The Influence of Carious Lesion and Bleeding Time on the Success of Partial Pulpotomy in Permanent Molars with Irreversible Pulpitis: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design and Sample Size Calculation
2.3. Participants
2.4. Inclusion and Exclusion Criteria
2.5. Patient Assessment and Operative Procedure
2.6. Outcome Evaluation
2.7. Scanning Electron Microscope (SEM) and Energy Dispersive X-ray Analysis (EDX)
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Möller, Å.J.; Fabricius, L.; Dahlen, G.; Öhmang, A.E.; Heyden, G.U.Y. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Eur. J. Oral Sci. 1981, 89, 475–484. [Google Scholar] [CrossRef] [PubMed]
- American Association of Endodontists. Endodontic Diagnosis. [WWW Document]. Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/endodonticdiagnosisfal2013.pdf (accessed on 5 May 2021).
- Ricucci, D.; Loghin, S.; Siqueira, J.F., Jr. Correlation between clinical and histologic pulp diagnoses. J. Endod. 2014, 40, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, S.; Bender, I.B.; Ziontz, M. The dynamics of pulp inflammation: Correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg. Oral Med. Oral Radiol. 1963, 16, 846–871. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Cooper, P.R.; Smith, A.J. Dissecting dentine–pulp injury and wound healing responses: Consequences for regenerative endodontics. Int. Endod. J. 2019, 52, 261–266. [Google Scholar] [CrossRef]
- Hachem, C.E.; Chedid, J.C.A.; Nehme, W.; Kaloustian, M.K.; Ghosn, N.; Sahnouni, H.; Mancino, D.; Haikel, Y.; Kharouf, N. Physicochemical and antibacterial properties of conventional and two premixed root canal filling materials in primary teeth. J. Funct. Biomater. 2022, 13, 177. [Google Scholar] [CrossRef]
- Kharouf, N.; Arntz, Y.; Eid, E.; Zghal, J.; Sauro, S.; Haike, Y.; Mancino, D. Physicochemical and antibacterial properties of novel, premixed calcium silicate-based sealer compared to powder–liquid bioceramic sealer. J. Clin. Med. 2020, 9, 3096. [Google Scholar] [CrossRef]
- Nair, P.N.R.; Duncan, H.F.; Pitt Ford, T.R.; Luder, H.U. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: A randomized controlled trial. Int. Endod. J. 2008, 41, 128–150. [Google Scholar]
- Ricucci, D.; Siqueira, J.F., Jr.; Li, Y.; Tay, F.R. Vital pulp therapy: Histopathology and histobacteriology-based guidelines to treat teeth with deep caries and pulp exposure. J. Dent. 2019, 86, 41–52. [Google Scholar] [CrossRef]
- Wolters, W.J.; Duncan, H.F.; Tomson, P.L.; Karim, I.E.; McKenna, G.; Dorri, M.; Stangvaltaite, L.; Van Der Sluis, L.W.M. Minimally invasive endodontics: A new diagnostic system for assessing pulpitis and subsequent treatment needs. Int. Endod. J. 2017, 50, 825–829. [Google Scholar] [CrossRef]
- Duncan, H.F. Present status and future directions—Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55, 497–511. [Google Scholar] [CrossRef]
- Eid, A.; Mancino, D.; Rekab, M.S.; Haike, Y.; Kharouf, N. Effectiveness of three agents in pulpotomy treatment of permanent molars with incomplete root development: A randomized controlled trial. Healthcare 2022, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- European Society of Endodontology (ESE); Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [PubMed]
- Taha, N.A.; Abdelkhader, S.Z. Outcome of full pulpotomy using Biodentine in adult patients with symptoms indicative of irreversible pulpitis. Int. Endod. J. 2018, 51, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Khazali, M.A. Partial pulpotomy in mature permanent teeth with clinical signs indicative of irreversible pulpitis: A randomized clinical trial. J. Endod. 2017, 43, 1417–1421. [Google Scholar] [CrossRef]
