Efficient Bioremediation of Petroleum-Contaminated Soil by Immobilized Bacterial Agent of Gordonia alkanivorans W33
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Soil, Biocarrier, and Biosurfactant
2.2. Cultivation of Bacteria
2.3. Optimization of the Fermentation Conditions of G. alkanivorans W33
2.3.1. Single Factor Test for the Medium
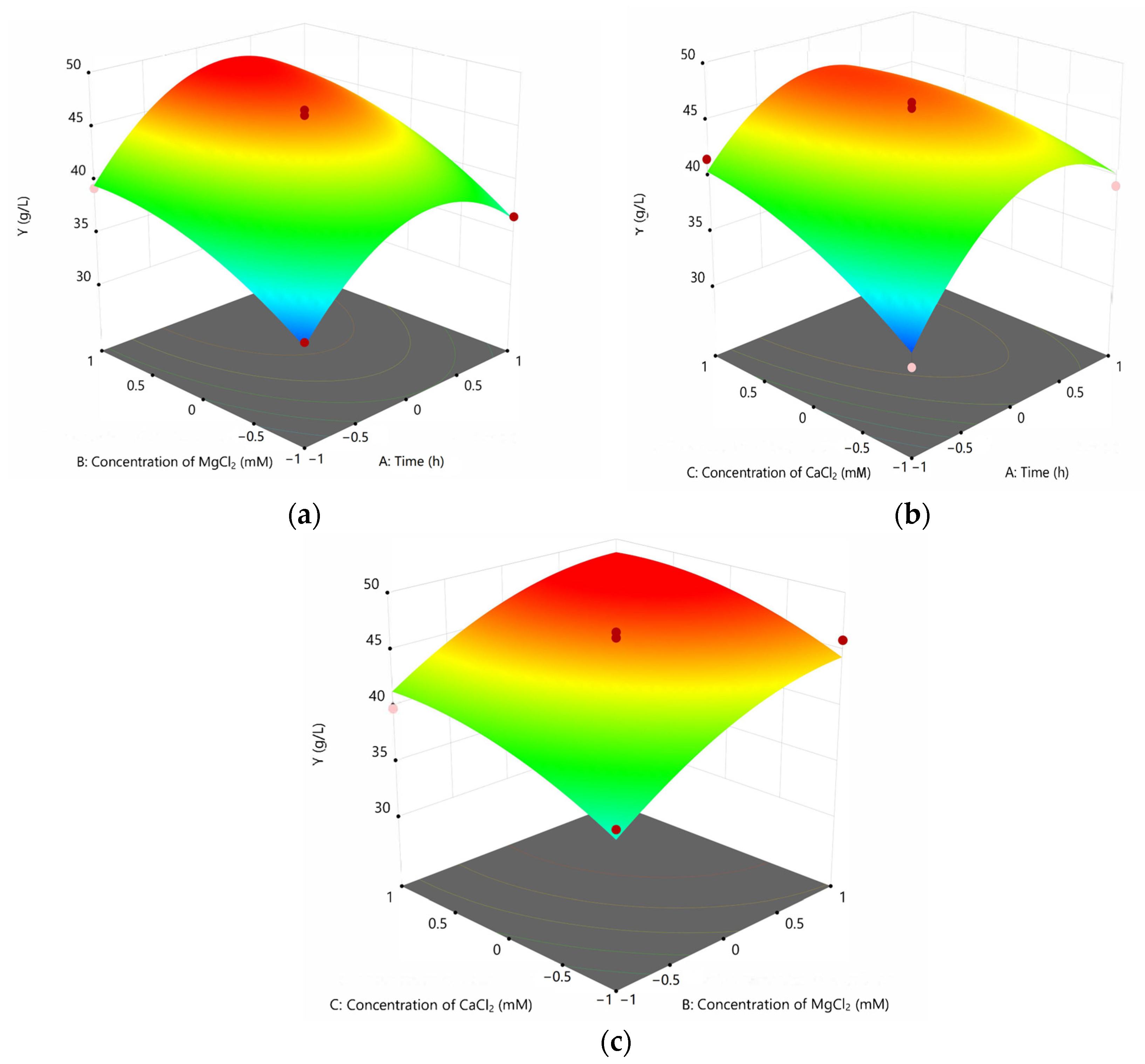
2.3.2. Box–Behnken Center Combination Design Experiment
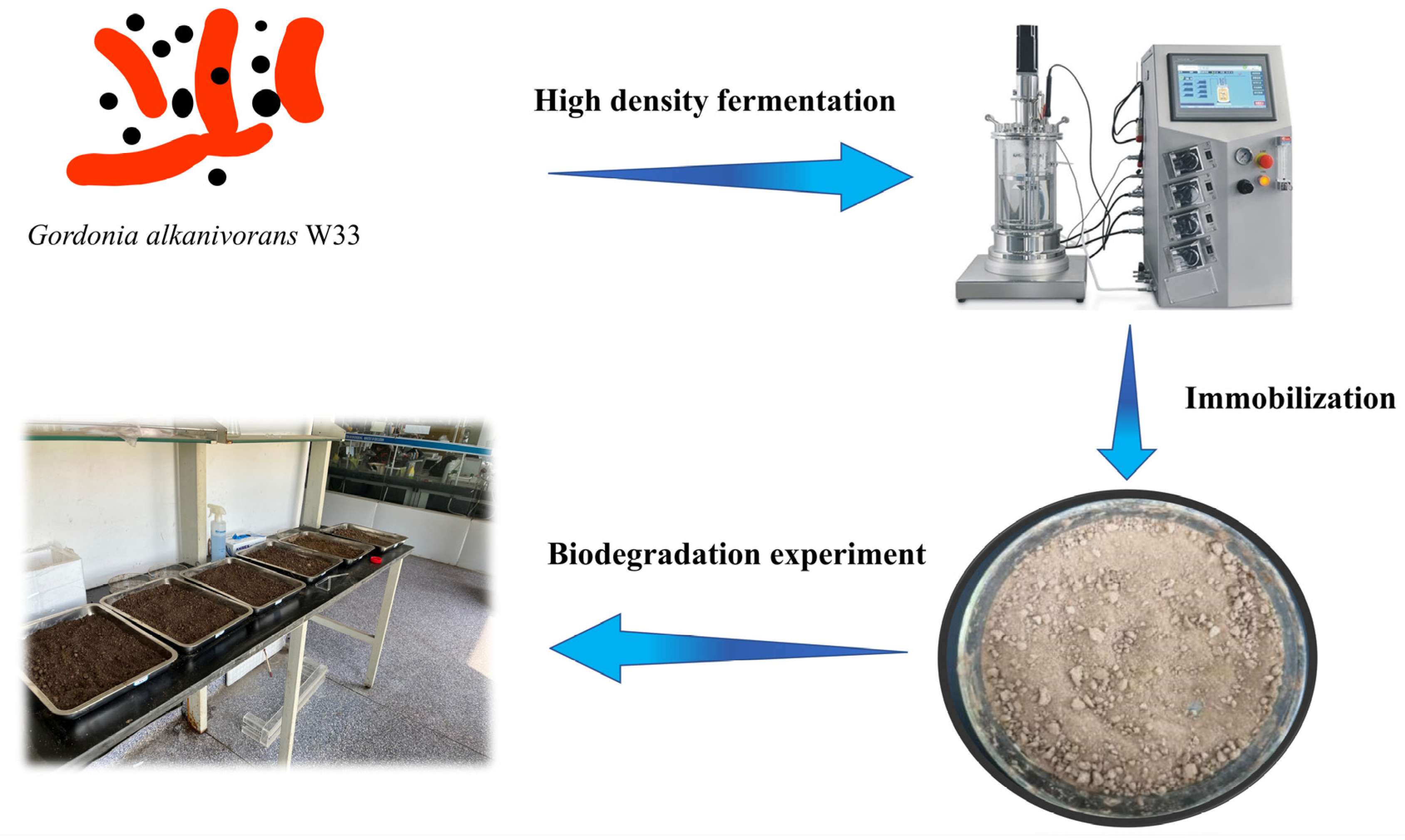
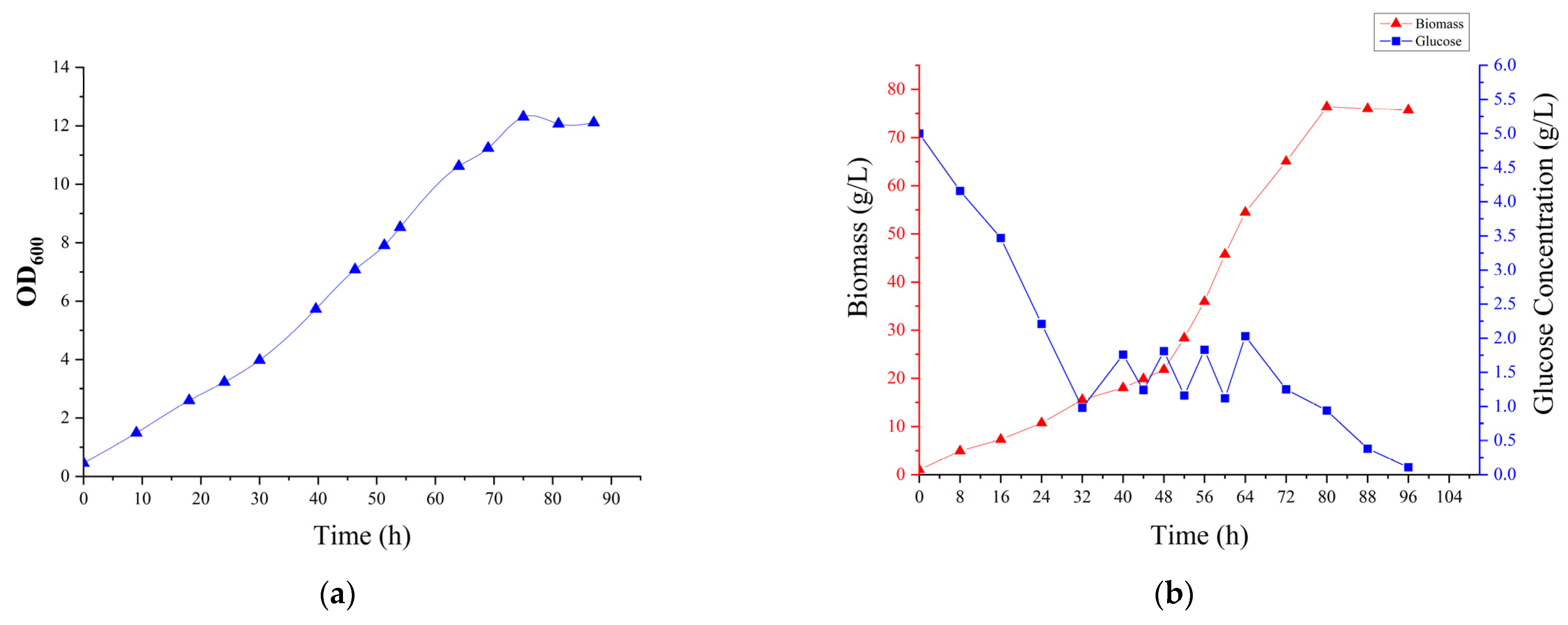
2.4. 5 L Fermenter Fermentation
2.5. Immobilization of the Bacteria and Detection of the Immobilized Consequent
2.6. Biodegradation of the Petroleum-Contaminated Soil
2.7. Determination of Petroleum Content in the Contaminated Soil
2.8. Observation of the Contaminated Soil by an Electron Microscope
3. Results
3.1. Shake Flask Fermentation for the Single-Factor Experiment
3.2. Box–Behnke Optimization
3.3. 5 L Fermenter Fermentation
3.4. Immobilization Effect of W33 in Different Carriers
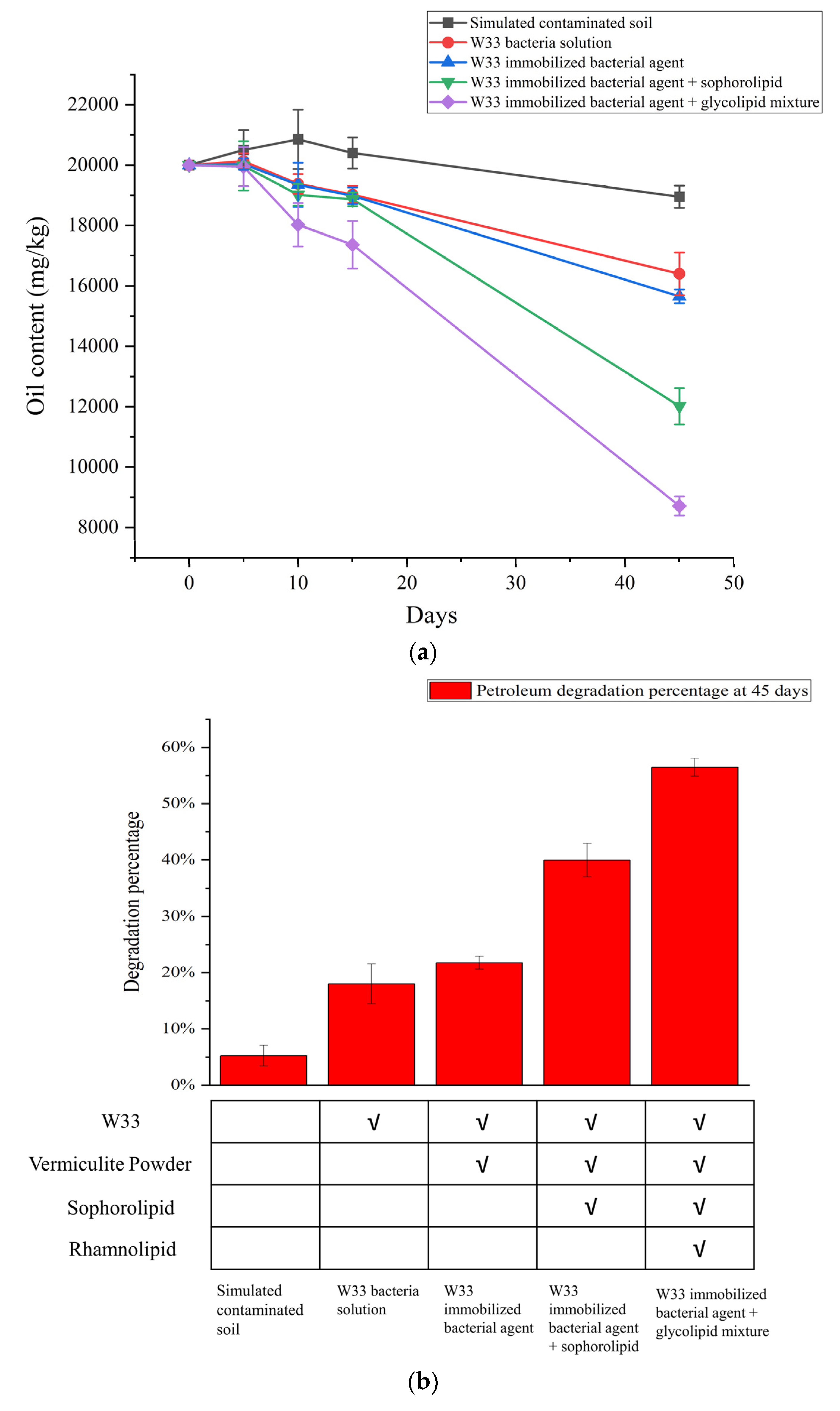
3.5. Biodegradation Analysis of Petroleum-Contaminated Soil between the Different W33 Bacterial Agents
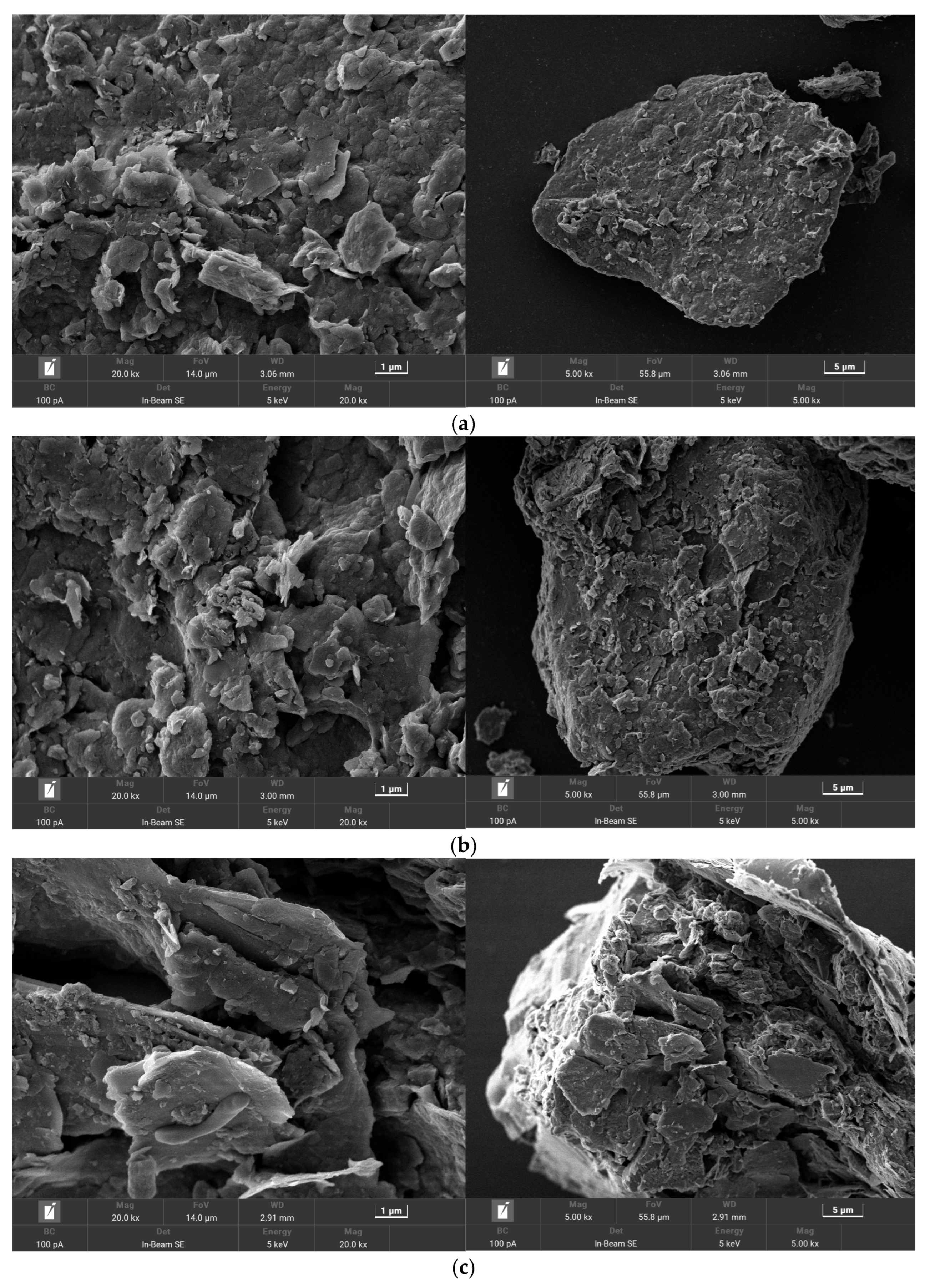
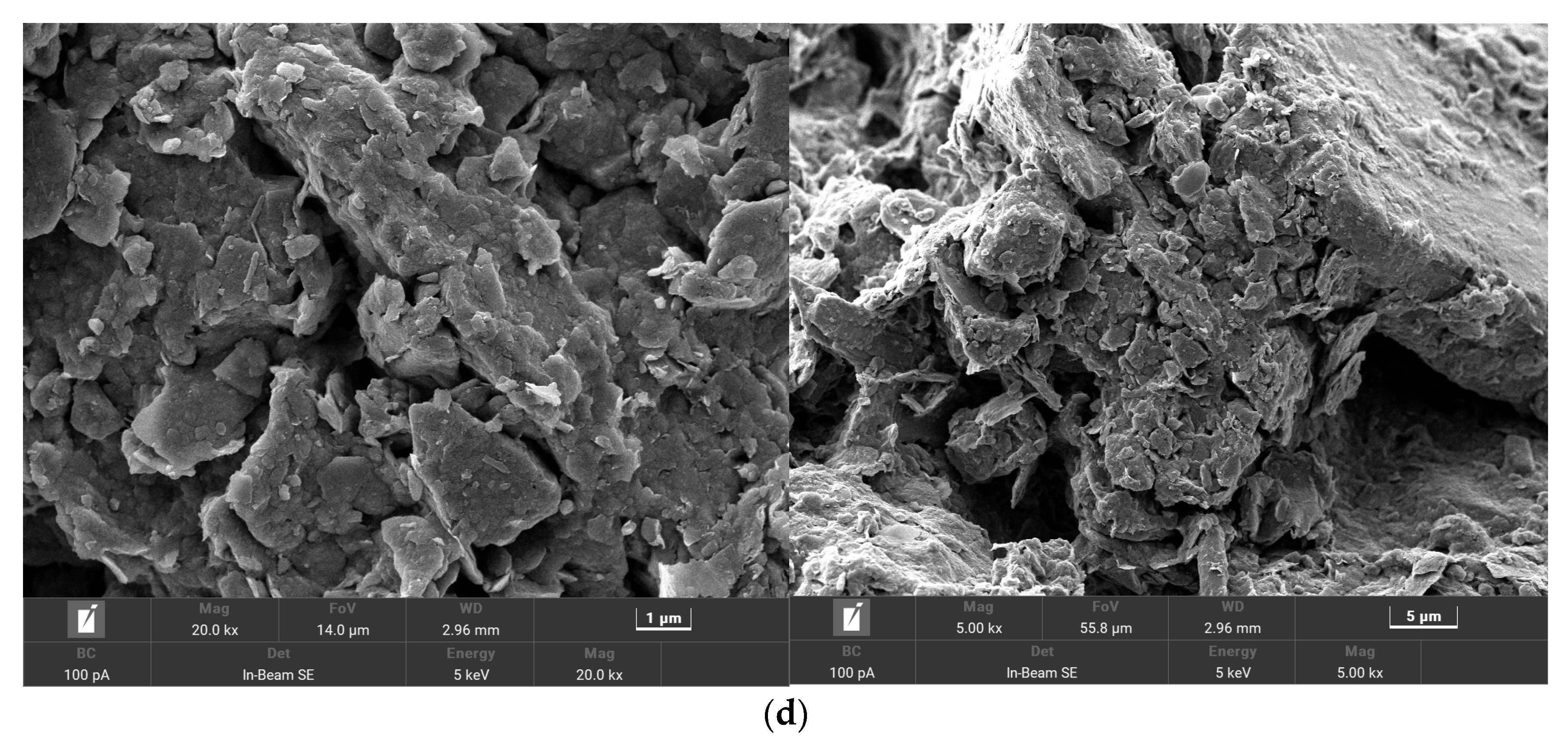
3.6. Analysis of the Microscopic Appearance of Each Petroleum Degradation Soil Group under an Electron Microscope
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, T.; Gondal, M.A. Monitoring and assessment of toxic metals in Gulf War oil spill contaminated soil using laser-induced breakdown spectroscopy. Environ. Monit. Assess. 2007, 136, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, F.; Lockington, R.; Megharaj, M.; Naidu, R. The Biodiversity Changes in the Microbial Population of Soils Contaminated with Crude Oil. Curr. Microbiol. 2016, 72, 663–670. [Google Scholar] [CrossRef]
