Robust Removal of Slow Artifactual Dynamics Induced by Deep Brain Stimulation in Local Field Potential Recordings Using SVD-Based Adaptive Filtering
Abstract
1. Introduction
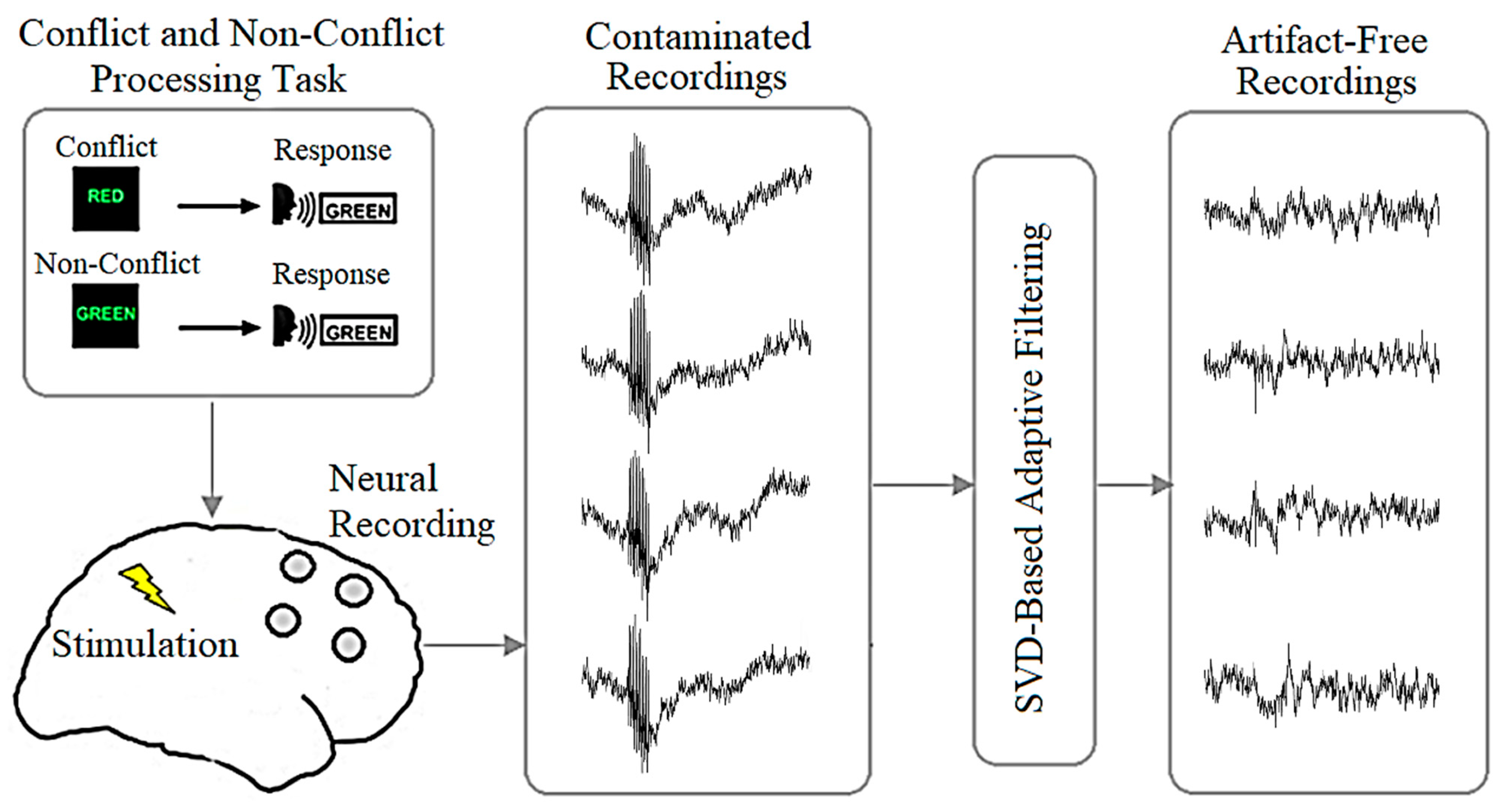
2. Materials and Methods
2.1. Data
2.1.1. Synthetic Data
2.1.2. Experimental Data
2.2. Benchmark DBS-Artifact Suppression Techniques in this Study
2.2.1. Normalized Least Mean Square (NLMS) Adaptive Filter Algorithm
2.2.2. Optimal FIR Wiener Filter
2.2.3. Gaussian Model Matching
2.2.4. Moving Average
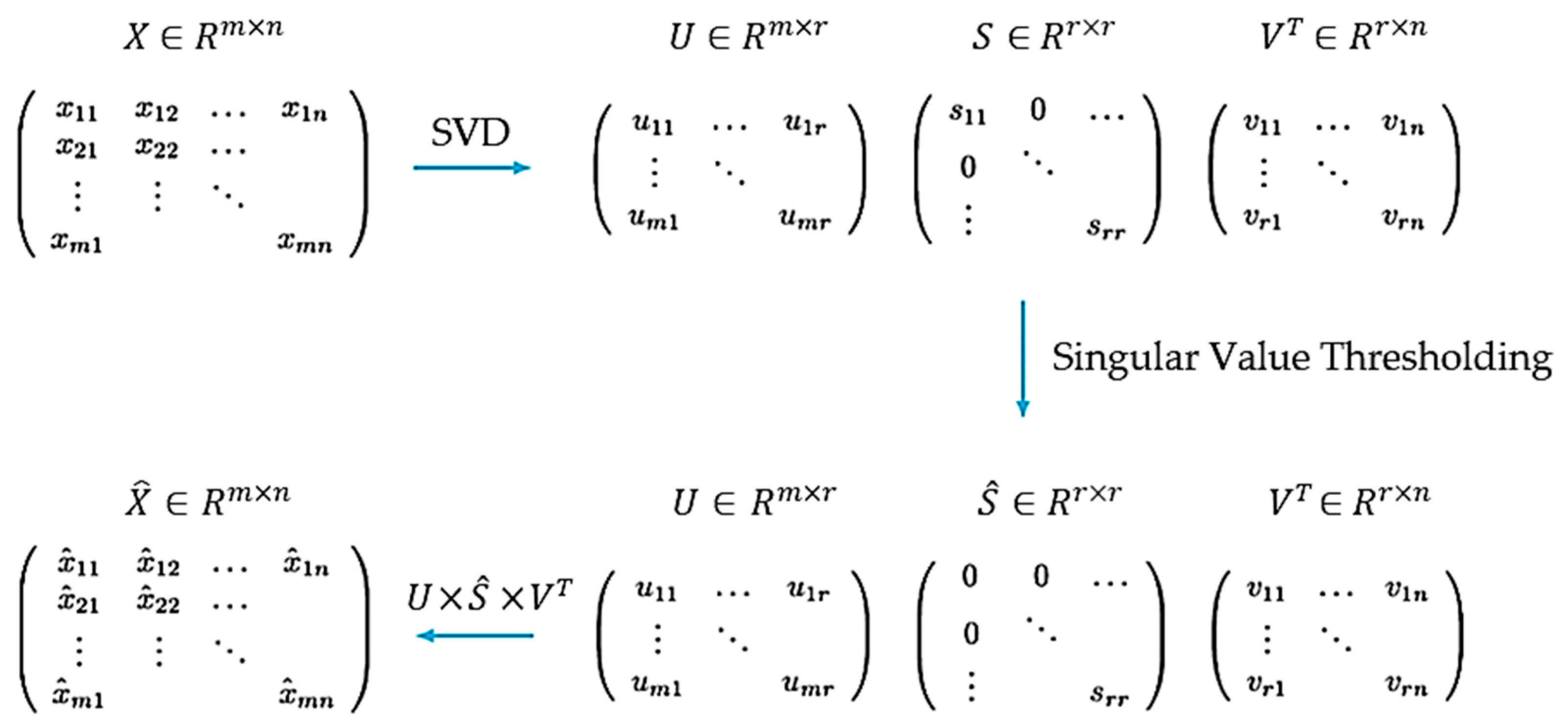
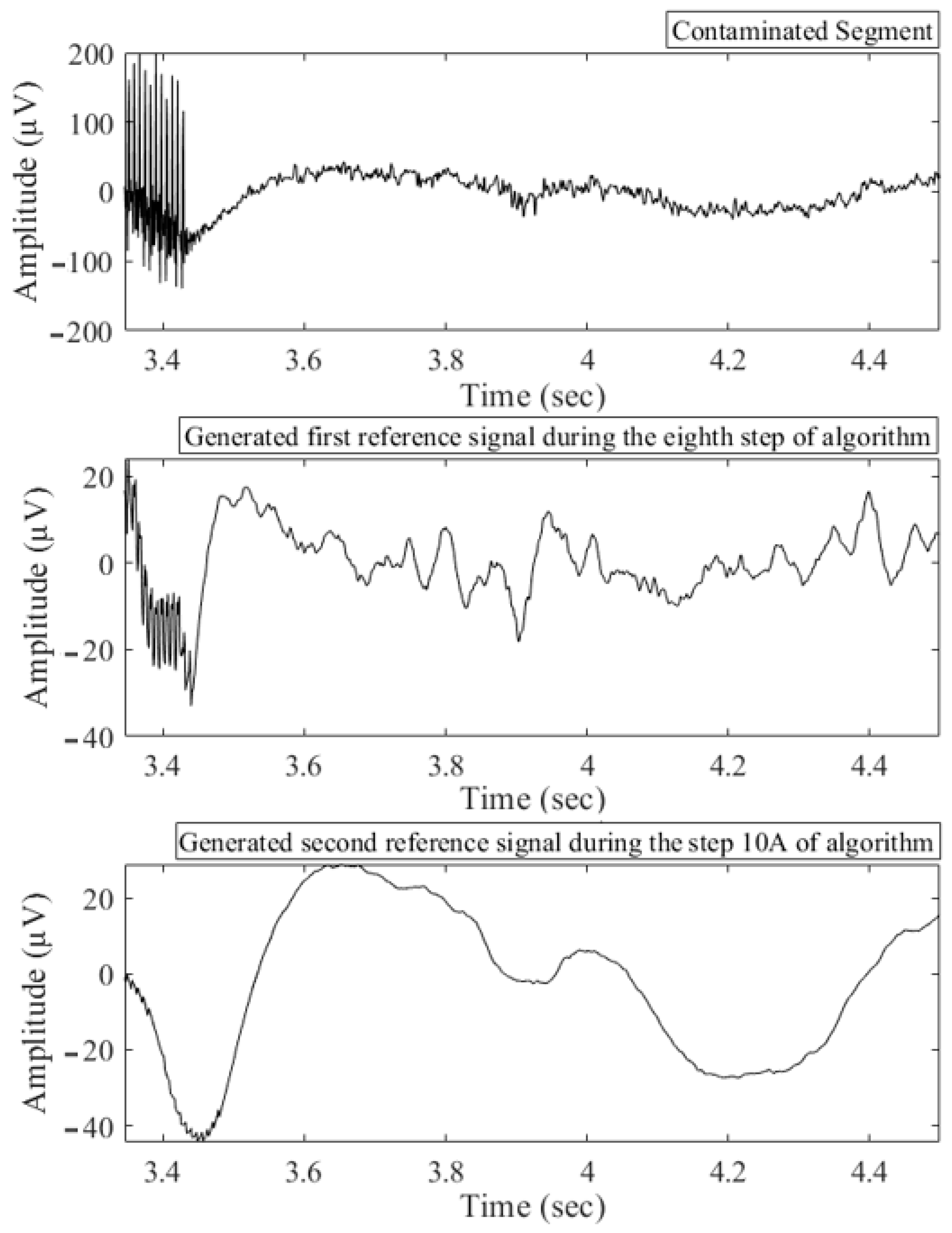
2.3. Proposed Algorithm
| Algorithm 1: SVD Adaptive Filtering |
|
3. Results
3.1. Validation of Artifact Removal Algorithms on Synthetic LFP Signal
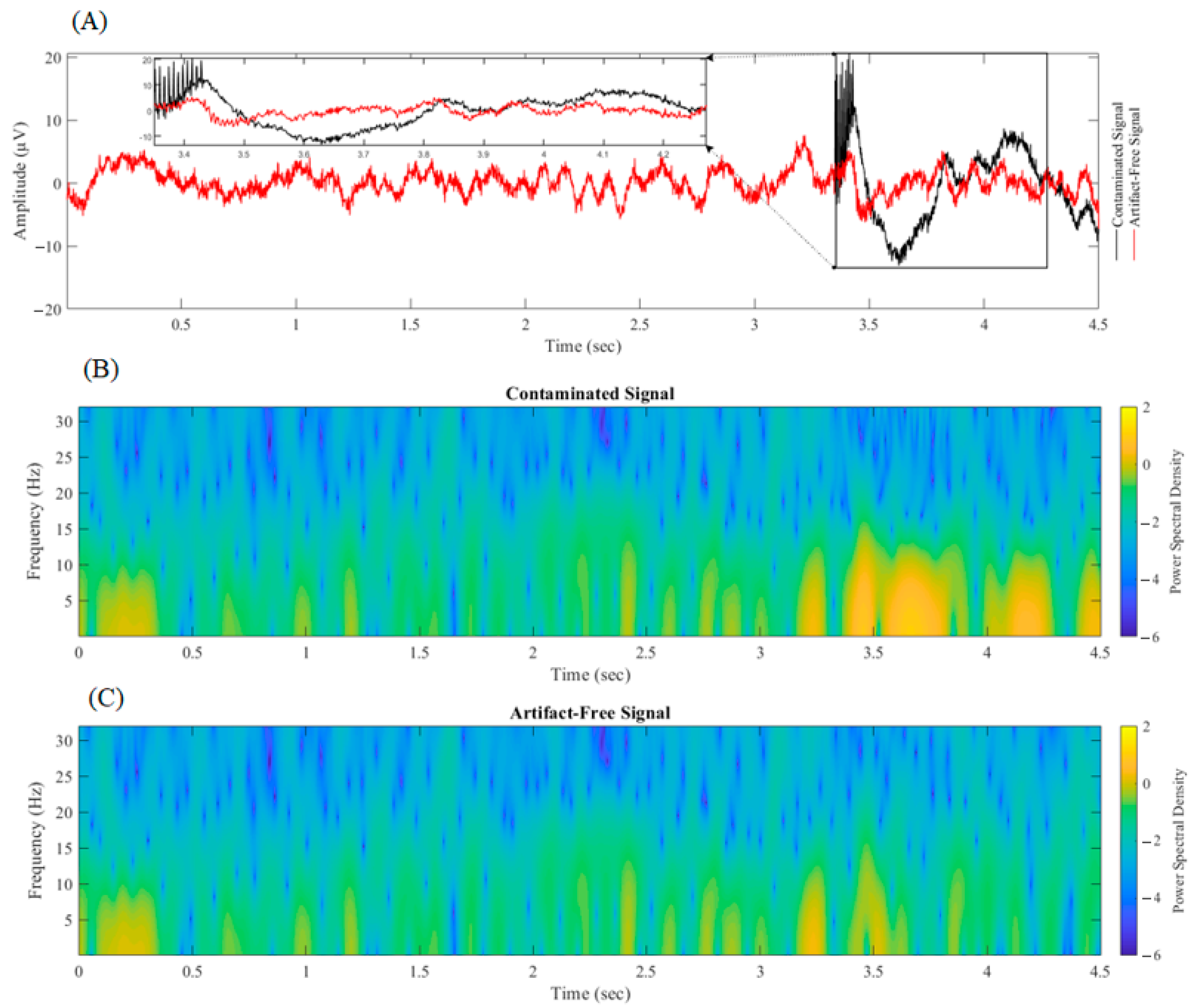
3.2. Validation of Artifact Removal Algorithms on Experimental LFP Signal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debarros, J.; Gaignon, L.; He, S.; Pogosyan, A.; Benjaber, M.; Denison, T.; Brown, P.; Tan, H. Artefact-free recording of local field potentials with simultaneous stimulation for closed-loop Deep-Brain Stimulation. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3367–3370. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Potter, S.M. Real-time multi-channel stimulus artifact suppression by local curve fitting. J. Neurosci. Methods 2002, 120, 113–120. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Foffani, G.; Marceglia, S.; Bracchi, F.; Barbieri, S.; Priori, A. An electronic device for artefact suppression in human local field potential recordings during deep brain stimulation. J. Neural Eng. 2007, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Petkos, K.; Guiho, T.; Degenaar, P.; Jackson, A.; Brown, P.; Denison, T.; Drakakis, E.M. A high-performance 4 nV/√ analog front-end architecture for artefact suppression in local field potential recordings during deep brain stimulation. J. Neural Eng. 2019, 16, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Stanslaski, S.; Afshar, P.; Cong, P.; Giftakis, J.; Stypulkowski, P.; Carlson, D.; Linde, D.; Ullestad, D.; Avestruz, A.