Author Contributions
Conceptualization, C.-W.L. and J.C.-H.C.; methodology, E.-R.C. and S.-H.C.; software, H.-J.L.; validation, P.C. and E.-R.C.; formal analysis, J.C.-H.C.; investigation, C.-W.L.; resources, C.-W.L.; data curation, P.C.; writing—original draft preparation, C.-W.L.; writing—review and editing, J.C.-H.C.; visualization, H.-J.L.; supervision, C.-W.L.; project administration, C.-W.L.; funding acquisition, C.-W.L. and J.C.-H.C. All authors have read and agreed to the published version of the manuscript.
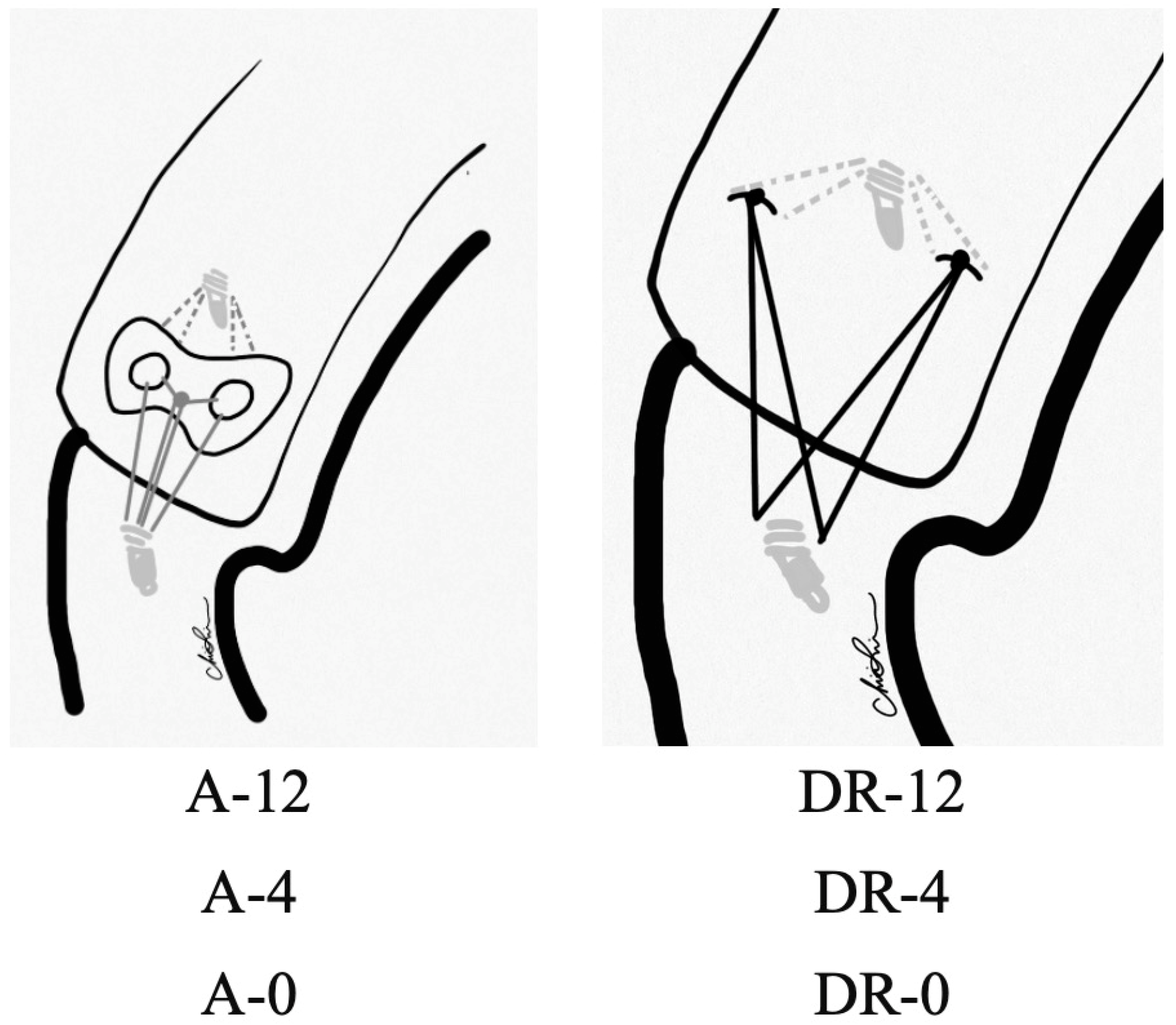
Figure 1.
