Tele-Rehabilitation Interventions for Motor Symptoms in COVID-19 Patients: A Narrative Review
Abstract
1. Introduction
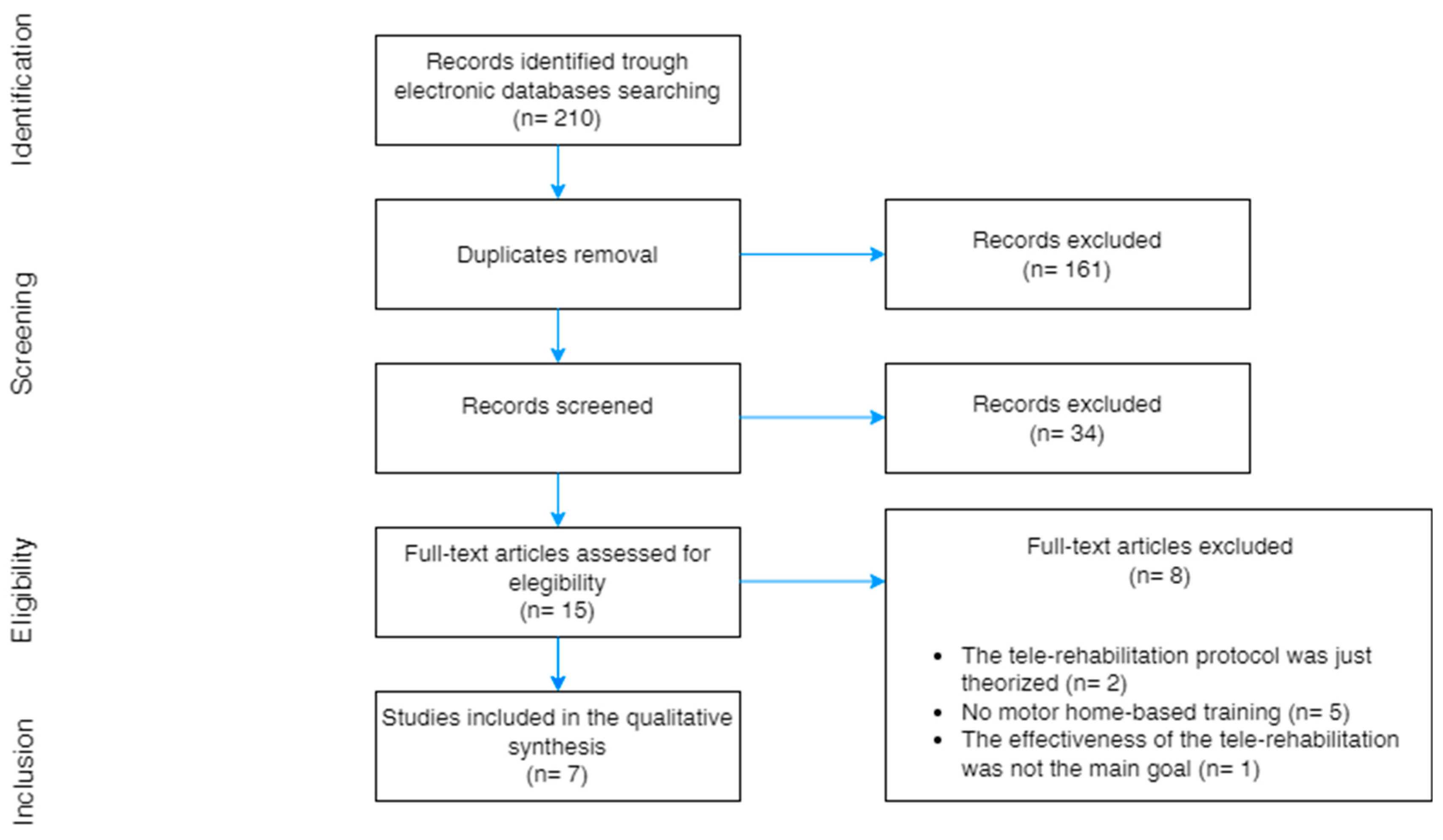
2. Materials and Methods
3. Results
3.1. Study Objectives, Participants, and Selection Criteria
3.2. Experimental Protocols and Methods for Home-Based Interventions
3.2.1. Target of Tele-Rehabilitation Interventions
3.2.2. Administration Modality and Tools
3.3. Functional Assessment and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Zhao, R.; Gao, L.; Gao, X.; Wang, D.; Gallagher, T. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Cai, L.; Cheng, Z.S.; Cheng, H.; Deng, T.; Fan, Y.P.; Fang, C.; Huang, D.; Huang, L.Q.; Huang, Q.; et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef]
- Lupia, T.; Scabini, S.; Mornese Pinna, S.; Di Perri, G.; De Rosa, F.G.; Corcione, S. 2019 novel coronavirus (2019-nCoV) outbreak: A new challenge. J. Glob. Antimicrob. Resist. 2020, 21, 22–27. [Google Scholar] [CrossRef]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831. [Google Scholar] [CrossRef]
- Brioni, M.; Meli, A.; Grasselli, G. Mechanical Ventilation for COVID-19 Patients. Semin. Respir. Crit. Care Med. 2022, 43, 405–416. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, C.; Gonzalez-Gerez, J.J.; Bernal-Utrera, C.; Anarte-Lazo, E.; Perez-Ale, M.; Saavedra-Hernandez, M. Short-term effects of a conditioning telerehabilitation program in confined patients affected by COVID-19 in the acute phase. A pilot randomized controlled trial. Medicina 2021, 57, 684. [Google Scholar] [CrossRef]
- Wittmer, V.L.; Paro, F.M.; Duarte, H.; Capellini, V.K.; Barbalho-Moulim, M.C. Early mobilization and physical exercise in patients with COVID-19: A narrative literature review. Complement. Ther. Clin. Pract. 2021, 43, 101364. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Estebanez-Pérez, M.J.; Pastora-Bernal, J.M.; Martín-Valero, R. The Effectiveness of a Four-Week Digital Physiotherapy Intervention to Improve Functional Capacity and Adherence to Intervention in Patients with Long COVID-19. Int. J. Environ. Res. Public Health 2022, 19, 9566. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Malani, P.N. 2019 Novel Coronavirus—Important Information for Clinicians. JAMA 2020, 323, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, J.; Leth, S.; Pedersen, T.H.; Harbo, T.; Blicher, J.U.; Karlsson, P.; Østergaard, L.; Andersen, H.; Tankisi, H. Myopathic changes in patients with long-term fatigue after COVID-19. Clin. Neurophysiol. 2021, 132, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Hama Amin, B.J.; Kakamad, F.H.; Ahmed, G.S.; Ahmed, S.F.; Abdulla, B.A.; Mohammed, S.H.; Mikael, T.M.; Salih, R.Q.; Ali, R.K.; Salh, A.M.; et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann. Med. Surg. 2022, 77, 103590. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef]
- Tirelli, U.; Taibi, R.; Chirumbolo, S. Post COVID syndrome: A new challenge for medicine. Eur. Rev. Med Pharmacol. Sci. 2021, 25, 4422–4425. [Google Scholar] [PubMed]
- Li, J.; Xia, W.; Zhan, C.; Liu, S.; Yin, Z.; Wang, J.; Chong, Y.; Zheng, C.; Fang, X.; Cheng, W.; et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): A randomised controlled trial. Thorax 2022, 77, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, C.; Duan, C.; Zhang, Y.; Li, Q.; Dou, Z.; Li, J.; Xia, W. Rehabilitation needs of the first cohort of post-acute COVID-19 patients in Hubei, China. Eur. J. Phys. Rehabil. Med. 2020, 56, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Guo, L.; Tian, F.; Dai, T.; Xing, X.; Zhao, J.; Li, Q. Rehabilitation of patients with COVID-19. Expert Rev. Respir. Med. 2020, 14, 1249–1256. [Google Scholar] [CrossRef]