- Baoune, H.; Aparicio, J.D.; Pucci, G.; El Hadj-Khelil, A.O.; Polti, M.A. Bioremediation of petroleum-contaminated soils using Streptomyces sp. Hlh1. J. Soils Sedim. 2019, 19, 2222–2230. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, J.-I.; Lee, S.S.; Lee, K.H.; Lee, J.; Kwon, E.E. Decontamination of petroleum-contaminated soil via pyrolysis under carbon dioxide atmosphere. J. Clean. Prod. 2019, 236, 117724. [Google Scholar] [CrossRef]
- Dorn, P.B.; Vipond, T.E.; Salanitro, J.P.; Wisniewski, H.L. Assessment of the acute toxicity of crude oils in soils using earthworms, Microtox (R) and plants. Chemosphere 1998, 37, 845–860. [Google Scholar] [CrossRef]
- Bidja Abena, M.T.; Li, T.; Shah, M.N.; Zhong, W. Biodegradation of total petroleum hydrocarbons (TPH) in highly contaminated soils by natural attenuation and bioaugmentation. Chemosphere 2019, 234, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Lin, X.; Zhang, C.; Li, Q.; Gong, Z. Biodegradation of aged polycyclic aromatic hydrocarbons (PAHs) by microbial consortia in soil and slurry phases. J. Hazard. Mater. 2008, 150, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, Z.; Jia, X.; Lu, W. Distribution of Bacterial Communities in Petroleum-Contaminated Soils from the Dagang Oilfield, China. Trans. Tianjin Univ. 2020, 26, 22–32. [Google Scholar] [CrossRef]
- Dickson, U.J.; Coffey, M.; Mortimer, R.J.G.; Di Bonito, M.; Ray, N. Mycoremediation of petroleum contaminated soils: Progress, prospects and perspectives. Environ. Sci. Process. Impacts 2019, 21, 1446–1458. [Google Scholar] [CrossRef]
- Meguro, N.; Kodama, Y.; Gallegos, M.-T.; Watanabe, K. Molecular characterization of resistance-nodulation-division transporters from solvent- and drug-resistant bacteria in petroleum-contaminated soil. Appl. Environ. Microbiol. 2005, 71, 580–586. [Google Scholar] [CrossRef]
- Cassidy, M.B.; Lee, H.; Trevors, J.T. Environmental applications of immobilized microbial cells: A review. J. Ind. Microbiol. 1996, 16, 79–101. [Google Scholar] [CrossRef]