-T.; Denison, T. Design and Validation of a Fully Implantable, Chronic, Closed-Loop Neuromodulation Device with Concurrent Sensing and Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 410–421. [Google Scholar] [CrossRef]
- Al-ani, T.; Cazettes, F.; Palfi, S.; Lefaucheur, J.-P. Automatic removal of high-amplitude stimulus artefact from neuronal signal recorded in the subthalamic nucleus. J. Neurosci. Methods 2011, 198, 135–146. [Google Scholar] [CrossRef]
- Qian, X.; Chen, Y.; Feng, Y.; Ma, B.; Hao, H.; Li, L. A Method for Removal of Deep Brain Stimulation Artifact from Local Field Potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 2217–2226. [Google Scholar] [CrossRef]
- Hashimoto, T.; Elder, C.M.; Vitek, J.L. A template subtraction method for stimulus artifact removal in high-frequency deep brain stimulation. J. Neurosci. Methods 2002, 113, 181–186. [Google Scholar] [CrossRef]
- Santillan-Guzman, A.; Heute, U.; Muthuraman, M.; Stephani, U.; Galka, A. DBS artifact suppression using a time-frequency domain filter. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4815–4818. [Google Scholar]
- Sahai, S.; Effendi, E.T.; Mahoney, E.C.; Tucker, H.R.; Moolick, B.J.; Mamone, G.; Mikkilineni, S.; Gupta, M.; Nicholson, A.; Chua, F.Y.; et al. Effects of subthalamic nucleus deep brain stimulation on neuronal spiking activity in the substantia nigra pars compacta in a rat model of Parkinson’s disease. Neurosci. Lett. 2020, 739, 135443. [Google Scholar] [CrossRef]
- Erez, Y.; Tischler, H.; Moran, A.; Bar-Gad, I. Generalized framework for stimulus artifact removal. J. Neurosci. Methods 2010, 191, 45–59. [Google Scholar] [CrossRef]
