Refined Techniques for Enabling Long-Term Cryo-Repository Using Vitrification and Laser Warming
Abstract
1. Introduction
2. Materials and Methods
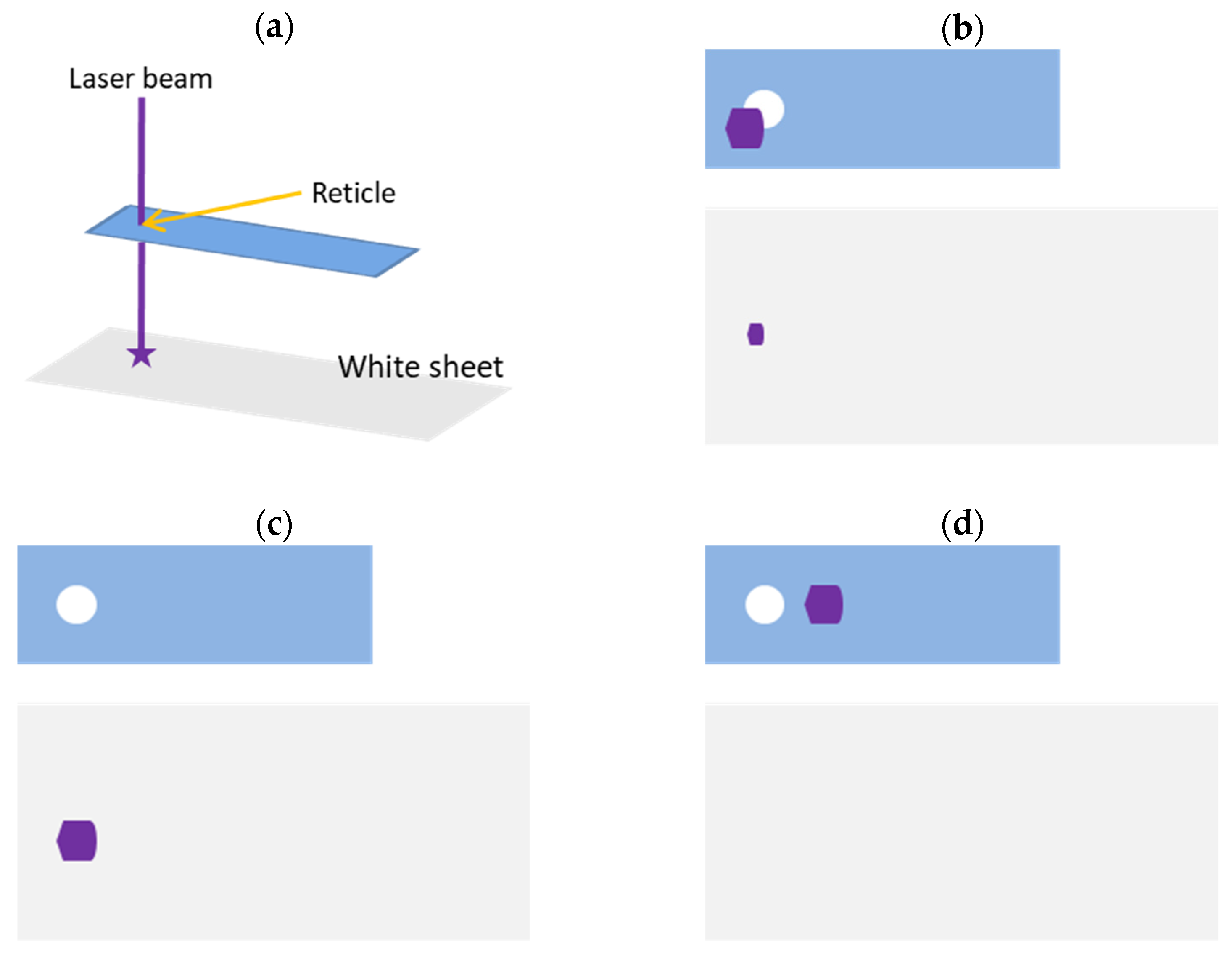
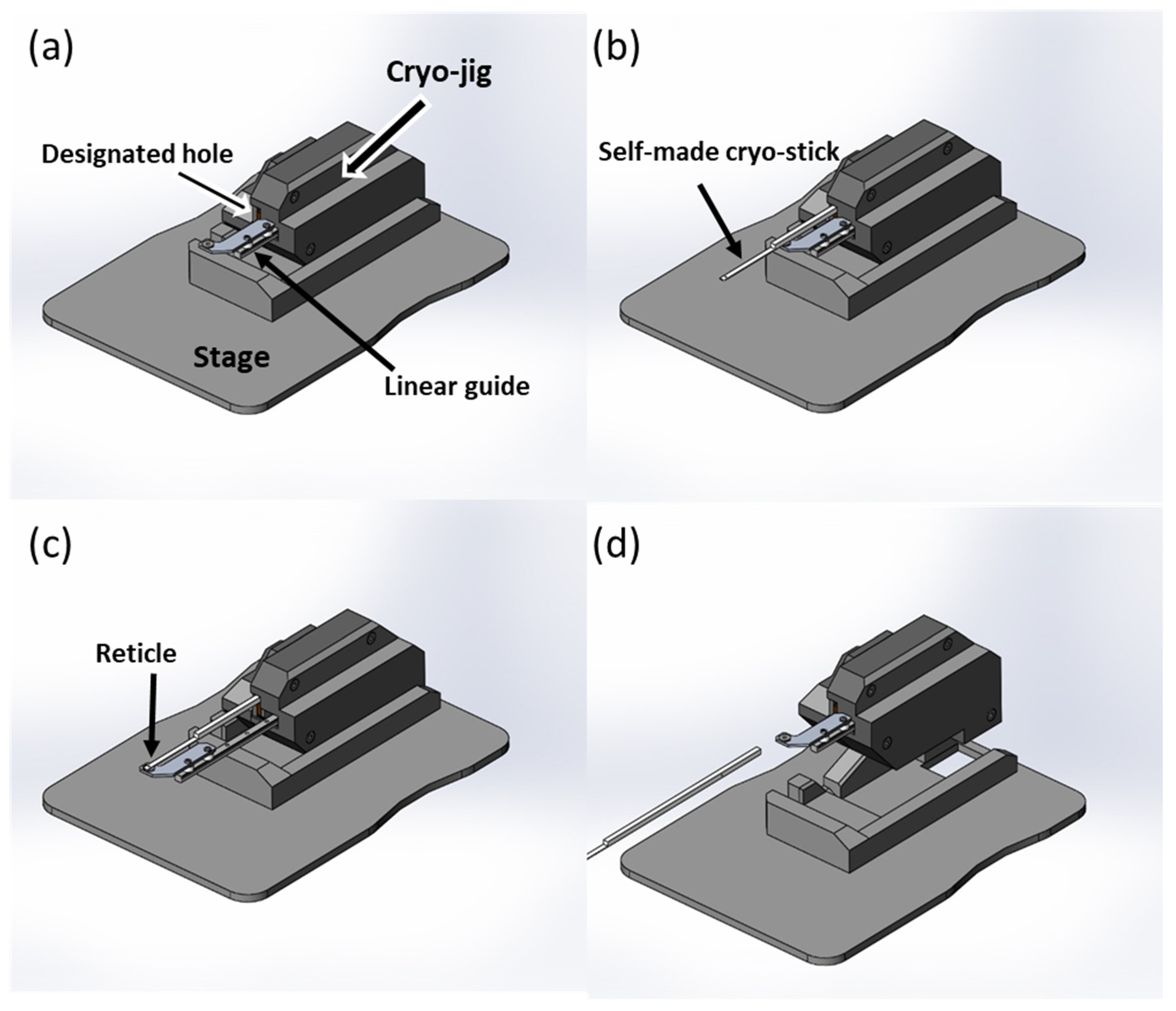
2.1. Alignment of Customized Device and Laser Machine
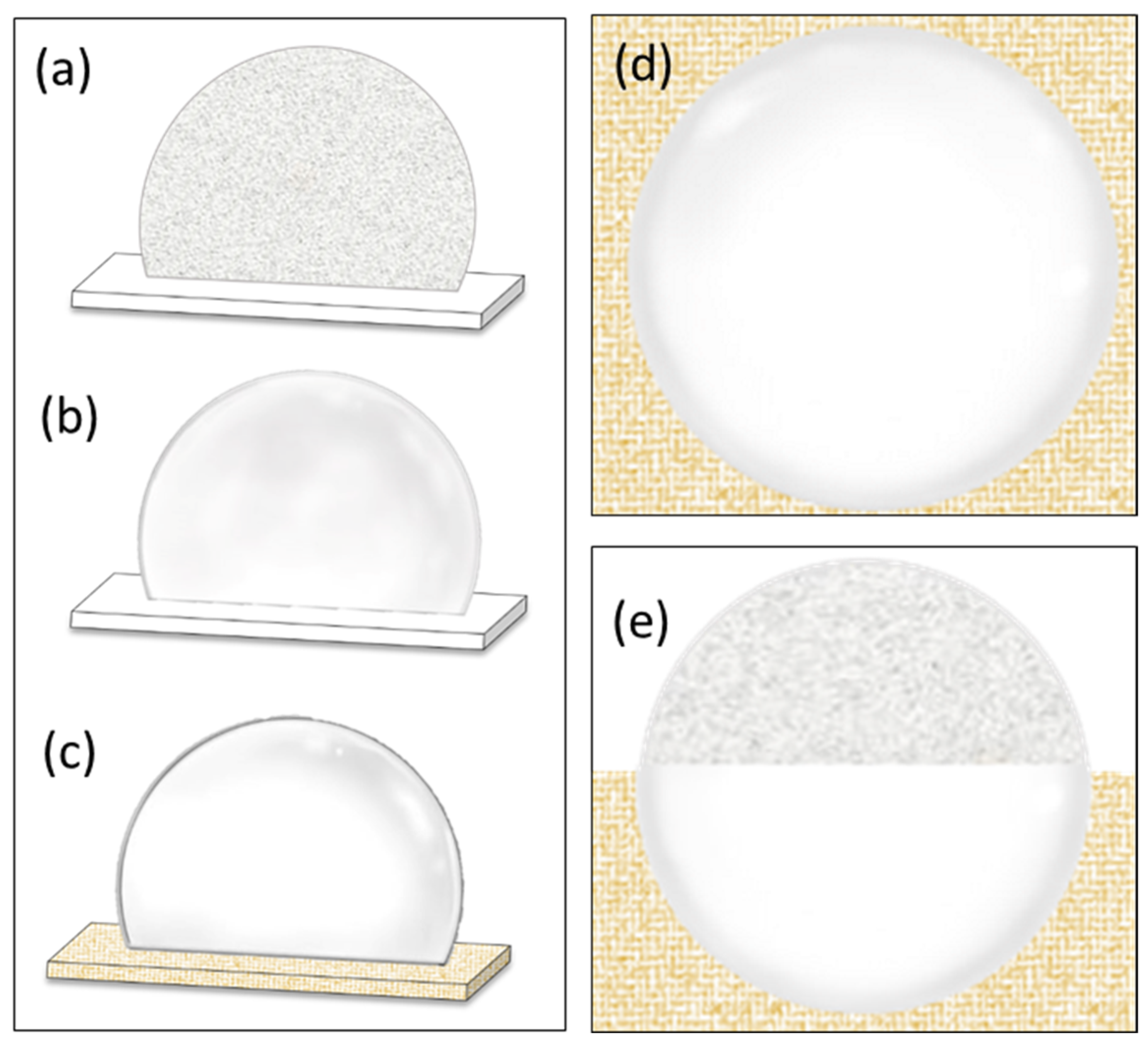
2.2. Cryopreservation
2.3. Testing of Laser Accuracy and Successful Rewarming Rate
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leibo, S.P. Preservation of ova and embryos by freezing. In New Technologies in Animal Breeding; Brackett, E.G., Scidel, G.E., Seidel, S.M., Eds.; Academic Press: New York, NY, USA, 1981; pp. 127–139. [Google Scholar]
- Parmegiani, L.; Cognigni, G.E.; Bernardi, S.; Cuomo, S.; Ciampaglia, W.; Infante, F.E.; de Fatis, C.T.; Arnone, A.; Maccarini, A.M.; Filicori, M. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reprod. Biomed. Online 2011, 23, 505–512. [Google Scholar] [CrossRef]
- Hwang, I.S.; Hochi, S. Recent Progress in Cryopreservation of Bovine Oocytes. Biomed. Res. Int. 2014, 2014, 570647. [Google Scholar] [CrossRef]
- Jin, B.; Mazur, P. High survival of mouse oocytes/embryos after vitrification without permeating cryoprotectants followed by ultra-rapid warming with an IR laser pulse. Sci. Rep. 2015, 5, 9271. [Google Scholar] [CrossRef]