- van Elsas, J.D.; Heijnen, C.E. Methods for the introduction of bacteria into soil: A review. Biol. Fertil. Soils 1990, 10, 127–133. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.-W.; Liu, R.-X.; Ju, H.-Y.; Bian, X.-K.; Zhang, W.-Z.; Zhang, C.-B.; Yang, T.; Guo, B.; Xiao, C.-L.; et al. Research progress in bioremediation of petroleum pollution. Environ. Sci. Pollut. Res. Int. 2021, 28, 46877–46893. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Onwosi, C.O. Bioremediation of gasoline contaminated agricultural soil by bioaugmentation. Environ. Technol. Innov. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Ebadi, A.; Sima, N.A.K.; Olamaee, M.; Hashemi, M.; Nasrabadi, R.G. Effective bioremediation of a petroleum-polluted saline soil by a surfactant-producing Pseudomonas aeruginosa consortium. J. Adv. Res. 2017, 8, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Bao, M.; Sun, P.; Li, Y. Study on bioadsorption and biodegradation of petroleum hydrocarbons by a microbial consortium. Bioresour. Technol. 2013, 149, 22–30. [Google Scholar] [CrossRef]
- Suja, F.; Rahim, F.; Taha, M.R.; Hambali, N.; Razali, M.R.; Khalid, A.; Hamzah, A. Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int. Biodeterior. Biodegrad. 2014, 90, 115–122. [Google Scholar] [CrossRef]
- Grace Liu, P.; Chang, T.C.; Whang, L.-M.; Kao, C.-H.; Pan, P.-T.; Cheng, S.-S. Bioremediation of petroleum hydrocarbon contaminated soil: Effects of strategies and microbial community shift. Int. Biodeterior. Biodegrad. 2011, 65, 1119–1127. [Google Scholar] [CrossRef]
- Safdari, M.S.; Kariminia, H.-R.; Rahmati, M.; Fazlollahi, F.; Polasko, A.; Mahendra, S.; Wilding, W.V.; Fletcher, T.H. Development of bioreactors for comparative study of natural attenuation, biostimulation, and bioaugmentation of petroleum-hydrocarbon contaminated soil. J. Hazard. Mater. 2018, 342, 270–278. [Google Scholar] [CrossRef]
- Masy, T.; Demanèche, S.; Tromme, O.; Thonart, P.; Jacques, P.; Hiligsmann, S.; Vogel, T.M. Hydrocarbon biostimulation and bioaugmentation in organic carbon and clay-rich soils. Soil Biol. Biochem. 2016, 99, 66–74. [Google Scholar] [CrossRef]
