar] [CrossRef]
- Ni, G.; Cheng, J.; Dai, X.; Guo, Z.; Ling, X.; Yu, T.; Sun, Z. Integrating Ultrathin Polypyrrole Framework on Nickel-Cobalt Layered Double Hydroxide as an Amperometric Sensor for Non-enzymatic Glucose Determination. Electroanalysis 2018, 30, 2366–2373. [Google Scholar] [CrossRef]
- Chen, S.; Liu, D.; Song, N.; Wang, C.; Lu, X. Promoting non-enzymatic electrochemical sensing performance toward glucose by the integration of conducting polypyrrole with metal-organic framework. Compo. Commun. 2022, 30, 101074. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, S.; Zou, L.; Drake, H.; Zhang, Y.; Qin, J.; Alsalme, A.; Zhou, H.-C. One-Step Synthesis of Hybrid Core–Shell Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2018, 57, 3927–3932. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, M.; Chen, B.; Zhang, Z.; Huang, Y.; Dai, F.; Lai, Z.; Cui, X.; Tan, C.; Zhang, H. Hybridization of MOFs and COFs: A New Strategy for Construction of MOF@COF Core-Shell Hybrid Materials. Adv. Mater. 2018, 30, 1705454. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Li, Y.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Core-shell MOF@MOF composites for sensitive nonenzymatic glucose sensing in human serum. Anal. Chim. Acta 2020, 1110, 35–43. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, T.; Asif, M.; Yu, Y.; Wang, W.; Wang, H.; Xiao, F.; Liu, H. Rimelike Structure-Inspired Approach toward in Situ-Oriented Self-Assembly of Hierarchical Porous MOF Films as a Sweat Biosensor. ACS Appl. Mater. Interfaces 2018, 10, 27936–27946. [Google Scholar] [CrossRef] [PubMed]
- Arif, D.; Hussain, Z.; Sohail, M.; Liaqat, M.A.; Khan, M.A.; Noor, T. A Non-enzymatic Electrochemical Sensor for Glucose Detection Based on Ag@TiO2@ Metal-Organic Framework (ZIF-67) Nanocomposite. Front. Chem. 2020, 8, 573510. [Google Scholar] [CrossRef] [PubMed]
- Elizbit; Liaqat, U.; Hussain, Z.; Baig, M.M.; Khan, M.A.; Arif, D. Preparation of porous ZIF-67 network interconnected by MWCNTs and decorated with Ag nanoparticles for improved non-enzymatic electrochemical glucose sensing. J. Korean Ceram. Soc. 2021, 58, 598–605. [Google Scholar] [CrossRef]
- Dong, S.; Niu, H.; Sun, L.; Zhang, S.; Wu, D.; Yang, Z.; Xiang, M. Highly dense Ni-MOF nanoflake arrays supported on conductive graphene/carbon fiber substrate as flexible microelectrode for electrochemical sensing of glucose. J. Electroanal. Chem. 2022, 911, 116219. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Peng, Y.; Cai, J.; Bai, Y.; Li, Q.; Pang, H. Recent Advances in Metal–Organic Frameworks (MOFs) and Their Composites for Non-Enzymatic Electrochemical Glucose Sensors. Bioengineering 2023, 10, 733. https://doi.org/10.3390/bioengineering10060733
Li P, Peng Y, Cai J, Bai Y, Li Q, Pang H. Recent Advances in Metal–Organic Frameworks (MOFs) and Their Composites for Non-Enzymatic Electrochemical Glucose Sensors. Bioengineering. 2023; 10(6):733. https://doi.org/10.3390/bioengineering10060733
Chicago/Turabian StyleLi, Panpan, Yi Peng, Jinpeng Cai, Yang Bai, Qing Li, and Huan Pang. 2023. "Recent Advances in Metal–Organic Frameworks (MOFs) and Their Composites for Non-Enzymatic Electrochemical Glucose Sensors" Bioengineering 10, no. 6: 733. https://doi.org/10.3390/bioengineering10060733
APA StyleLi, P., Peng, Y., Cai, J., Bai, Y., Li, Q., & Pang, H. (2023). Recent Advances in Metal–Organic Frameworks (MOFs) and Their Composites for Non-Enzymatic Electrochemical Glucose Sensors. Bioengineering, 10(6), 733. https://doi.org/10.3390/bioengineering10060733