- Montgomery, E.B., Jr.; Gale, J.T.; Huang, H. Methods for isolating extracellular action potentials and removing stimulus artifacts from microelectrode recordings of neurons requiring minimal operator intervention. J. Neurosci. Methods 2005, 144, 107–125. [Google Scholar] [CrossRef]
- Hammer, L.H.; Kochanski, R.B.; Starr, P.A.; Little, S. Artifact Characterization and a Multipurpose Template-Based Offline Removal Solution for a Sensing-Enabled Deep Brain Stimulation Device. Stereotact. Funct. Neurosurg. 2022, 100, 168–183. [Google Scholar] [CrossRef]
- Abbasi, O.; Hirschmann, J.; Storzer, L.; Özkurt, T.E.; Elben, S.; Vesper, J.; Wojtecki, L.; Schmitz, G.; Schnitzler, A.; Butz, M. Unilateral deep brain stimulation suppresses alpha and beta oscillations in sensorimotor cortices. Neuroimage 2018, 174, 201–207. [Google Scholar] [CrossRef]
- Abbasi, O.; Hirschmann, J.; Schmitz, G.; Schnitzler, A.; Butz, M. Rejecting deep brain stimulation artefacts from MEG data using ICA and mutual information. J. Neurosci. Methods 2016, 268, 131–141. [Google Scholar] [CrossRef]
- Taulu, S.; Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006, 51, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Dattada, V.V.M.; Sasidharan, S.; Højlund, A.; Sridharan, K.S. How Does Deep Brain Stimulation Affect Magnetoencephalography Data? In Proceedings of the 2021 IEEE International Conference on Distributed Computing, VLSI, Electrical Circuits and Robotics (DISCOVER), Nitte, India, 19–20 November 2021; pp. 307–312. [Google Scholar] [CrossRef]
- Harmsen, I.E.; Rowland, N.C.; Wennberg, R.A.; Lozano, A.M. Characterizing the effects of deep brain stimulation with magnetoencephalography: A review. Brain Stimul. 2018, 11, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, R.R.; Kopell, B.H.; Butson, C.R.; Hiner, B.C.; Baillet, S. Spectral signal space projection algorithm for frequency domain MEG and EEG denoising, whitening, and source imaging. Neuroimage 2011, 56, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, A.L.; Litvak, V.; Florin, E. The comparative performance of DBS artefact rejection methods for MEG recordings. NeuroImage 2020, 219, 117057. [Google Scholar] [CrossRef]