- Ramadass, K.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Bioavailability of weathered hydrocarbons in engine oil-contaminated soil: Impact of bioaugmentation mediated by Pseudomonas spp. on bioremediation. Sci. Total Environ. 2018, 636, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Beškoski, V.P.; Gojgić-Cvijović, G.; Milić, J.; Miletić, S.; Šolević, T.; Vrvić, M.M. Ex situ bioremediation of a soil contaminated by mazut (heavy residual fuel oil)—A field experiment. Chemosphere 2011, 83, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Nutrient Leaching in a Colombian Savanna Oxisol Amended with Biochar. J. Environ. Qual. 2012, 41, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Kureel, M.K.; Geed, S.R.; Giri, B.S.; Rai, B.N.; Singh, R.S. Biodegradation and kinetic study of benzene in bioreactor packed with PUF and alginate beads and immobilized with Bacillus sp. M3. Bioresour. Technol. 2017, 242, 92–100. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, Y.; Hou, Y.; Arp, H.P.H.; Reid, B.J.; Cai, C. Enhanced biodegradation of PAHs in historically contaminated soil by M. gilvum inoculated biochar. Chemosphere 2017, 182, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Luth, M.; Kenney, R.; Crowley, D. Evaluation of pinewood biochar as a carrier of bacterial strain Enterobacter cloacae UW5 for soil inoculation. Appl. Soil Ecol. 2014, 84, 192–199. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhao, F.; Lu, C.; Zhao, G.-R.; Lu, W. Production of sesquiterpenoid zerumbone from metabolic engineered Saccharomyces cerevisiae. Metab. Eng. 2018, 49, 28–35. [Google Scholar] [CrossRef]
- Zhu, Y.; Ai, M.; Jia, X. Optimization of a Two-Species Microbial Consortium for Improved mcl-PHA Production From Glucose-Xylose Mixtures. Front. Bioeng. Biotechnol. 2022, 9, 794331. [Google Scholar] [CrossRef]