- Uesrichai, N.; Nirunsittirat, A.; Chuveera, P.; Srisuwan, T.; Sastraruji, T.; Chompu-Inwai, P. Partial pulpotomy with two bioactive cements in permanent teeth of 6-to 18-year-old patients with signs and symptoms indicative of irreversible pulpitis: A noninferiority randomized controlled trial. Int. Endod. J. 2019, 52, 749–759. [Google Scholar] [CrossRef]
- Elmsmari, F.; Ruiz, X.F.; Miró, Q.; Feijoo-Pato, N.; Durán-Sindreu, F.; Olivieri, J.G. Outcome of partial pulpotomy in cariously exposed posterior permanent teeth: A systematic review and meta-analysis. J. Endod. 2019, 45, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Yu, V.S.H.; Lim, K.C.; Tan, B.C.K.; Neo, C.L.J.; Shen, M.L.; Messer, H.H. Long-term pulpal and restorative outcomes of pulpotomy in mature permanent teeth. J. Endod. 2020, 46, 383–390. [Google Scholar] [CrossRef]
- Mejare, I.; Axelsson, S.; Davidson, T.; Frisk, F.; Hakeberg, M.; Kvist, T.; Norlund, A.; Petersson, A.; Portenier, I.; Sandberg, H. Diagnosis of the condition of the dental pulp: A systematic review. Int. Endod. J. 2012, 45, 597–613. [Google Scholar] [CrossRef]
- Demant, S.; Dabelsteen, S.; Bjørndal, L. A macroscopic and histological analysis of radiographically well-defined deep and extremely deep carious lesions: Carious lesion characteristics as indicators of the level of bacterial penetration and pulp response. Int. Endod. J. 2021, 54, 319–330. [Google Scholar] [CrossRef]
- Bjørndal, L.; Demant, S.; Dabelsteen, S. Depth and activity of carious lesions as indicators for the regenerative potential of dental pulp after intervention. J. Endod. 2014, 40, S76–S81. [Google Scholar] [CrossRef]
- Ashi, T.; Mancino, D.; Hardan, L.; Bourgi, R.; Zghal, J.; Macaluso, V.; Al-Ashkar, S.; Alkhouri, S.; Haikel, Y.; Kharouf, N. Physicochemical and antibacterial properties of bioactive retrograde filling materials. Bioengineering 2022, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Ashi, T.; Richert, R.; Mancino, D.; Jmal, H.; Alkhouri, S.; Addiego, F.; Kharouf, N.; Haïkel, Y. Do the mechanical properties of calcium-silicate-based cements influence the stress distribution of different retrograde cavity preparations? Materials 2023, 16, 3111. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Sauro, S.; Eid, A.; Zghal, J.; Jmal, H.; Seck, A.; Maculuso, V.; Addiego, F.; Inchingolo, F.; Affolter-Zbaraszczuk, C. Physicochemical and mechanical properties of premixed calcium silicate and resin sealers. J. Funct. Biomater. 2022, 14, 9. [Google Scholar] [CrossRef]
- Kassab, P.; El Hachem, C.; Habib, M.; Nehme, W.; Zogheib, V.; Tonini, R.; Kaloustian, M.K. The Pushout Bond Strength of Three Calcium Silicate-based Materials in Furcal Perforation Repair and the Effect of a Novel Irrigation Solution: A Comparative In Vitro Study. J. Contemp. Dent. Pract. 2022, 23, 289–294. [Google Scholar] [PubMed]
- Careddu, R.; Duncan, H.F. A prospective clinical study investigating the effectiveness of partial pulpotomy after relating preoperative symptoms to a new and established classification of pulpitis. Int. Endod. J. 2021, 54, 2156–2172. [Google Scholar] [CrossRef]
- Taha, N.; Ahmad, M.; Ghanim, A. Assessment of mineral trioxide aggregate pulpotomy in mature permanent teeth with carious exposures. Int. Endod. J. 2017, 50, 117–125. [Google Scholar] [CrossRef]
- Bjørndal, L.; Simon, S.; Tomson, P.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef]
- Bjørndal, L.; Larsen, T.; Thylstrup, A. A clinical and microbiological study of deep carious lesions during stepwise excavation using long treatment intervals. Caries Res. 1997, 31, 411–417. [Google Scholar] [CrossRef]
- Orhan, A.I.; Oz, F.T.; Ozcelik, B.; Orhan, K. A clinical and microbiological comparative study of deep carious lesion treatment in deciduous and young permanent molars. Clin. Oral Investig. 2008, 12, 369–378. [Google Scholar] [CrossRef]