- Boring, M.J.; Jessen, Z.F.; Wozny, T.A.; Ward, M.J.; Whiteman, A.C.; Richardson, R.M.; Ghuman, A.S. Quantitatively validating the efficacy of artifact suppression techniques to study the cortical consequences of deep brain stimulation with magnetoencephalography. NeuroImage 2019, 199, 366–374. [Google Scholar] [CrossRef]
- Nie, Y.; Guo, X.; Li, X.; Geng, X.; Li, Y.; Quan, Z.; Zhu, G.; Yin, Z.; Zhang, J.; Wang, S. Real-time removal of stimulation artifacts in closed-loop deep brain stimulation. J. Neural Eng. 2021, 18, 066031. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, B.; Hao, H.; Li, L. Removal of Electrocardiogram Artifacts from Local Field Potentials Recorded by Sensing-Enabled Neurostimulator. Front. Neurosci. 2021, 15, 637274. [Google Scholar] [CrossRef]
- Najafabadi, M.S.; Chen, L.; Dutta, K.; Norris, A.; Feng, B.; Schnupp, J.W.H.; Rosskothen-Kuhl, N.; Read, H.L.; Escabí, M.A. Optimal Multichannel Artifact Prediction and Removal for Neural Stimulation and Brain Machine Interfaces. Front. Neurosci. 2020, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Alagapan, S.; Shin, H.W.; Fröhlich, F.; Wu, H.-T. Diffusion geometry approach to efficiently remove electrical stimulation artifacts in intracranial electroencephalography. J. Neural Eng. 2019, 16, 036010. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hinrichs, H. Moving average template subtraction to remove stimulation artefacts in EEGs and LFPs recorded during deep brain stimulation. J. Neurosci. Methods 2016, 266, 126–136. [Google Scholar] [CrossRef]
- Rijn, E.M.D.-V.; Provenza, N.R.; Calvert, J.S.; Gilron, R.; Allawala, A.B.; Darie, R.; Syed, S.; Matteson, E.; Vogt, G.S.; Avendano-Ortega, M.; et al. Uncovering biomarkers during therapeutic neuromodulation with PARRM: Period-based Artifact Reconstruction and Removal Method. Cell Rep. Methods 2021, 1, 100010. [Google Scholar] [CrossRef]