Experimental groups. A: Augment; DR: Double-row.
Figure 1.
Experimental groups. A: Augment; DR: Double-row.
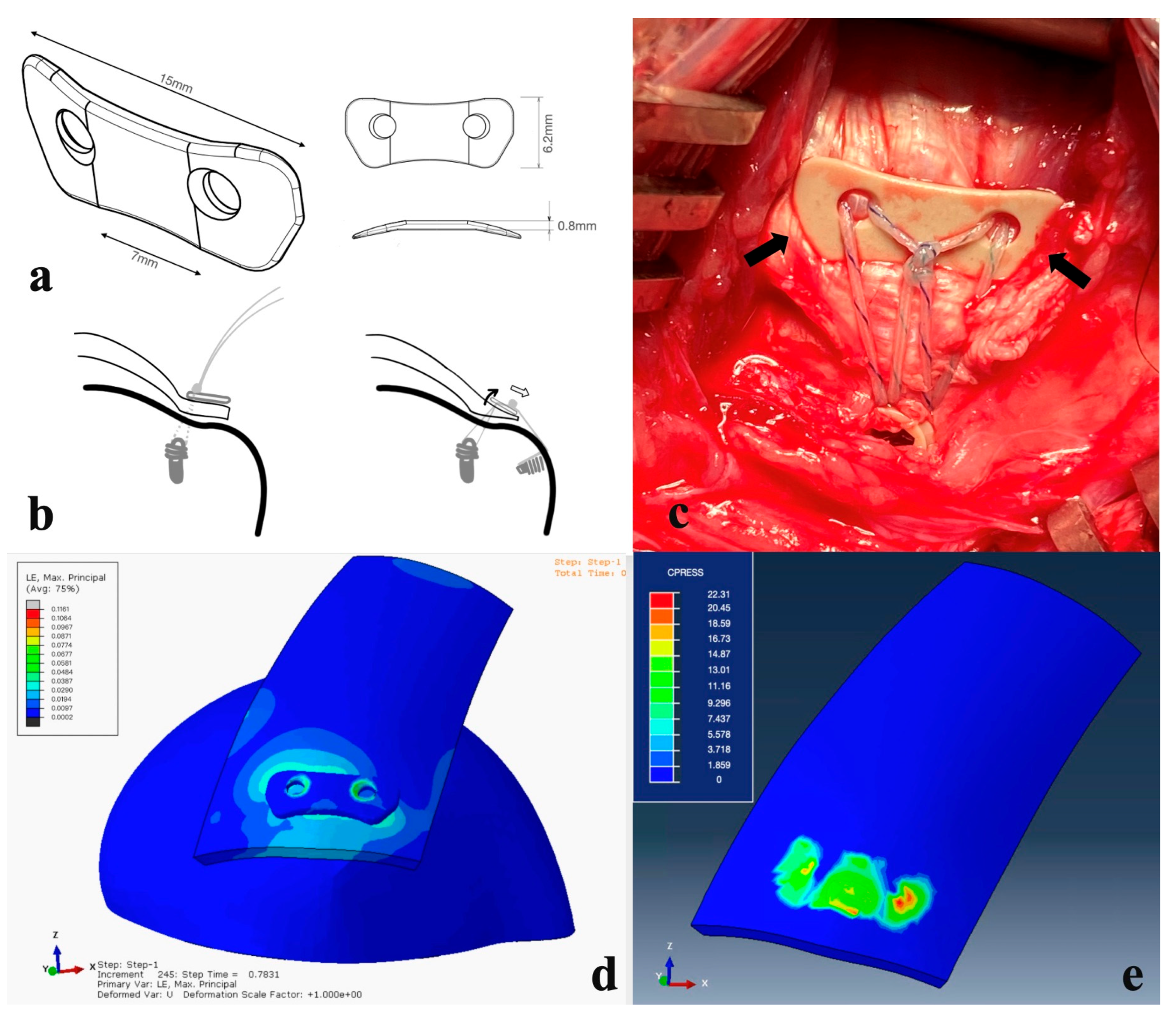
Figure 2.