- Ballal, N.V.; Duncan, H.F.; Wiedemeier, D.; Rai, N.; Jalan, P.; Bhat, V.; Belle, V.; Zehnder, M. MMP-9 levels and NaOCl lavage in randomized trial on direct pulp capping. J. Dent. Res. 2022, 101, 414–419. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Azizi, N.; Farahat, F. Evaluation of microhardness of mineral trioxide aggregate after immediate placement of different coronal restorations: An in vitro study. J. Dent. 2018, 15, 116. [Google Scholar]
- Cuevas-Suárez, C.E.; da Rosa, W.L.O.; Lund, R.G.; da Silva, A.F.; Piva, E. Bonding Performance of Universal Adhesives: An Updated Systematic Review and Meta-Analysis. J. Adhe Dent. 2019, 21, 7–26. [Google Scholar]
- Linsuwanont, P.; Wimonsutthikul, K.; Pothimoke, U.; Santiwong, B. Treatment outcomes of mineral trioxide aggregate pulpotomy in vital permanent teeth with carious pulp exposure: The retrospective study. J. Endod. 2017, 43, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.; Smith, A.; Windsor, L.; Mjör, I. Remaining dentine thickness and human pulp responses. Int. Endod. J. 2003, 36, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Nakanishi, T.; Shimizu, H.; Ebisu, S. A clinical study of direct pulp capping applied to carious-exposed pulps. J. Endod. 1996, 22, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Holder, M.J.; Smith, A.J. Inflammation and regeneration in the dentin-pulp complex: A double-edged sword. J. Endod. 2014, 40, S46–S51. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, V.; Campus, G.; Doméjean, S. Managing carious lesions: Consensus recommendations on carious tissue removal. J. Adv. Dent. Res. 2016, 28, 58–67. [Google Scholar] [CrossRef]
- Bjørndal, L.; Ricucci, D. Pulp inflammation: From the reversible pulpitis to pulp necrosis during caries progression. In The Dental Pulp: Biology, Pathology, and Regenerative Therapies; Goldberg, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 125–139. [Google Scholar]
- Warfvinge, J.; Bergenholtz, G. Healing capacity of human and monkey dental pulps following experimentally-induced pulpitis. Dent. Traumatol. 1986, 2, 256–262. [Google Scholar] [CrossRef]
- Chu, W.S.; Park, S.H.; Ahn, D.K.; Kim, S.K. Effect of local anesthesia on pulpal blood flow in mechanically stimulated teeth. J. Korean Acad. 2006, 31, 257–262. [Google Scholar] [CrossRef]
- Oulis, C.J.; Vadiakas, G.; Vasilopoulou, A. The effectiveness of mandibular infiltration compared to mandibular block anesthesia in treating primary molars in children. Pediatr. Dent. 1996, 18, 301–305. [Google Scholar]
- Kharouf, N.; Sauro, S.; Hardan, L.; Fawzi, A.; Suhanda, I.E.; Zghal, J.; Addiego, F.; Affolter-Zbaraszczuk, C.; Arntz, Y.; Ball, V.; et al. Impacts of Resveratrol and Pyrogallol on Physicochemical, Mechanical and Biological Properties of Epoxy-Resin Sealers. Bioengineering 2022, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Farrayeh, A.; Akil, S.; Eid, A.; Macaluso, V.; Mancino, D.; Haïkel, Y.; Kharouf, N. Effectiveness of Two Endodontic Instruments in Calcium Silicate-Based Sealer Retreatment. Bioengineering 2023, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, K.A.; Ghabraei, S.; Bolhari, B.; Kafili, M.; Meraji, N.; Nekoofar, M.; Dummer, P. Microstructure and chemical analysis of four calcium silicate-based cements in different environmental conditions. Clin. Oral Investig. 2019, 23, 43–52. [Google Scholar] [CrossRef]
- Oskoee, S.S.; Kimyai, S.; Bahari, M.; Motahari, P.; Eghbal, M.J.; Asgary, S. Comparison of shear bond strength of calcium-enriched mixture cement and mineral trioxide aggregate to composite resin. J. Contemp. Dent. Pract. 2011, 12, 457–462. [Google Scholar] [CrossRef] [PubMed]
- American Association of Endodontists. AAE Clinical Considerations for a Regenerative Procedure. Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/currentregenerativeendodonticconsiderations.pdf (accessed on 25 May 2018).