- Bures, M.; Neumannova, K.; Blazek, P.; Klima, M.; Schvach, H.; Nema, J.; Kopecky, M.; Dygryn, J.; Koblizek, V. A Sensor Network Utilizing Consumer Wearables for Telerehabilitation of Post-acute COVID-19 Patients. IEEE Internet Things J. 2022, 9, 23795–23809. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, E.; Palalı, İ.; Atan, S.G.; Turan, D.; Çınarka, H.; Çetinkaya, E. The effectiveness of POST-DISCHARGE telerehabilitation practices in COVID-19 patients: Tele-COVID study-randomized controlled trial. Ann. Thorac. Med. 2022, 17, 110–117. [Google Scholar] [CrossRef]
- Belli, S.; Balbi, B.; Prince, I.; Cattaneo, D.; Masocco, F.; Zaccaria, S.; Bertalli, L.; Cattini, F.; Lomazzo, A.; Dal Negro, F.; et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur. Respir. J. 2020, 56, 2002096. [Google Scholar] [CrossRef]
- Letizia, S.; Angelo, G. Rehabilitation in the Treatment of the Post COVID-19 Patient. J. Adv. Heal. CARE. 2022, 4, 1–14. [Google Scholar]
- Zampogna, E.; Paneroni, M.; Belli, S.; Aliani, M.; Gandolfo, A.; Visca, D.; Bellanti, M.T.; Ambrosino, N.; Vitacca, M. Pulmonary Rehabilitation in Patients Recovering from COVID-19. Respiration 2021, 100, 416–422. [Google Scholar] [CrossRef]
- Dalbosco-Salas, M.; Torres-Castro, R.; Leyton, A.R.; Zapata, F.M.; Salazar, E.H.; Bastías, G.E.; Díaz, M.E.B.; Allers, K.T.; Fonseca, D.M.; Vilaró, J. Effectiveness of a primary care telerehabilitation program for post-COVID-19 patients: A feasibility study. J. Clin. Med. 2021, 10, 4428. [Google Scholar] [CrossRef]
- Franchini, S.; Spessot, M.; Landoni, G.; Piani, C.; Cappelletti, C.; Mariani, F.; Mauri, S.; Taglietti, M.V.; Fortunato, M.; Furlan, F.; et al. Stranger Months: How SARS-CoV-2, Fear of Contagion, and Lockdown Measures Impacted Attendance and Clinical Activity During February and March 2020 at an Urban Emergency Department in Milan. Disaster Med. Public Health Prep. 2021, 15, e33–e42. [Google Scholar] [CrossRef] [PubMed]
- Carda, S.; Invernizzi, M.; Bavikatte, G.; Bensmaïl, D.; Bianchi, F.; Deltombe, T.; Draulans, N.; Esquenazi, A.; Francisco, G.E.; Gross, R.; et al. COVID-19 pandemic. What should Physical and Rehabilitation Medicine specialists do? A clinician’s perspective. Eur. J. Phys. Rehabil. Med. 2020, 56, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Pastora-Bernal, J.-M.; Estebanez-Pérez, M.-J.; Molina-Torres, G.; García-López, F.-J.; Sobrino-Sánchez, R.; Martín-Valero, R. Telerehabilitation Intervention in Patients with COVID-19 after Hospital Discharge to Improve Functional Capacity and Quality of Life. Study Protocol for a Multicenter Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 2924. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Lillo, G.; Torres-Castro, R.; Fregonezi, G.; Vilaró, J.; Puppo, H. Challenge for Rehabilitation After Hospitalization for COVID-19. Arch. Phys. Med. Rehabil. 2020, 101, 1470–1471. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.G. Physical rehabilitation using telemedicine. J. Telemed. Telecare 2007, 13, 217–220. [Google Scholar] [CrossRef]
- Seelman, K.D.; Hartman, L.M. Telerehabilitation: Policy issues and research tools. Int. J. Telerehabil. 2009, 1, 47–58. [Google Scholar] [CrossRef]