(a) Perspective view of the PEEK augment. (b) See-saw effect. When the sutures are pulled laterally (right), more compressive force is applied on the lateral side and loosened at the medial part of the PEEK augment, preventing circulation compromise. (c) In the A-4 group, the PEEK augment was applied onto the rotator cuff. Bilateral fins (arrows) yielded compressive force onto the rotator cable and fit local anatomic structures well. (d,e) The pressure is dispersed averagely underneath the PEEK button, while the sutures used during rotator cuff repairs are thin and the stress concentration lies in the suture-tendon area. The PEEK material sustains the shear force of the sutures, converting them into a compression force and providing a homogeneous pressure distribution, which could further benefit the tendon healing proved in our animal study.
Figure 2.
(a) Perspective view of the PEEK augment. (b) See-saw effect. When the sutures are pulled laterally (right), more compressive force is applied on the lateral side and loosened at the medial part of the PEEK augment, preventing circulation compromise. (c) In the A-4 group, the PEEK augment was applied onto the rotator cuff. Bilateral fins (arrows) yielded compressive force onto the rotator cable and fit local anatomic structures well. (d,e) The pressure is dispersed averagely underneath the PEEK button, while the sutures used during rotator cuff repairs are thin and the stress concentration lies in the suture-tendon area. The PEEK material sustains the shear force of the sutures, converting them into a compression force and providing a homogeneous pressure distribution, which could further benefit the tendon healing proved in our animal study.