- Heffer, L.F.; Fallon, J.B. A novel stimulus artifact removal technique for high-rate electrical stimulation. J. Neurosci. Methods 2008, 170, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Cho, W.; Ramos-Murguialday, A.; Keller, T. Detection and removal of stimulation artifacts in electroencephalogram recordings. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar] [CrossRef]
- Waddell, C.; Pratt, J.A.; Porr, B.; Ewing, S. Deep brain stimulation artifact removal through under-sampling and cubic-spline interpolation. In Proceedings of the 2009 2nd International Congress on Image and Signal Processing, Tianjin, China, 17–19 October 2009; pp. 1–5. [Google Scholar]
- Zhou, A.; Johnson, B.C.; Muller, R. Toward true closed-loop neuromodulation: Artifact-free recording during stimulation. Curr. Opin. Neurobiol. 2018, 50, 119–127. [Google Scholar] [CrossRef]
- Tiruvadi, V.; James, S.; Howell, B.; Obatusin, M.; Crowell, A.; Riva-Posse, P.; Gross, R.E.; McIntyre, C.C.; Mayberg, H.S.; Butera, R. Mitigating Mismatch Compression in Differential Local Field Potentials. arXiv 2022, arXiv:2204.03778. [Google Scholar] [CrossRef] [PubMed]
- Kasdin, N.J. Discrete Simulation of Colored Noise and Stochastic Processes and 1/fα Power Law Noise Generation. Proc. IEEE 1995, 83, 802–827. [Google Scholar] [CrossRef]
- Mansouri, F. Towards Achieving a Home-Based Brain Stimulation System. Ph.D. Thesis, Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 2019. [Google Scholar]
- Oweiss, K.G. Statistical Signal Processing for Neuroscience and Neurotechnology; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Ghahremani, A.; Aron, A.R.; Udupa, K.; Saha, U.; Reddy, D.; Hutchison, W.D.; Kalia, S.K.; Hodaie, M.; Lozano, A.M.; Chen, R. Event-related deep brain stimulation of the subthalamic nucleus affects conflict processing. Ann. Neurol. 2018, 84, 515–526. [Google Scholar] [CrossRef]