- Rosen, K.; Patel, M.; Lawrence, C.; Mooney, B. Delivering Telerehabilitation to COVID-19 Inpatients: A Retrospective Chart Review Suggests It Is a Viable Option. HSS J. 2020, 16, 64–70. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef]
- He, J.; Yang, T. In the era of long COVID, can we seek new techniques for better rehabilitation? Chronic Dis. Transl. Med. 2022, 8, 149–153. [Google Scholar] [CrossRef]
- Keshner, E.A.; Weiss, P. Introduction to the special issue from the proceedings of the 2006 International Workshop on Virtual Reality in Rehabilitation. J. Neuroeng. Rehabil. 2007, 4, 18. [Google Scholar] [CrossRef]
- Larson, E.B.; Feigon, M.; Gagliardo, P.; Dvorkin, A.Y. Virtual reality and cognitive rehabilitation: A review of current outcome research. NeuroRehabilitation 2014, 34, 759–772. [Google Scholar] [CrossRef]
- Kenyon, R.V.; Leigh, J.; Keshner, E.A. Considerations for the future development of virtual technology as a rehabilitation tool. J. Neuroeng. Rehabil. 2004, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Burdea, G.C.; Cioi, D.; Kale, A.; Janes, W.E.; Ross, S.A.; Engsberg, J.R. Robotics and gaming to improve ankle strength, motor control, and function in children with cerebral palsy—A case study series. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 165–173. [Google Scholar] [CrossRef]
- Ferraris, C.; Ronga, I.; Pratola, R.; Coppo, G.; Bosso, T.; Falco, S.; Amprimo, G.; Pettiti, G.; Lo Priore, S.; Priano, L.; et al. Usability of the REHOME Solution for the Telerehabilitation in Neurological Diseases: Preliminary Results on Motor and Cognitive Platforms. Sensors 2022, 22, 9467. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Sharma, S.; Omar, B.; Paungmali, A.; Joseph, L. Validity and reliability of Internet-based physiotherapy assessment for musculoskeletal disorders: A systematic review. J. Telemed. Telecare 2016, 23, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Gal, N.; Andrei, D.; Nemeş, D.I.; Nădăşan, E.; Stoicu-Tivadar, V. A Kinect based intelligent e-rehabilitation system in physical therapy. Stud. Health Technol. Inform. 2015, 210, 489–493. [Google Scholar]
- Jagos, H.; David, V.; Haller, M.; Kotzian, S.; Hofmann, M.; Schlossarek, S.; Eichholzer, K.; Winkler, M.; Frohner, M.; Reichel, M.; et al. A Framework for (Tele-) Monitoring of the Rehabilitation Progress in Stroke Patients: eHealth 2015 Special Issue. Appl. Clin. Inform. 2015, 6, 757–768. [Google Scholar] [CrossRef]
- Ferraris, C.; Cimolin, V.; Vismara, L.; Votta, V.; Amprimo, G.; Cremascoli, R.; Galli, M.; Nerino, R.; Mauro, A.; Priano, L. Monitoring of gait parameters in post-stroke individuals: A feasibility study using rgb-d sensors. Sensors 2021, 21, 5945. [Google Scholar] [CrossRef]
- Amprimo, G.; Masi, G.; Priano, L.; Azzaro, C.; Galli, F.; Pettiti, G.; Mauro, A.; Ferraris, C. Assessment Tasks and Virtual Exergames for Remote Monitoring of Parkinson’s Disease: An Integrated Approach Based on Azure Kinect. Sensors 2022, 22, 8173. [Google Scholar] [CrossRef]
- Cox, N.S.; Dal Corso, S.; Hansen, H.; McDonald, C.F.; Hill, C.J.; Zanaboni, P.; Alison, J.A.; O’Halloran, P.; Macdonald, H.; Holland, A.E. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst. Rev. 2021, 1, CD013040. [Google Scholar] [CrossRef]
- Reychler, G.; Piraux, E.; Beaumont, M.; Caty, G.; Liistro, G. Telerehabilitation as a Form of Pulmonary Rehabilitation in Chronic Lung Disease: A Systematic Review. Healthcare 2022, 10, 1795. [Google Scholar] [CrossRef] [PubMed]