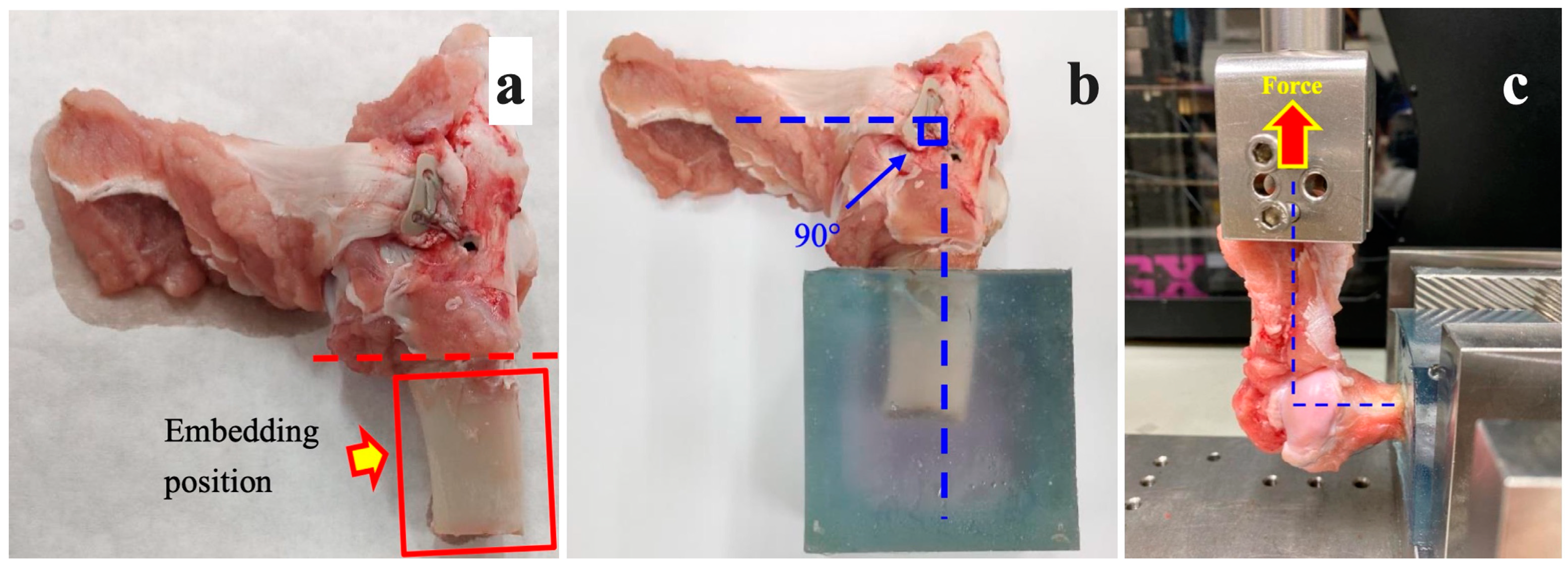
Figure 3.
(a) The soft tissues on the bone were removed. (b,c) The infraspinatus muscle specimen was fixed and erected 90 degrees from the humerus during the biomechanical testing.
Figure 3.
(a) The soft tissues on the bone were removed. (b,c) The infraspinatus muscle specimen was fixed and erected 90 degrees from the humerus during the biomechanical testing.
Figure 4.
(a) Before testing. (b) The specimen was pulled at a rate of 75 mm/min. (c) Failure was observed at the infraspinatus muscle, but not the enthesis.
Figure 4.
(a) Before testing. (b) The specimen was pulled at a rate of 75 mm/min. (c) Failure was observed at the infraspinatus muscle, but not the enthesis.
Figure 5.
Macroscopic findings of the repaired tendon and bone. (a) The specimen before dissection. A well-developed fibrotic appearance was observed in the A-4 group. (b) The specimen of the A-4 group after dissection. Retracted native tendon (Native) and well-developed, thicker (7.5 mm) new entheses (New) are observed. (c) New enthesis of specimen in DR-4 group has thinner (3.5 mm) appearance than that in A-4 group.
Figure 5.
Macroscopic findings of the repaired tendon and bone. (a) The specimen before dissection. A well-developed fibrotic appearance was observed in the A-4 group. (b) The specimen of the A-4 group after dissection. Retracted native tendon (Native) and well-developed, thicker (7.5 mm) new entheses (New) are observed. (c) New enthesis of specimen in DR-4 group has thinner (3.5 mm) appearance than that in A-4 group.