- Morshedzadeh, T.; Drummond, N.M.; Saha, U.; Chen, R.; Lankarany, M. Adaptive Filters to Remove Deep Brain Stimulation Artifacts from Local Field Potentials. In Proceedings of the 2019 Conference on Cognitive Computational Neuroscience (CCN 2019), Berlin, Germany, 13–16 September 2019. [Google Scholar] [CrossRef]
- Shah, S.A.; Tan, H.; Tinkhauser, G.; Brown, P. Towards Real-Time, Continuous Decoding of Gripping Force from Deep Brain Local Field Potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Bayford, R.; Demosthenous, A. A Framework for Adapting Deep Brain Stimulation Using Parkinsonian State Estimates. Front Neurosci. 2020, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.H. Statistical Digital Signal Processing and Modeling; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Pepper, K. Newtonian Noise Simulation and Suppression for Gravitational-Wave Interferometers; LIGO: Pasadena, CA, USA, 2007. [Google Scholar]
- Rouhollahi, K.; Emadi Andani, M.; Karbassi, S.M.; Izadi, I. Design of robust adaptive controller and feedback error learning for rehabilitation in Parkinson’s disease: A simulation study. IET Syst. Biol. 2017, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Rouhollahi, K.; Emadi Andani, M.; Karbassi, S.M.; Izadi, I. Designing a robust backstepping controller for rehabilitation in Parkinson’s disease: A simulation study. IET Syst. Biol. 2016, 10, 136–146. [Google Scholar] [CrossRef]






| Frequency domain analysis | Frequency domain techniques identify artifact based on narrow frequency peaks in the frequency spectrum. The effectiveness of these methods depends on the chosen window size and threshold parameter C, which require careful selection and evaluation to achieve satisfactory results. DBS artifacts can be present across the entire frequency spectrum, including frequencies that are relevant for studying brain activity. Therefore, defining specific frequency bands for artifact removal may not be practical. |