- Wuytack, F.; Devane, D.; Stovold, E.; McDonnell, M.; Casey, M.; McDonnell, T.J.; Gillespie, P.; Raymakers, A.; Lacasse, Y.; McCarthy, B. Comparison of outpatient and home-based exercise training programmes for COPD: A systematic review and meta-analysis. Respirology 2018, 23, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, E.; Baranowski, R.; Bilinska, M.; Stepnowska, M.; Korewicki, J.; Chojnowska, L.; Piotrowska, M.; Wo, A.; Malek, L.A.; Klopotowski, M.; et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: Effectiveness, quality of life, and adherence. Eur. J. Heart Fail. 2010, 12, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Cacciante, L.; della Pietà, C.; Rutkowski, S.; Cieślik, B.; Szczepańska-Gieracha, J.; Agostini, M.; Kiper, P. Cognitive telerehabilitation in neurological patients: Systematic review and meta-analysis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022, 43, 847–862. [Google Scholar] [CrossRef]
- Seron, P.; Oliveros, M.-J.; Gutierrez-Arias, R.; Fuentes-Aspe, R.; Torres-Castro, R.C.; Merino-Osorio, C.; Nahuelhual, P.; Inostroza, J.; Jalil, Y.; Solano, R.; et al. Effectiveness of Telerehabilitation in Physical Therapy: A Rapid Overview. Phys. Ther. 2021, 101, pzab053. [Google Scholar] [CrossRef]
- Cottrell, M.A.; Russell, T.G. Telehealth for musculoskeletal physiotherapy. Musculoskelet. Sci. Pract. 2020, 48, 102193. [Google Scholar] [CrossRef]
- Baroni, M.P.; Jacob, M.F.A.; Rios, W.R.; Fandim, J.V.; Fernandes, L.G.; Chaves, P.I.; Fioratti, I.; Saragiotto, B.T. The state of the art in telerehabilitation for musculoskeletal conditions. Arch. Physiother. 2023, 13, 1. [Google Scholar] [CrossRef]
- Fekete, M.; Fazekas-Pongor, V.; Balazs, P.; Tarantini, S.; Nemeth, A.N.; Varga, J.T. Role of new digital technologies and telemedicine in pulmonary rehabilitation: Smart devices in the treatment of chronic respiratory diseases. Wien. Klin. Wochenschr. 2021, 133, 1201–1207. [Google Scholar] [CrossRef]
- Kim, S.Y.; Daley, K.; Pruski, A.D.; AlFarra, T.; Azola, A.; Gonzalez Fernandez, M.; Keszler, M.S.; Friedel, S.; Haaf, H.; Segall, H.; et al. Implementation of a Framework for Telerehabilitation in Clinical Care Across the Continuum During COVID-19 and Beyond. Am. J. Phys. Med. Rehabil. 2022, 101, 53–60. [Google Scholar] [CrossRef]
- Rinaldo, R.F.; Mondoni, M.; Parazzini, E.M.; Pitari, F.; Brambilla, E.; Luraschi, S.; Balbi, M.; Sferrazza Papa, G.F.; Sotgiu, G.; Guazzi, M.; et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur. Respir. J. 2021, 58, 2100870. [Google Scholar] [CrossRef]
- Skjørten, I.; Ankerstjerne, O.A.W.; Trebinjac, D.; Brønstad, E.; Rasch-Halvorsen, Ø.; Einvik, G.; Lerum, T.V.; Stavem, K.; Edvardsen, A.; Ingul, C.B. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur. Respir. J. 2021, 58, 2100996. [Google Scholar] [CrossRef]