- Kayahan, M.B.; Nekoofar, M.H.; Kazandağ, M.; Canpolat, C.; Malkondu, O.; Kaptan, F.; Dummer, P.M.H. Effect of acid-etching procedure on selected physical properties of mineral trioxide aggregate. Int. Endod. J. 2009, 42, 1004–1014. [Google Scholar] [CrossRef]
- Shie, M.Y.; Huang, T.H.; Kao, C.T.; Huang, C.H.; Ding, S.J. The effect of a physiologic solution pH on properties of white mineral trioxide aggregate. J. Endod. 2009, 35, 98–101. [Google Scholar] [CrossRef]
- Jefferson, J.C.; Manhães, F.C.; Bajo, H.; Duque, T.M. Efficiency of different concentrations of sodium hypochlorite during endodontic treatment. Dental Press Endod. J. 2012, 2, 32–37. [Google Scholar]
- Pai, A.V. Factors influencing the occurrence and progress of sodium hypochlorite accident: A narrative and update review. J. Conserv. Dent. 2023, 26, 3. [Google Scholar]
- Eggmann, F.; Gasser, T.J.; Hecker, H.; Amato, M.; Weiger, R.; Zaugg, L.K. Partial pulpotomy without age restriction: A retrospective assessment of permanent teeth with carious pulp exposure. Clin. Oral Investig. 2022, 26, 365–373. [Google Scholar] [CrossRef]
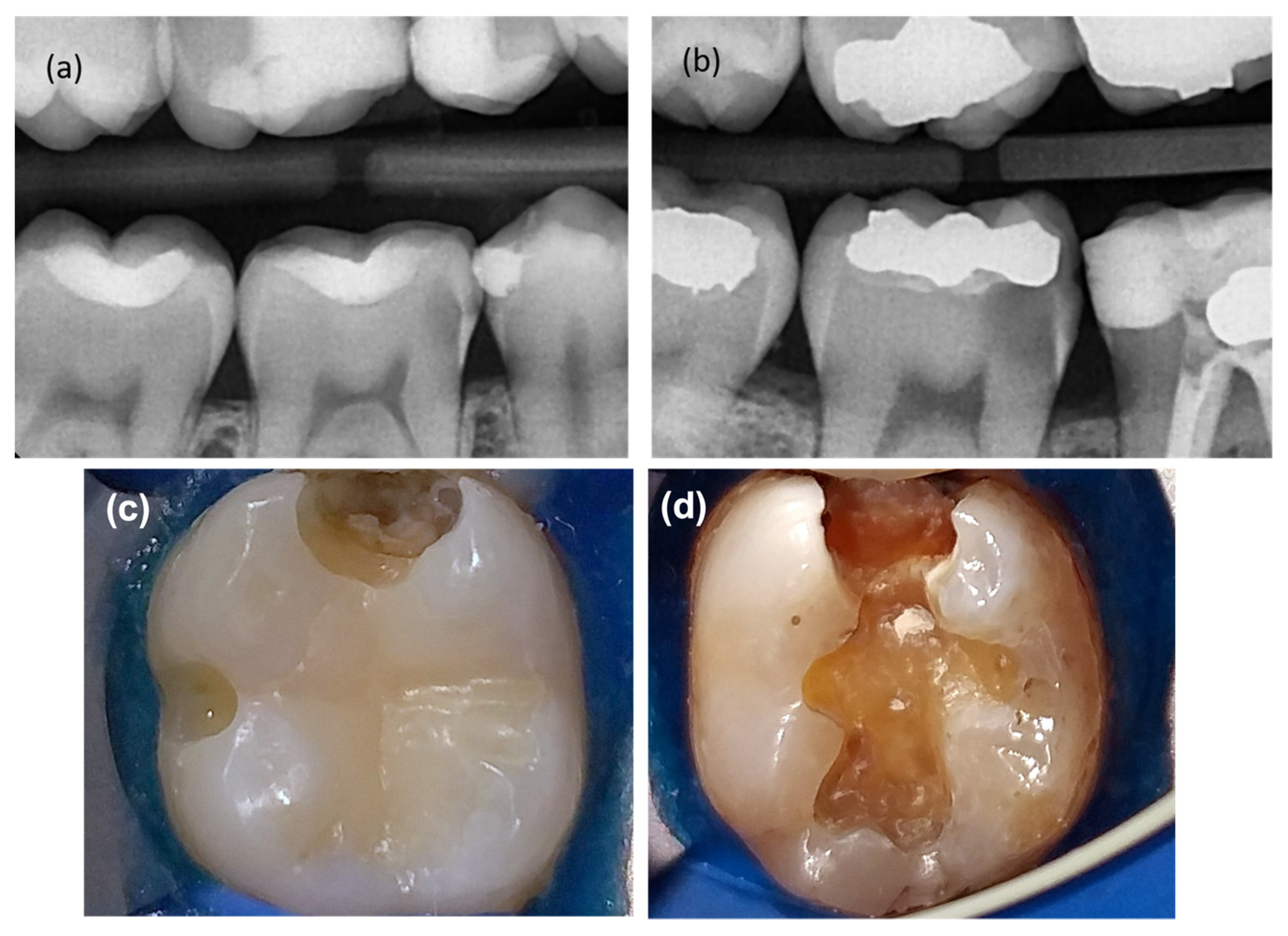


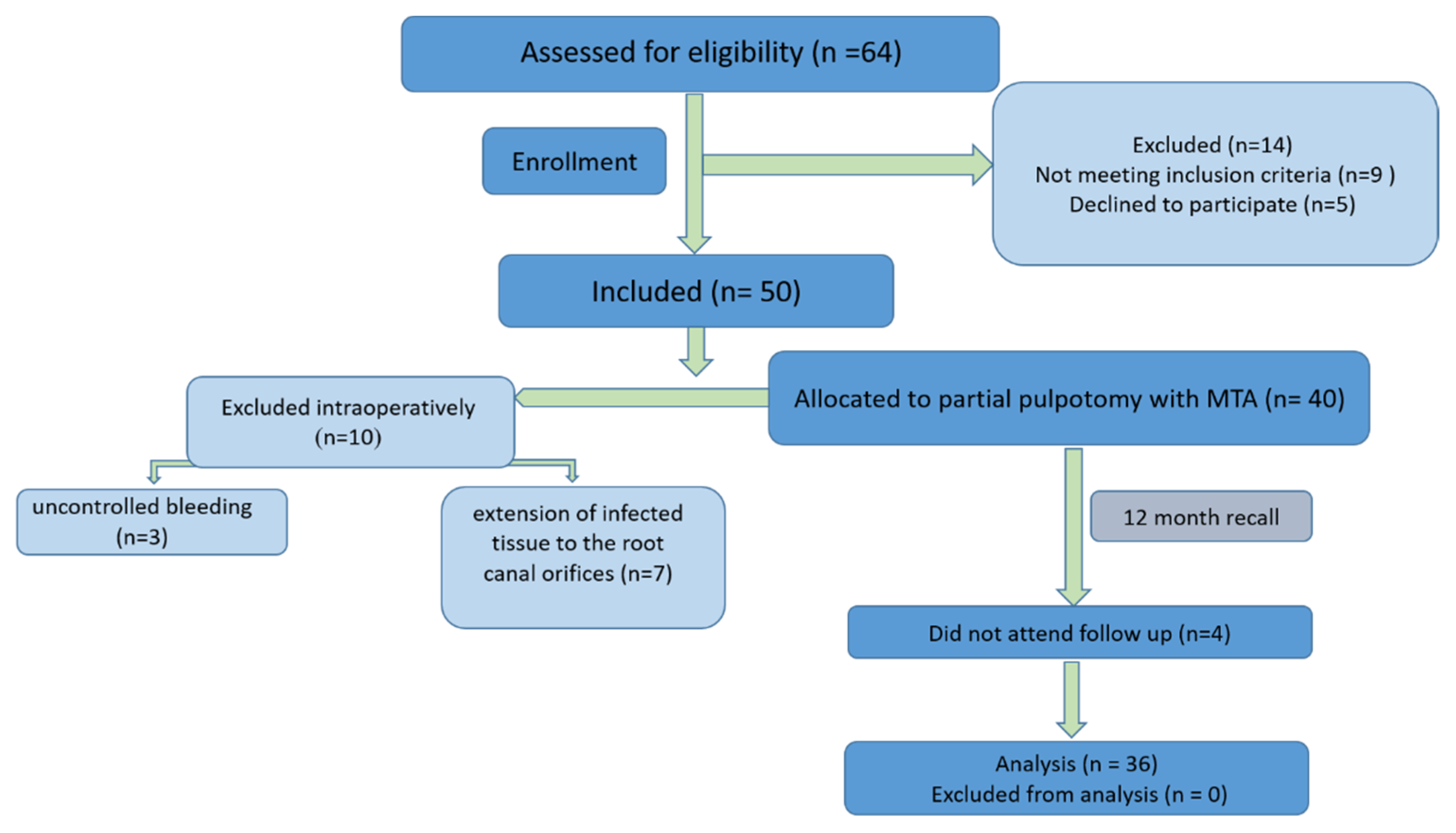
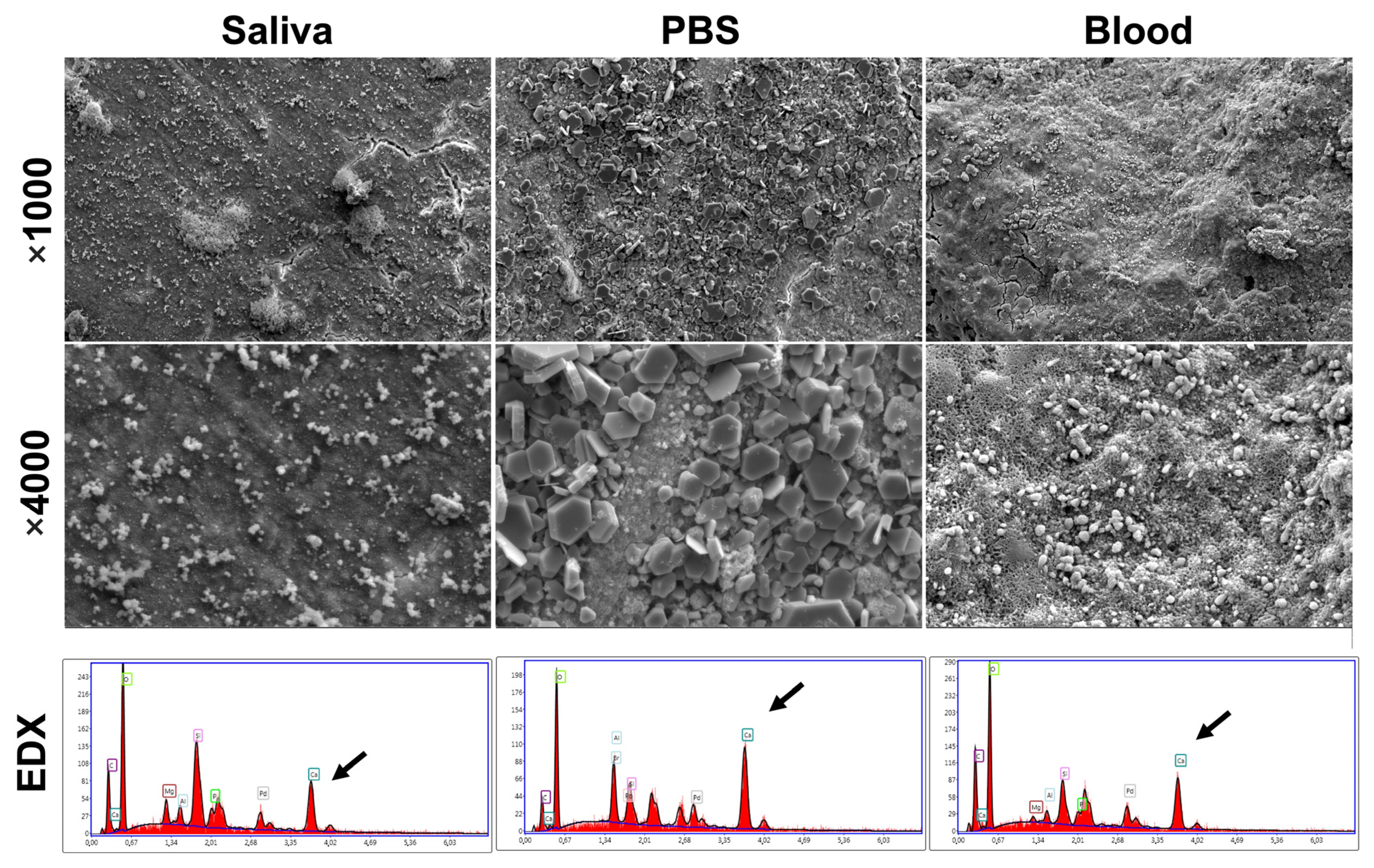
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Variables | Overall Outcome | p-Value | |||
|---|---|---|---|---|---|
| n (%) | Success (%) | Failure (%) | |||
| Activity of carious lesion | Rapid progression | 24 (66.7) | 20 (83.3) | 4 (16.7) | 0.18 |
| Slow progression | 12 (33.3) | 12 (100) | 0 (0) | ||
| Carious lesion depth | Deep | 20 (55.5) | 19 (95) | 1 (5) | 0.221 |
| Extremely deep | 16 (44.5) | 13 (81.3) | 3 (18.7) | ||
| Bleeding time | 1–3 min | 15 (41.7) | 15 (100) | 0 (0) | 0.102 |
| 4–6 min | 21 (58.3) | 17 (81) | 4 (19) | ||
| Overall Outcome | ||||