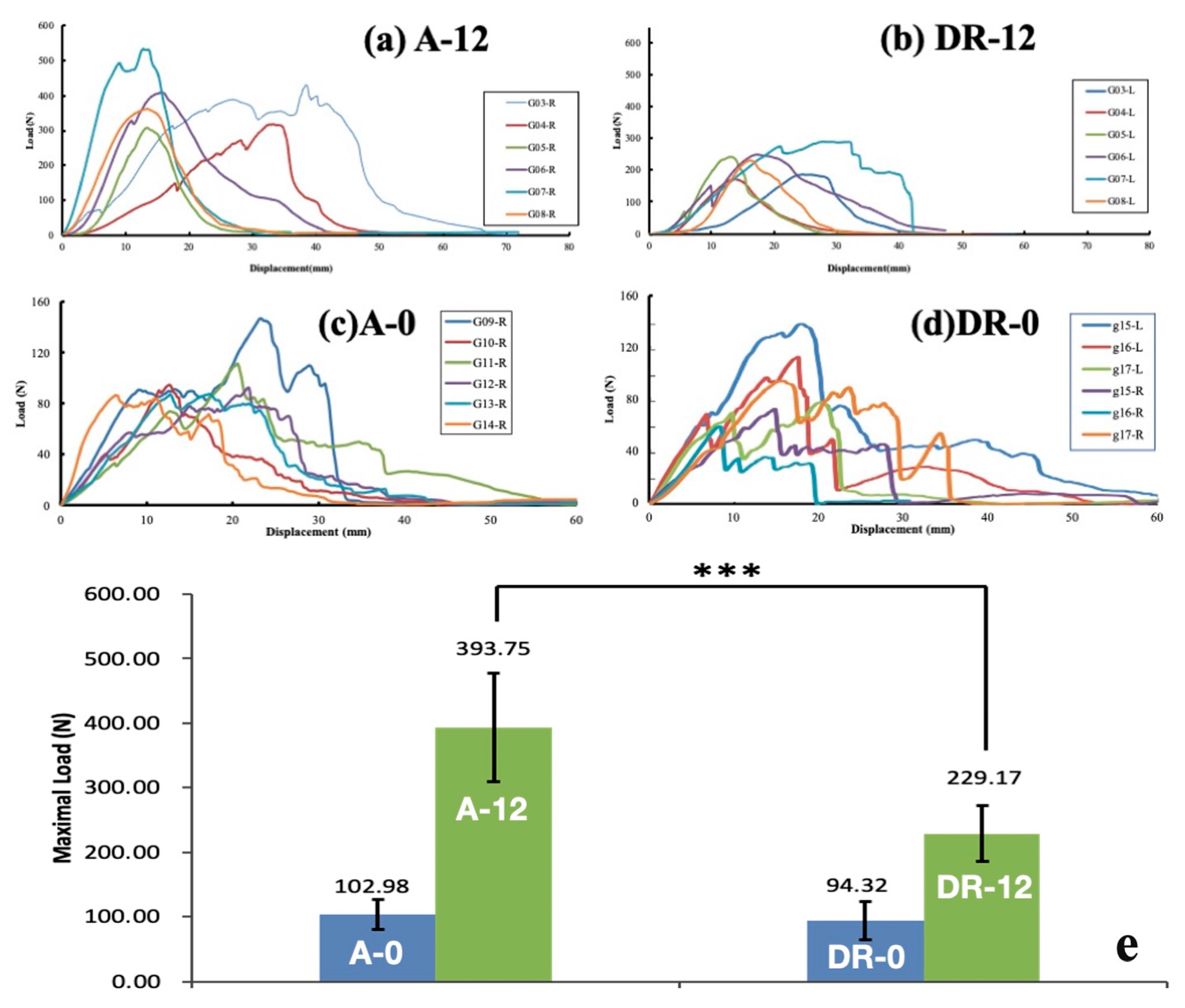
Figure 6.
Load-displacement plot of (a) A-12 group, (b) DR-12 group, (c) A-0 group, and (d) DR-0 group and (e) comparison among the 4 groups. The pull-out strength of A-0 vs. DR-0 and A-12 vs. DR-12 group. The error bar stands for standard deviation (n = 6). Statistical significance between A-12 and DR-12 is observed (*** p < 0.001, one-way ANOVA for independent samples).
Figure 6.
Load-displacement plot of (a) A-12 group, (b) DR-12 group, (c) A-0 group, and (d) DR-0 group and (e) comparison among the 4 groups. The pull-out strength of A-0 vs. DR-0 and A-12 vs. DR-12 group. The error bar stands for standard deviation (n = 6). Statistical significance between A-12 and DR-12 is observed (*** p < 0.001, one-way ANOVA for independent samples).
Figure 7.
Histopathological cell response and tissue alternation in tendon-to-bone insertion site with (A-4) and without (DR-4) PEEK augment.
Figure 7.
Histopathological cell response and tissue alternation in tendon-to-bone insertion site with (A-4) and without (DR-4) PEEK augment.