- Peçanha, T.; Goessler, K.F.; Roschel, H.; Gualano, B. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1441–H1446. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Luisa Schmidt, M.; et al. Detection of 2019 -nCoV by RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Kortianou, E.A.; Tsimouris, D.; Mavronasou, A.; Lekkas, S.; Kazatzis, N.; Apostolara, Z.E.; Isakoglou, M.; Dimakou, G.; Barmparessou, Z.; Tsikrika, S.; et al. Application of a home-based exercise program combined with tele-rehabilitation in previously hospitalized patients with COVID-19: A feasibility, single-cohort interventional study. Pneumon 2022, 35, 12. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, C.; Bernal-Utrera, C.; Anarte-Lazo, E.; Saavedra-Hernandez, M.; De-La-Barrera-Aranda, E.; Serrera-Figallo, M.A.; Gonzalez-Martin, M.; Gonzalez-Gerez, J.J. Breathing exercises versus strength exercises through telerehabilitation in coronavirus disease 2019 patients in the acute phase: A randomized controlled trial. Clin. Rehabil. 2022, 36, 486–497. [Google Scholar] [CrossRef]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Rajala, K.; Lehto, J.T.; Sutinen, E.; Kautiainen, H.; Myllärniemi, M.; Saarto, T. mMRC dyspnoea scale indicates impaired quality of life and increased pain in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2017, 3, 84. [Google Scholar] [CrossRef]
- Pramuka, M.; van Roosmalen, L. Telerehabilitation technologies: Accessibility and usability. Int. J. Telerehabil. 2009, 1, 85–98. [Google Scholar] [CrossRef]
- Suso-Martí, L.; La Touche, R.; Herranz-Gómez, A.; Angulo-Díaz-Parreño, S.; Paris-Alemany, A.; Cuenca-Martínez, F. Effectiveness of Telerehabilitation in Physical Therapist Practice: An Umbrella and Mapping Review With Meta—Meta-Analysis. Phys. Ther. 2021, 101, pzab075. [Google Scholar] [CrossRef]
- Del Corral, T.; Fabero-Garrido, R.; Plaza-Manzano, G.; Fernández-de-las-Peñas, C.; Navarro-Santana, M.; López-de-Uralde-Villanueva, I. Home-based respiratory muscle training on quality of life and exercise tolerance in long-term post-COVID-19: Randomized controlled trial. Ann. Phys. Rehabil. Med. 2023, 66, 101709. [Google Scholar] [CrossRef]
- Calvo-Paniagua, J.; Díaz-Arribas, M.J.; Valera-Calero, J.A.; Gallardo-Vidal, M.I.; Fernández-De-las-Peñas, C.; López-De-Uralde-Villanueva, I.; del Corral, T.; Plaza-Manzano, G. A tele-health primary care rehabilitation program improves self-perceived exertion in COVID-19 survivors experiencing Post-COVID fatigue and dyspnoea: A quasi-experimental study. PLoS ONE 2022, 17, e0271802. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gerez, J.J.; Saavedra-Hernandez, M.; Anarte-Lazo, E.; Bernal-Utrera, C.; Perez-Ale, M.; Rodriguez-Blanco, C. Short-Term Effects of a Respiratory Telerehabilitation Program in Confined COVID-19 Patients in the Acute Phase: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 7511. [Google Scholar] [CrossRef]
- Storm, F.A.; Cesareo, A.; Reni, G.; Biffi, E. Wearable inertial sensors to assess gait during the 6-minute walk test: A systematic review. Sensors 2020, 20, 2660. [Google Scholar] [CrossRef] [PubMed]
- Morino, A.; Takahashi, H.; Chiba, H.; Ishiai, S. Factors affecting dyspnoea after the 6-minute walk test in idiopathic pulmonary fibrosis patients presenting with exercise-induced hypoxemia. J. Phys. Ther. Sci. 2017, 29, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European respiratory society/American thoracic society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Cimolin, V.; Gobbi, M.; Buratto, C.; Ferraro, S.; Fumagalli, A.; Galli, M.; Capodaglio, P. A Comparative Analysis of Shoes Designed for Subjects with Obesity Using a Single Inertial Sensor: Preliminary Results. Sensors 2022, 22, 782. [Google Scholar] [CrossRef]
- Issues, S.; Test, M.W.; Equipment, R.; Preparation, P. American Thoracic Society ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Crouch, R. 1-Minute Sit-to-Stand Test: Systematic review of procedures, performance, and clinimetric properties. J. Cardiopulm. Rehabil. Prev. 2019, 39, 2–8. [Google Scholar] [CrossRef]