| Template-based methods | Estimating a general template for DBS artifacts is challenging due to the wide variety of shapes they can have and their variability over time. Moreover, conventional template-based methods, which have primarily been used in single-pulse stimulation studies, may not yield optimal results when dealing with high-frequency DBS. |
| Low-pass and notch filters | Low-pass and notch filters may not be efficient if the stimulation peaks overlap other frequency bands. |
| Threshold-based methods | Underestimating the threshold can lead to a slight amplitude bias and potentially result in missing parts of the physiological data. Overestimating the threshold can leave residual artifacts. |
| Methods based on principal component analysis (PCA) | PCA can lead to the loss of information due to the reduction in dimensionality it induces. |
| Methods based on signal space separation (SSS) | SSS assumes that the natural brain activity is not correlated with any artifacts or unwanted signals. However, in reality, there can be situations where strong sources of activity leak into the intermediate part, leading to the false identification of sources as artifact. |
| Average of Power Differences between | ||
|---|---|---|
| Original Signal and Contaminated Signal | Original Signal and Artifact-Free Signal | |
| Delta band | 0.1025 | 0.0469 |
| Theta band | 0.1026 | 0.0317 |
| Alpha band | 0.0485 | 0.0076 |
| Beta band | 0.0384 | 0.0552 |
| Technique | Mean-Squared Error (MSE) between the Original Signal and | Percentage Difference in MSE% | |
|---|---|---|---|
| Contaminated Signal (μV2) | Artifact-Free Signal (μV2) | ||
| SVD | 1230 | 58.57 | −95.26 |
| Gaussian model matching | 1230 | 1540 | +24.91 |