- Tsai, S.; Lin, C. Advantages and applications of cryopreservation in fisheries science. Braz. Arch. Biol. Technol. 2012, 55, 425–434. [Google Scholar] [CrossRef]
- Tiersch, T.R.; Yang, H.; Jenkins, J.A.; Dong, Q. Sperm cryopreservation in fish and shellfish. Soc. Reprod. Fertil. Suppl. 2007, 65, 493–508. [Google Scholar] [PubMed]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitri-fication to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Kuwayama, M.; Vajta, G.; Kato, O.; Leibo, S.P. Highly efficient vitrification method for cryopreservation of human oo-cytes. Reprod. Biomed. Online 2005, 11, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Saragusty, J.; Arav, A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011, 141, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Yen, W.; Chavanich, S.; Viyakarn, V.; Lin, C. Development of Cryopreservation Techniques for Gorgonian (Junceella juncea) Oocytes through Vitrification. PLoS ONE 2015, 10, e0123409. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.; Yang, V.; Lin, C. Comparison of the cryo-tolerance of vitrified gorgonian oocytes. Sci. Rep. 2016, 6, 23290. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lu, C.; Dou, M.; Zhang, C.; Chang, H.; Liu, J.; Rao, W. Soft liquid metal nanoparticles achieve reduced crystal nucleation and ultrarapid rewarming for human bone marrow stromal cell and blood vessel cryopreservation. Acta Biomater. 