- Crook, S.; Büsching, G.; Schultz, K.; Lehbert, N.; Jelusic, D.; Keusch, S.; Wittmann, M.; Schuler, M.; Radtke, T.; Frey, M.; et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur. Respir. J. 2017, 49, 1601871. [Google Scholar] [CrossRef]
- Pereira, M.C.; Lima, L.N.G.; Moreira, M.M.; Mendes, F.A.R. One minute sit-to-stand test as an alternative to measure functional capacity in patients with pulmonary arterial hypertension. J. Bras. Pneumol. 2022, 48, e20210483. [Google Scholar]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Martin, I.; Braem, F.; Baudet, L.; Poncin, W.; Fizaine, S.; Aboubakar, F.; Froidure, A.; Pilette, C.; Liistro, G.; De Greef, J.; et al. Follow-up of functional exercise capacity in patients with COVID-19: It is improved by telerehabilitation. Respir. Med. 2021, 183, 106438. [Google Scholar] [CrossRef]
- Kohlbrenner, D.; Benden, C.; Radtke, T. The 1-minute sit-to-stand test in lung transplant candidates: An alternative to the 6-minute walk test. Respir. Care 2020, 65, 437–443. [Google Scholar] [CrossRef]
- Cimolin, V.; Cau, N.; Malchiodi Albedi, G.; Aspesi, V.; Merenda, V.; Galli, M.; Capodaglio, P. Do wearable sensors add meaningful information to the Timed Up and Go test? A study on obese women. J. Electromyogr. Kinesiol. 2019, 44, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Borel, B.; Wilkinson-Maitland, C.A.; Hamilton, A.; Bourbeau, J.; Perrault, H.; Jensen, D.; Maltais, F. Three-minute constant rate step test for detecting exertional dyspnoea relief after bronchodilation in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2991–3000. [Google Scholar] [CrossRef]
- Cho, M. The effects of modified wall squat exercises on average adults’ deep abdominal muscle thickness and lumbar stability. J. Phys. Ther. Sci. 2013, 25, 689–692. [Google Scholar] [CrossRef]
- Ora, J.; Prendi, E.; Attinà, M.L.; Cazzola, M.; Calzetta, L.; Rogliani, P. Efficacy of respiratory tele-rehabilitation in COPD patients: Systematic review and meta-analysis. Monaldi Arch. Chest Dis. 2022, 92. [Google Scholar] [CrossRef] [PubMed]
- Hermans, G.; Van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 2015, 19, 274. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, R.; Solis-Navarro, L.; Sitjà-Rabert, M.; Vilaró, J. Functional Limitations Post-COVID-19: A Comprehensive Assessment Strategy Limitaciones funcionales post COVID-19: Una estrategia de evaluación integral. Arch. Bronconeumol. 2021, 57, 7–8. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Alawna, M. Role of increasing the aerobic capacity on improving the function of immune and respiratory systems in patients with coronavirus (COVID-19): A review. Diabetes Metab. Syndr. 2020, 14, 489–496. [Google Scholar] [CrossRef]
- Bulut, C.; Kato, Y. Epidemiology of COVID-19. Turk. J. Med. Sci. 2020, 50, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Tiruneh, S.A.; Tesema, Z.T.; Azanaw, M.M.; Angaw, D.A. The effect of age on the incidence of COVID-19 complications: A systematic review and meta-analysis. Syst. Rev. 2021, 10, 80. [Google Scholar] [CrossRef]
- Cerfoglio, S.; Ferraris, C.; Vismara, L.; Amprimo, G.; Priano, L.; Pettiti, G.; Galli, M.; Mauro, A.; Cimolin, V. Kinect-Based Assessment of Lower Limbs during Gait in Post-Stroke Hemiplegic Patients: A Narrative Review. Sensors 2022, 22, 4910. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R. Writing narrative style literature reviews. Med. Writ. 2015, 24, 230–235. [Google Scholar] [CrossRef]