- Sun, X.; Peng, Z.; Li, C.; Zheng, Y.; Cheng, Y.; Zong, J.; Lu, F.; Li, Y.; Li, Q. Combinatorial metabolic engineering and tolerance evolving of Escherichia coli for high production of 2′-fucosyllactose. Bioresour. Technol. 2023, 372, 128667. [Google Scholar] [CrossRef]
- Chen, Y. Research on Degradation Performance, Mechanism and Application of Petroleum Hydrocarbon Degrading Bacteria; Tianjin University: Tianjin, China, 2014; p. 147. [Google Scholar]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V.; Plekhov, O.A.; Naimark, O.B.; Podorozhko, E.A.; Lozinsky, V.I. Biosurfactant-enhanced immobilization of hydrocarbon-oxidizing Rhodococcus ruber on sawdust. Appl. Microbiol. Biotechnol. 2013, 97, 5315–5327. [Google Scholar] [CrossRef]
- Mathew, A.K.; Crook, M.; Chaney, K.; Humphries, A.C. Comparison of entrapment and biofilm mode of immobilisation for bioethanol production from oilseed rape straw using Saccharomyces cerevisiae cells. Biomass Bioenergy 2013, 52, 1–7. [Google Scholar] [CrossRef]
- Boshagh, F.; Rostami, K.; Moazami, N. Immobilization of Enterobacter aerogenes on carbon fiber and activated carbon to study hydrogen production enhancement. Biochem. Eng. J. 2019, 144, 64–72. [Google Scholar] [CrossRef]
- Shamsollahi, Z.; Partovinia, A. Recent advances on pollutants removal by rice husk as a bio-based adsorbent: A critical review. J. Environ. Manag. 2019, 246, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Tezer, M.M.; Bundur, Z.B. Use of Natural Minerals to Immobilize Bacterial Cells for Remediating Cracks in Cement-Based Materials. J. Mater. Civil Eng. 2022, 34. [Google Scholar] [CrossRef]
- Lu, W.; Zhu, B.; XIaoqiang, J. Development and Optimization of Oil Spill Dispersant for High Viscosity Oil Spill. J. Tianjin Univ. 2019, 52, 26–32. [Google Scholar]
- Wyndham, R.C.; Costerton, J.W. Heterotrophic potentials and hydrocarbon biodegradation potentials of sediment microorganisms within the athabasca oil sands deposit. Appl. Environ. Microbiol. 1981, 41, 783–790. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Feng, C. Determination of Petroleum Content in Soil by Ultrasonic-Ultraviolet Method. J. China Univ. Pet. 1999, 82-83+93+122-123. [Google Scholar]