2019, 102, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, F.; Chen, Z.; Hossain, S.M.C.; Wang, M.; Haider, Z.; Memon, K.; Chen, P.; Zhao, G. Near-infrared laser mediated modulation of ice crystallization by two-dimensional nanosheets enables high-survival recovery of biological cells from cryogenic temperatures. Nanoscale 2018, 10, 11760–11774. [Google Scholar] [CrossRef] [PubMed]
- Khosla, K.; Wang, Y.; Hagedorn, M.; Qin, Z.; Bischof, J. Gold Nanorod Induced Warming of Embryos from the Cryogenic State Enhances Viability. ACS Nano. 2017, 11, 7869–7878. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Kleinhans, F.; Mazur, P. Survivals of mouse oocytes approach 100% after vitrification in 3-fold diluted media and ultra-rapid warming by an IR laser pulse. Cryobiology 2014, 68, 419–430. [Google Scholar] [CrossRef]
- Daly, J.; Zuchowicz, N.; Lendo, C.I.N.; Khosla, K.; Lager, C.; Henley, E.M.; Bischof, J.; Kleinhans, F.W.; Lin, C.; Peters, E.C.; et al. Successful cryopreservation of coral larvae using vitrification and laser warming. Sci. Rep. 2018, 8, 15714. [Google Scholar] [CrossRef]