- Teixeira Do Amaral, V.; Viana, A.A.; Heubel, A.D.; Linares, S.N.; Martinelli, B.; Witzler, P.H.C.; Orikassa De Oliveira, G.Y.; Zanini, G.D.S.; Borghi Silva, A.; Mendes, R.G.; et al. Cardiovascular, Respiratory, and Functional Effects of Home-Based Exercise Training after COVID-19 Hospitalization. Med. Sci. Sports Exerc. 2022, 54, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Hanada, M.; Kasawara, K.T.; Mathur, S.; Rozenberg, D.; Kozu, R.; Ahmed Hassan, S.; Darlene Reid, W. Aerobic and breathing exercises improve dyspnoea, exercise capacity and quality of life in idiopathic pulmonary fibrosis patients: Systematic review and meta-analysis. J. Thorac. Dis. 2020, 12, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- De Souza, Y.; Suzana, M.E.; Medeiros, S.; Macedo, J.; da Costa, C.H. Respiratory muscle weakness and its association with exercise capacity in patients with chronic obstructive pulmonary disease. Clin. Respir. J. 2022, 16, 162–166. [Google Scholar] [CrossRef]
- Negm, A.M.; Salopek, A.; Zaide, M.; Meng, V.J.; Prada, C. Rehabilitation at the Time of Pandemic: Patient Journey Recommendations. Front. Aging Neurosci. 2022, 14, 172. [Google Scholar] [CrossRef]
- Verga, M.; Viganò, G.L.; Capuzzo, M.; Duri, C.; Ignoti, L.M.; Picozzi, P.; Cimolin, V. The digitization process and the evolution of Clinical Risk Management concept: The role of Clinical Engineering in the operational management of biomedical technologies. Front. Public Health 2023, 11, 1121243. [Google Scholar] [CrossRef]
- Sakai, T.; Hoshino, C.; Yamaguchi, R.; Hirao, M.; Nakahara, R.; Okawa, A. Remote rehabilitation for patients with COVID-19. J. Rehabil. Med. 2020, 52, jrm00095. [Google Scholar] [CrossRef]
| Tele-Rehabilitation Program | |||||||
|---|---|---|---|---|---|---|---|
| Source | Year and Country | Study Type | #Participants, Age (Years), Gender (#M/F) and Stage of the Disease | Type | Mode, Tools, and Duration | Interventions | Functional Assessment of Physical Capacity |
| Dalbosco-Salas et al. [30] | 2021 Chile | Multicentric, observational, and prospective study | Total = 115 (M: 46/F: 66) Age = 55.6 ± 12.7 years Hospitalized (n = 57) and Non-Hospitalized (n = 58) Post-COVID patients | Motor and Respiratory | 2–3 d/w for 9 weeks (24 sessions) Weekly phone calls | Warm up (5 min), breathing exercises (3 min), aerobic and/or strength exercises (20–30 min), and stretching (5 min) | 1 min sit-to-stand test (1 min STST) |
| Estebanez-Pérez et al. [11] | 2022 Spain | One arm quasi-experimental clinical trial | Total = 32 (M: 9/F: 23) Age = 45.93 ± 10.65 years Long-COVID patients | Motor | 3–5 d/w for 4 weeks Synchronous sessions via video conference and sessions via smartphone app | Personalized recommendations (e.g., walking, jogging) and progressive strength training, working 1–3 muscle groups with a load of 8–12 reps, with 2 min training intervals | 1 min STST Short performance physical battery test (SPPB) |
| Kortianou et al. [64] | 2022 Greece | Single-cohort interventional study | Total = 22 (M: 18/F: 4) Age = 50.1 ± 13.2 years Hospitalized Post-COVID Patients | Motor and respiratory | 5 d/w for 8 weeks Daily unsupervised self-practice via smartphone app 3 supervised sessions with a physiotherapist over the duration of the program | Warm-up (5–10 min), aerobic and total body strengthening exercises (15–20 min), and stretching (5–10 min) | 1 min STST SPPB 3 min step test (3MST) |