- Lagesson, V.; Lagesson-Andrasko, L.; Andrasko, J.; Baco, F. Identification of compounds and specific functional groups in the wavelength region 168-330 nm using gas chromatography with UV detection. J. Chromatogr. A 2000, 867, 187–206. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Ye, Z.; Borthwick, A.G.; Ni, J. Oil field wastewater treatment in Biological Aerated Filter by immobilized microorganisms. Process. Biochem. 2006, 41, 1475–1483. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.; Peng, S.; Tian, X.; Li, Z.; Zhou, R. Degradation of petroleum hydrocarbons by embedding immobilized crude oil degrading bacteria. Water Sci. Technol. 2020, 82, 2296–2303. [Google Scholar] [CrossRef]
- Pi, Y.; Meng, L.; Bao, M.; Sun, P.; Lu, J. Degradation of crude oil and relationship with bacteria and enzymatic activities in laboratory testing. Int. Biodeterior. Biodegrad. 2016, 106, 106–116. [Google Scholar] [CrossRef]
- Rigden, D.J.; Jedrzejas, M.J.; Moroz, O.V.; Galperin, M.Y. Structural diversity of calcium-binding proteins in bacteria: Single-handed EF-hands? Trends Microbiol. 2003, 11, 295–297. [Google Scholar] [CrossRef]
- Matthies, D.; Dalmas, O.; Borgnia, M.J.; Dominik, P.K.; Merk, A.; Rao, P.; Reddy, B.G.; Islam, S.; Bartesaghi, A.; Perozo, E.; et al. Cryo-EM Structures of the Magnesium Channel CorA Reveal Symmetry Break upon Gating. Cell 2016, 164, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Cowan, J.A. Metallobiochemistry of the magnesium ion: Characterization of the essential metal-binding site in Escherichia coli ribonuclease H. Eur. J. Biochem. 