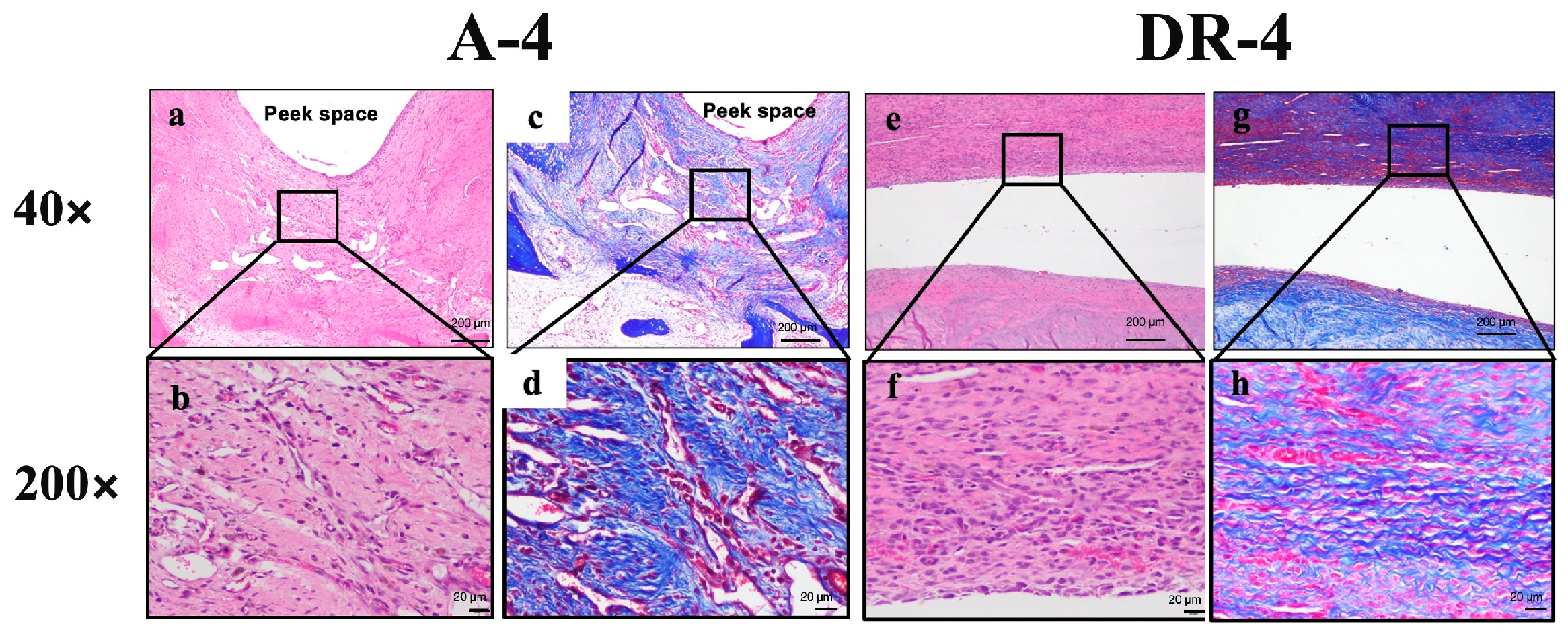
Figure 8.
Low- and high-power fields section of both A-4 and DR-4 groups. (a,b) 40× and 200× of A-4 group under H&E stain and Masson’s trichrome stain (c,d) showed more sub-acute to sub-chronic inflammatory response, such as fibrin deposition and cell apoptosis, than DR-4 group. (e,f) H&E stain and (g,h) Masson’s trichrome stain of DR-4 group.
Figure 8.
Low- and high-power fields section of both A-4 and DR-4 groups. (a,b) 40× and 200× of A-4 group under H&E stain and Masson’s trichrome stain (c,d) showed more sub-acute to sub-chronic inflammatory response, such as fibrin deposition and cell apoptosis, than DR-4 group. (e,f) H&E stain and (g,h) Masson’s trichrome stain of DR-4 group.