| FIR wiener filtering | 1230 | 671.55 | −45.76 |
| Adaptive filtering | 1230 | 656.27 | −46.99 |
| Moving average | 1230 | 1400 | +16.01 |
| Average Power | ||||||
|---|---|---|---|---|---|---|
| Baseline | SVD | Adaptive Filtering | FIR Wiener Filtering | Gaussian Model Matching | Moving Average | |
| Delta | 0.1930 | 0.1868 | 0.1427 | 0.0982 | 0.2805 | 0.0921 |
| Theta | 0.2246 | 0.2263 | 0.1797 | 0.1443 | 0.2875 | 0.1445 |
| Alpha | 0.1783 | 0.1880 | 0.1600 | 0.1621 | 0.1730 | 0.1697 |
| Beta | 0.2141 | 0.2198 | 0.2167 | 0.2760 | 0.1284 | 0.2866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahador, N.; Saha, J.; Rezaei, M.R.; Utpal, S.; Ghahremani, A.; Chen, R.; Lankarany, M. Robust Removal of Slow Artifactual Dynamics Induced by Deep Brain Stimulation in Local Field Potential Recordings Using SVD-Based Adaptive Filtering. Bioengineering 2023, 10, 719. https://doi.org/10.3390/bioengineering10060719
Bahador N, Saha J, Rezaei MR, Utpal S, Ghahremani A, Chen R, Lankarany M. Robust Removal of Slow Artifactual Dynamics Induced by Deep Brain Stimulation in Local Field Potential Recordings Using SVD-Based Adaptive Filtering. Bioengineering. 2023; 10(6):719. https://doi.org/10.3390/bioengineering10060719
Chicago/Turabian StyleBahador, Nooshin, Josh Saha, Mohammad R. Rezaei, Saha Utpal, Ayda Ghahremani, Robert Chen, and Milad Lankarany. 2023. "Robust Removal of Slow Artifactual Dynamics Induced by Deep Brain Stimulation in Local Field Potential Recordings Using SVD-Based Adaptive Filtering" Bioengineering 10, no. 6: 719. https://doi.org/10.3390/bioengineering10060719
APA StyleBahador, N., Saha, J., Rezaei, M. R., Utpal, S., Ghahremani, A., Chen, R., & Lankarany, M. (2023). Robust Removal of Slow Artifactual Dynamics Induced by Deep Brain Stimulation in Local Field Potential Recordings Using SVD-Based Adaptive Filtering. Bioengineering, 10(6), 719. https://doi.org/10.3390/bioengineering10060719