| Pehlivan et al. [26] | 2022 Turkey | Randomized controlled study | Total = 34 TeleG = 17 (M:14/F:3) Age = 50.76 years CG = 17 (M:11/F:6) Age = 43.24 years Post-COVID patients | Motor and respiratory | 3 d/w for 6 weeks Synchronous video conference with a physiotherapist | Paced running/self-walking in the corridor, breathing exercises, active cycle of breathing technique, range of motion exercises, and standing squat | Timed up and go test (TUG) Short physical performance battery (SPPB) |
| Li et al. [22] | 2022 China | Parallel-group randomized controlled trial | Total = 119 TeleG = 59 (M:27/F: 32) Age = 49.17 ± 10.75 years CG = 60 (M: 26 F:43) Age = 52.03 ± 11.10 years Post-COVID patients (formerly hospitalized) | Motor and respiratory | 3–4 d/w for 6 weeks Daily self-practice via smartphone app | Breathing control and thoracic expansion, aerobic exercise, and LMS exercise | 6 min walking test (6MWT) Squat test |
| Rodriguez-Blanco et al. [8] | 2021 Spain | Randomized controlled trial | Total = 36 TeleG = 18 (M: 9/F: 9) Age = 39.39 ± 11.74 years CG = 18 (M:8/F:10) Age = 41.33 ± 12.13 years Acute patients with mild to moderate symptomatology | Motor | Daily 10–30 min session for one week Daily text messages and videoconferences, if needed | 10 non-specific toning exercises of resistance and strength up to 12 reps | 6 min walking test (6MWT) Thirty second sit-to-stand test (30STST) |
| Rodriguez-Blanco et al. [65] | 2022 Spain | Total = 77 TeleG (1) = 26 (M: 14/F: 12) Age = 34.81 ± 11.82 years TeleG (2) = 29 (M:13/F: 16) Age = 41.93 ± 10.19 years CG = 22 (M: 13/F: 12) Age = 42.36 ± 11.84 years Acute patients, with mild to moderate symptomatology | Motor or respiratory | Daily 10–30 min session for 14 days Daily text messages and videoconferences, if needed | TeleG (1): 10 strength exercises to improve the physical deconditioning and physiological deterioration TeleG (2): 10 exercises based on the active cycle of breathing techniques | 6MWT 30STST | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerfoglio, S.; Capodaglio, P.; Rossi, P.; Verme, F.; Boldini, G.; Cvetkova, V.; Ruggeri, G.; Galli, M.; Cimolin, V. Tele-Rehabilitation Interventions for Motor Symptoms in COVID-19 Patients: A Narrative Review. Bioengineering 2023, 10, 650. https://doi.org/10.3390/bioengineering10060650
Cerfoglio S, Capodaglio P, Rossi P, Verme F, Boldini G, Cvetkova V, Ruggeri G, Galli M, Cimolin V. Tele-Rehabilitation Interventions for Motor Symptoms in COVID-19 Patients: A Narrative Review. Bioengineering. 2023; 10(6):650. https://doi.org/10.3390/bioengineering10060650
Chicago/Turabian StyleCerfoglio, Serena, Paolo Capodaglio, Paolo Rossi, Federica Verme, Gabriele Boldini, Viktoria Cvetkova, Graziano Ruggeri, Manuela Galli, and Veronica Cimolin. 2023. "Tele-Rehabilitation Interventions for Motor Symptoms in COVID-19 Patients: A Narrative Review" Bioengineering 10, no. 6: 650. https://doi.org/10.3390/bioengineering10060650
APA StyleCerfoglio, S., Capodaglio, P., Rossi, P., Verme, F., Boldini, G., Cvetkova, V., Ruggeri, G., Galli, M., & Cimolin, V. (2023). Tele-Rehabilitation Interventions for Motor Symptoms in COVID-19 Patients: A Narrative Review. Bioengineering, 10(6), 650. https://doi.org/10.3390/bioengineering10060650