Figure 9.
H&E stain and IHC stain results in new footprints area of A-4 group (a–c) and DR-4 group (d–f). The dashed border represents the interface between osseous region and soft tissue. Fibrocartilage maturation is obvious in new enthesis area, with positive Collagen II and Collagen III secretion in A-4 group, but not in DR-4 group. (a) The fibrocartilage maturation score was Grade 3, where fibrous tissue ingrowth and fibrocartilage cells (arrow) were obvious in new enthesis. (b) Positive collagen II signal in fibrocartilage cells (arrow). (c) Positive collagen III signal in fibrocartilage cells (arrow). (d) The fibrocartilage maturation score was Grade 2 in DR-4 group. (e) Negative collagen II signal in fibrocartilage cells. (f) Negative collagen III signal in fibrocartilage cells.
Figure 9.
H&E stain and IHC stain results in new footprints area of A-4 group (a–c) and DR-4 group (d–f). The dashed border represents the interface between osseous region and soft tissue. Fibrocartilage maturation is obvious in new enthesis area, with positive Collagen II and Collagen III secretion in A-4 group, but not in DR-4 group. (a) The fibrocartilage maturation score was Grade 3, where fibrous tissue ingrowth and fibrocartilage cells (arrow) were obvious in new enthesis. (b) Positive collagen II signal in fibrocartilage cells (arrow). (c) Positive collagen III signal in fibrocartilage cells (arrow). (d) The fibrocartilage maturation score was Grade 2 in DR-4 group. (e) Negative collagen II signal in fibrocartilage cells. (f) Negative collagen III signal in fibrocartilage cells.
Table 1.
Material testing machine characteristics.
Table 1.
Material testing machine characteristics.
| Model | Capacity | Speed Accuracy | Load Accuracy | Capacity |
|---|
| HT-2402EC | 500 Kgf | ±1% | 0.01% | 0.005~500 mm/min |
Table 2.
Scoring system of cell response after surgical repair.
Table 2.
Scoring system of cell response after surgical repair.
| Cell Type/Response | Score |
|---|
| 0 | 1 | 2 | 3 | 4 |
|---|
| Polymorphonuclear cells | 0 | 1–5/phf a | 5–10/phf | Heavy infiltrate | Packed |
| Lymphocytes | 0 |
| Plasma cells | 0 |
| Macrophages | 0 |
| Giant cells | 0 | 1–2/phf | 3–5/phf | Sheets |
| Necrosis | 0 | Minimal | Mild | Moderate | Severe |
Table 3.
Scoring system of tissue alternation.
Table 3.
Scoring system of tissue alternation.
| Response | Score |
|---|
| 0 | 1 | 2 | 3 | 4 |
|---|
| Neovascularization | 0 | Minimal capillary proliferation, focal, 1–3 buds | Groups of 4–7 capillaries with supporting fibroblastic structures | Broad band of capillaries with supporting structures | Extensive band with supporting fibroblastic structures |
| Fibrosis | 0 | Narrow band | Moderately thick band | Thick band | Extensive band |
| Fatty infiltrate | 0 | Minimal amount of fat associated with fibrosis | Several layers of fat and fibrosis | Elongated and broad accumulation of fat cells about the implant site | Extensive fat completely surrounding the implant |
Table 4.
Scoring system of the overall rating of the surgical impact.
Table 4.
Scoring system of the overall rating of the surgical impact.
| Rating | Score |
|---|
| Minimal or no reaction | (0.0 up to 2.9) |
| Slight reaction | (3.0 up to 8.9) |
| Moderate reaction | (9.0 up to 15.0) |
| Severe reaction | (>15) |
Table 5.
Semi-quantitative scoring system of the enthesis maturation.
Table 5.