1994, 219, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Sreedhara, A.; Cowan, J.A. Structural and catalytic roles for divalent magnesium in nucleic acid biochemistry. Biometals 2002, 15, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.C.; Margolin, W. Ca2+-mediated GTP-dependent dynamic assembly of bacterial cell division protein FtsZ into asters and polymer networks in vitro. EMBO J. 1997, 16, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.O.; Tian, Y.M.; Zhao, X.H. The influence of dual-substrate-layer extensive green roofs on rainwater runoff quantity and quality. Sci. Total Environ. 2017, 592, 465–476. [Google Scholar] [CrossRef] [PubMed]
- van Veen, J.A.; van Overbeek, L.S.; van Elsas, J.D. Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. MMBR 1997, 61, 121–135. [Google Scholar]
- Sriram, K.; Lin, G.X.; Jefferson, A.M.; Goldsmith, W.T.; Jackson, M.; McKinney, W.; Frazer, D.G.; Robinson, V.A.; Castranova, V. Neurotoxicity Following Acute Inhalation Exposure to the Oil Dispersant COREXIT EC9500A. J. Toxicol. Environ. Health Part A Curr. Issues 2011, 74, 1405–1418. [Google Scholar] [CrossRef]
- Negri, A.P.; Luter, H.M.; Fisher, R.; Brinkman, D.L.; Irving, P. Comparative toxicity of five dispersants to coral larvae. Sci. Rep. 2018, 8, 3043. [Google Scholar] [CrossRef]
- MacInnis, C.Y.; Brunswick, P.; Park, G.H.; Buday, C.; Schroeder, G.; Fieldhouse, B.; Brown, C.E.; van Aggelen, G.; Shang, D. Acute Toxicity of Corexit EC9500A and Assessment of Dioctyl Sulfosuccinate as an Indicator for Monitoring Four Oil Dispersants Applied to Diluted Bitumen. Environ. Toxicol. Chem. 2018, 37, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.E.; Osborn, H.L. Comparison of toxicity and transcriptomic profiles in a diatom exposed to oil, dispersants, dispersed oil. Aquat. Toxicol. 2012, 124, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Sasaki, M.; Komatsu, K.; Miura, A.; Matsuda, H. Oil spill remediation by using the remediation agent JE1058BS that contains a biosurfactant produced by Gordonia sp. strain JE-1058. Bioresour. Technol. 2009, 100, 572–577. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, J.; Garamus, V.M.; Almásy, L.; Willumeit, R.; Mu, B.; Zou, A. Interaction of the Biosurfactant, Surfactin with Betaines in Aqueous Solution. Langmuir 2013, 29, 10648–10657. [Google Scholar] [CrossRef]