- Cirino, L.; Wen, Z.H.; Hsieh, K.; Huang, C.L.; Leong, Q.L.; Wang, L.H.; Chen, C.S.; Daly, J.; Tsai, S.; Lin, C. First instance of settlement by cryopreserved coral larvae in symbiotic association with dinoflagellates. Sci Rep. 2019, 9, 18851. [Google Scholar] [CrossRef] [PubMed]
- Cirino, L.; Tsai, S.; Wang, L.H.; Chen, C.S.; Hsieh, W.C.; Huang, C.L.; Wen, Z.H.; Lin, C. Supplementation of exogenous lipids via liposomes improves coral larvae settlement post-cryopreservation and nano-laser warming. Cryobiology 2021, 98, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Narida, A.; Hsieh, W.C.; Huang, C.L.; Wen, Z.H.; Tsai, S.; Lin, C. Long-term cryopreservation for vitrification and laser warming. Biopreserv. Biobank 2022, in press. [Google Scholar] [CrossRef]
- Lane, M.; Bavister, B.D.; Lyons, E.A.; Forest, K.T. Containerless vitrification of mammalian oocytes and embryos. Nat. Biotechnol. 1999, 17, 1232–1236. [Google Scholar] [CrossRef]
- Inna, N.; Sanmee, U.; Saeng-anan, U.; Piromlertamorn, W.; Vutyavanich, T. Rapid freezing versus Cryotop vitrification of mouse two-cell embryos. Clin. Exp. Reprod. Med. 2018, 45, 110–115. [Google Scholar] [CrossRef]
- Seki, S.; Mazur, P. Ultra-rapid warming yields high survival of mouse oocytes cooled to −196 °C in dilutions of a standard vitrification solution. PLoS ONE 2012, 7, e36058. [Google Scholar] [CrossRef]


| Percentage (%) | |||
|---|---|---|---|