|---|---|---|---|---|
| State of Carious Lesion | n (%) | Success (%) | Failure (%) | p-Value |
| Rapidly progressing deep | 14 (38.88) | 13 (92.9) | 1 (7.1) | 0.149 |
| Rapidly progressing, extremely deep | 10 (27.8) | 7 (70) | 3 (30) | |
| Slowly progressing, deep | 6 (16.66) | 6 (100) | 0(0) | |
| Slowly progressing, extremely deep | 6 (16.66) | 6 (100) | 0(0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldeen, R.Z.; Aljabban, O.; Almanadili, A.; Alkurdi, S.; Eid, A.; Mancino, D.; Haikel, Y.; Kharouf, N. The Influence of Carious Lesion and Bleeding Time on the Success of Partial Pulpotomy in Permanent Molars with Irreversible Pulpitis: A Prospective Study. Bioengineering 2023, 10, 700. https://doi.org/10.3390/bioengineering10060700
Aldeen RZ, Aljabban O, Almanadili A, Alkurdi S, Eid A, Mancino D, Haikel Y, Kharouf N. The Influence of Carious Lesion and Bleeding Time on the Success of Partial Pulpotomy in Permanent Molars with Irreversible Pulpitis: A Prospective Study. Bioengineering. 2023; 10(6):700. https://doi.org/10.3390/bioengineering10060700
Chicago/Turabian StyleAldeen, Rami Zen, Ossama Aljabban, Ahmad Almanadili, Saleh Alkurdi, Ammar Eid, Davide Mancino, Youssef Haikel, and Naji Kharouf. 2023. "The Influence of Carious Lesion and Bleeding Time on the Success of Partial Pulpotomy in Permanent Molars with Irreversible Pulpitis: A Prospective Study" Bioengineering 10, no. 6: 700. https://doi.org/10.3390/bioengineering10060700
APA StyleAldeen, R. Z., Aljabban, O., Almanadili, A., Alkurdi, S., Eid, A., Mancino, D., Haikel, Y., & Kharouf, N. (2023). The Influence of Carious Lesion and Bleeding Time on the Success of Partial Pulpotomy in Permanent Molars with Irreversible Pulpitis: A Prospective Study. Bioengineering, 10(6), 700. https://doi.org/10.3390/bioengineering10060700