- Lessard, R.R.; Demarco, G. The significance of oil spill dispersants. Spill Sci. Technol. Bull. 2000, 6, 59–68. [Google Scholar] [CrossRef]
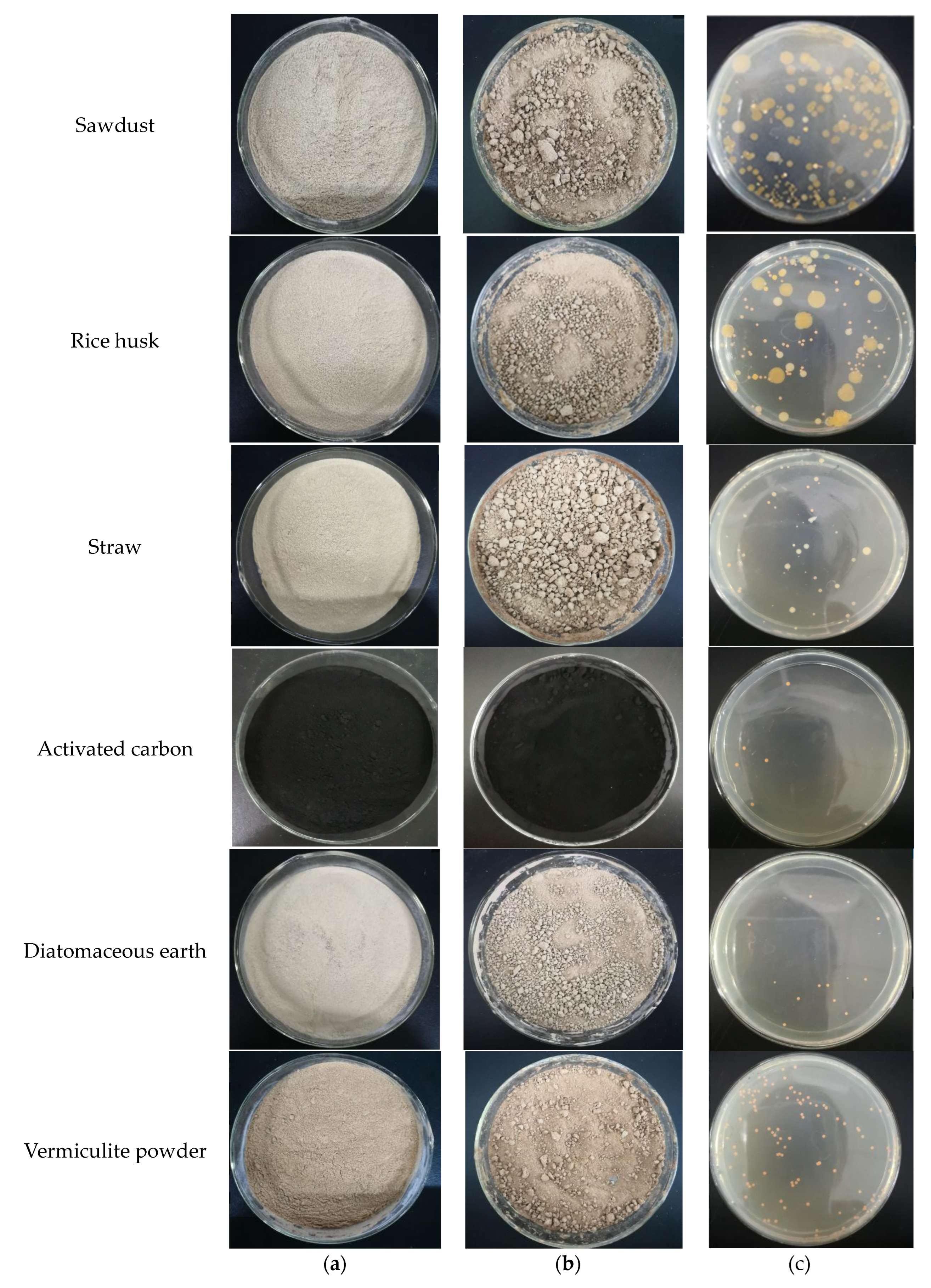
| Type of Carrier | Immobilization Rate of W33 (%) |
|---|---|
| Rice husk | 152 |
| Vermiculite powder | 82.6 |
| Sawdust | 56.2 |
| Diatomaceous earth | 20.6 |
| Activated carbon | 7.25 |
| Straw | 0.873 |
| Simulated Contaminated Soil | Simulated Contaminated Soil with the W33 Bacteria Solution added | Simulated Contaminated Soil with theW33-Immobilized Bacterial Agent added | Simulated Contaminated Soil with the W33-Immobilized Bacterial Agent with the Mixed Glycolipids added |
|---|---|---|---|
| 0 | 3.0 × 108 | 5.8 × 109 | 2.6 × 1010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, W.; Zhang, Z.; Yang, T.; Xu, Z.; Zhang, C.; Guo, B.; Lu, W. Efficient Bioremediation of Petroleum-Contaminated Soil by Immobilized Bacterial Agent of Gordonia alkanivorans W33. Bioengineering 2023, 10, 561. https://doi.org/10.3390/bioengineering10050561
Yang Y, Zhang W, Zhang Z, Yang T, Xu Z, Zhang C, Guo B, Lu W. Efficient Bioremediation of Petroleum-Contaminated Soil by Immobilized Bacterial Agent of Gordonia alkanivorans W33. Bioengineering. 2023; 10(5):561. https://doi.org/10.3390/bioengineering10050561
Chicago/Turabian StyleYang, Yong, Wanze Zhang, Zhanwei Zhang, Ting Yang, Zhuo Xu, Chuanbo Zhang, Bing Guo, and Wenyu Lu. 2023. "Efficient Bioremediation of Petroleum-Contaminated Soil by Immobilized Bacterial Agent of Gordonia alkanivorans W33" Bioengineering 10, no. 5: 561. https://doi.org/10.3390/bioengineering10050561
APA StyleYang, Y., Zhang, W., Zhang, Z., Yang, T., Xu, Z., Zhang, C., Guo, B., & Lu, W. (2023). Efficient Bioremediation of Petroleum-Contaminated Soil by Immobilized Bacterial Agent of Gordonia alkanivorans W33. Bioengineering, 10(5), 561. https://doi.org/10.3390/bioengineering10050561