| Tip travel speed | 1113 mm/s | 834 mm/s | 668 mm/s |
| Laser striking accuracy | 95.00 ± 0 # | 93.33 ± 2.89 # | 91.67 ± 2.89 # |
| Successful rewarming rate | 60.00 ± 5.00 * | 61.67 ± 2.89 * | 55.00 ± 0 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.; Hsieh, W.-C.; Loeslakwiboon, K.; Huang, C.-L.; Chen, T.-C.; Tsai, S. Refined Techniques for Enabling Long-Term Cryo-Repository Using Vitrification and Laser Warming. Bioengineering 2023, 10, 605. https://doi.org/10.3390/bioengineering10050605
Lin C, Hsieh W-C, Loeslakwiboon K, Huang C-L, Chen T-C, Tsai S. Refined Techniques for Enabling Long-Term Cryo-Repository Using Vitrification and Laser Warming. Bioengineering. 2023; 10(5):605. https://doi.org/10.3390/bioengineering10050605
Chicago/Turabian StyleLin, Chiahsin, Wen-Chung Hsieh, Kanokpron Loeslakwiboon, Cheng-Liang Huang, Ting-Chun Chen, and Sujune Tsai. 2023. "Refined Techniques for Enabling Long-Term Cryo-Repository Using Vitrification and Laser Warming" Bioengineering 10, no. 5: 605. https://doi.org/10.3390/bioengineering10050605
APA StyleLin, C., Hsieh, W.-C., Loeslakwiboon, K., Huang, C.-L., Chen, T.-C., & Tsai, S. (2023). Refined Techniques for Enabling Long-Term Cryo-Repository Using Vitrification and Laser Warming. Bioengineering, 10(5), 605. https://doi.org/10.3390/bioengineering10050605