Semi-quantitative scoring system of the enthesis maturation.
| Grading | C | I | F | T | Definition |
|---|
| 1 | + | | | | The insertion had continuity without fibrous tissue or bone ingrowth |
| 2 | + | + | | | The insertion had continuity with fibrous tissue ingrowth, but no fibrocartilage cells |
| 3 | + | + | + | | The insertion had continuity with fibrous tissue ingrowth and fibrocartilage cells, but no tidemark |
| 4 | + | + | + | + | The insertion had continuity with fibrous tissue ingrowth, fibrocartilage cells, and a tidemark |
Table 6.
Maximal load of separate specimens in the biomechanical test. A: Augmented; DR: Double-row.
Table 6.
Maximal load of separate specimens in the biomechanical test. A: Augmented; DR: Double-row.
| Group (n = 6) | Average Maximal Load (N) |
|---|
| A-12 | 393.75 (84.40) * |
| DR-12 | 229.17 (43.94) |
| A-0 | 102.98 (23.14) |
| DR-0 | 94.32 (29.32) |
Table 7.
Cell response and tissue alternation in tendon-to-bone insertion site with and without PEEK augment. A: Augmented; DR: Double-row.
Table 7.
Cell response and tissue alternation in tendon-to-bone insertion site with and without PEEK augment. A: Augmented; DR: Double-row.
| Group | A-4 (n = 6) | DR-4 (n = 6) |
| | Score |
| Average | 99/6 = 16.5 | 72/6 = 12.0 |
Difference between A-4 & DR-4:
16.5 − 12 = 4.5, Slight reaction |
Table 8.
Result of tissue remodeling. A: Augmented; DR: Double-row.
Table 8.
Result of tissue remodeling. A: Augmented; DR: Double-row.
| Group | A-4 | DR-4 | |
| Sample quantity | 6 | 6 | |
| Enthesis maturation grade | Native footprint part | p-value |
| Grade ≥ 3 | 3/6 (50.0%) | 1/6 (16.7%) | 0.545 |
| Median (Min:Max) | 2.5 (2:3) | 2 (2:3) | 0.545 |
| | New footprint part | |
| Grade ≥ 3 | 5/6 (83.3%) | 3/6 (50.0%) | 0.545 |
| Median (Min:Max) | 3 (2:3) | 2.5 (2:3) | 0.242 |
Table 9.
IHC stain from native footprint part. A: Augmented; DR: Double-row.
Table 9.
IHC stain from native footprint part. A: Augmented; DR: Double-row.
| Group | A-4 | DR-4 |
| Sample quantity | 6 | 6 |
| Native footprint part, bone | | |
| Collagen I (+) | 0/6 | 0/6 |
| Collagen II (+) | 0/6 | 2/6 |
| Collagen III (+) | 0/6 | 0/6 |
| Native footprint part, cartilage | | |
| Collagen I (+) | 1/5 | 1/3 |
| Collagen II (+) | 5/5 | 3/3 |
| Collagen III (+) | 5/5 | 3/3 |
| Native footprint part, tendon | | |
| Collagen I (+) | 0/6 | 0/6 |
| Collagen II (+) | 2/6 | 1/6 |
| Collagen III (+) | 2/6 | 2/6 |
Table 10.
IHC stain from new footprint part. A: Augmented; DR: Double-row.
Table 10.
IHC stain from new footprint part. A: Augmented; DR: Double-row.
| Group | A-4 | DR-4 |
| Sample quantity | 6 | 6 |
| New footprint part, bone | | |
| Collagen I (+) | 0/6 | 0/6 |
| Collagen II (+) | 1/6 | 3/6 |
| Collagen III (+) | 0/6 | 0/6 |
| New footprint part, cartilage | | |
| Collagen I (+) | 1/3 | 0/1 |
| Collagen II (+) | 3/3 | 1/1 |
| Collagen III (+) | 3/3 | 1/1 |
| New footprint part, tendon | | |
| Collagen I (+) | 0/6 | 0/6 |
| Collagen II (+) | 1/6 | 0/6 |
| Collagen III (+) | 4/6 | 0/6 |